Summary
Clinical observations stemming from widespread employment of restorative L-3,4-dihydroxyphenylalanine (L-DOPA) therapy for management of dyskinesia in Parkinson’s Disease (PD) patients implicate a regulatory role for endogenous morphine in central nervous system dopamine neurotransmission. Reciprocally, it appears that restorative L-DOPA administration has provided us with a compelling in vivo pharmacological model for targeting peripheral sites involved in endogenous morphine expression in human subjects. The biological activities underlying endogenous morphine expression and its interaction with its major precursor dopamine strongly suggest that endogenous morphine systems are reciprocally dysregulated in PD. These critical issues are examined from historical and current perspectives within our short review.
Keywords: dopamine, L-DOPA, Parkinson’s disease, morphine, tetrahydropapaveroline
Background
The discipline of endogenous morphine research has experienced a prolonged gestational period of nearly four decades, often marked by skepticism and prejudicial disregard from a significant portion of the scientific community. Within the last decade, however, a wealth of complementary biochemical, molecular, and physiological studies emanating from major independent laboratories has provided indispensable self-validating, foundation data sets to solidify the status of endogenous morphine research as a vital component of the biological sciences. Briefly, indisputable Q-TOF tandem mass spectrometry has confirmed the presence of low steady-state levels of chemically authentic morphine in diverse animal cells and organ systems [1–12]. Biochemical studies have characterized multiple enzyme-catalyzed reactions and chemically defined intermediate precursor molecules within a regulated biosynthetic pathway that display striking similarities to the plant biosynthetic scheme previously established in Papaver somniferum [1–35]. Preclinical and clinical studies have demonstrated regulated expression of endogenous morphine upon physiological demand in normative regulatory processes and in dysregulatory disease states [2–35]. As a unifying principle, our laboratory has identified and characterized a cellular “morphinergic” signaling pathway functionally linked to constitutive nitric oxide production and mediated by cognate mu3 and mu4 opiate receptors [13,18,22,36,37].
In light of the above, we engage the privilege of historical hindsight to propose that clinical observations stemming from widespread employment of restorative L-3,4-dihydroxyphenylalanine (L-DOPA) therapy for management of dyskinesia in Parkinson’s Disease (PD) patients implicate a regulatory role for endogenous morphine in central nervous system (CNS) dopamine (DA) neurotransmission. Reciprocally, it appears that restorative L-DOPA administration has provided us with a compelling in vivo pharmacological model for targeting peripheral sites involved in endogenous morphine expression in human subjects.
Urinary Excretion of Morphine, Codeine, and Tetrahydropapaveroline by Parkinson’s Disease Patients: Presumptive Evidence for L-DOPA as a Morphine Precursor
It is twenty years since the publication of a clinical report indicating an approximate 20 fold enrichment of morphine, codeine, and the benzylisoquinoline (BIQ) alkaloid tetrahydropapaveroline (THP, also called norlaudanosoline) in the urine of Parkinson’s disease (PD) patients receiving L-DOPA replacement therapy, as compared to naive healthy controls [38]. The study was closely followed by a 1993 case report that quantified THP concentrations in the urine of 3 PD patients treated with L-DOPA-Carbidopa formulated as Sinemet [39]. In confirmation of the earlier report, the second study demonstrated marked increases in urinary THP concentrations that were roughly correlated with low, medium, and high administered dosages of L-DOPA-Carbidopa. These collected results provide putative evidence that endogenous morphine and codeine are synthesized in vivo utilizing L-DOPA and/or DA via the well characterized Pictet-Spengler condensation product THP [40–42] as an early intermediate precursor molecule. A later report demonstrated stereoselective expression of the (S) enantiomer of THP in human brain, thereby providing additional support for a regulated pathway of de novo synthesis of endogenous morphine via enzymatic O- and N-methyl transferase conversion of (S)-THP to (R)-reticuline [43].
Biological Signficance of Tetrahydropapaveroline as a Peripherally Expressed Morphine Precursor
Interestingly, the reports cited above are also confirmatory of a 1987 preclinical study demonstrating dramatic increases in rat brain concentrations of THP subsequent to peripheral co-administration of L-DOPA and ethanol [44]. Despite an excessively high concentration of ethanol (3 g/kg) that was administrated via the intraperitoneal route, it is apparent that a rapid synthesis of THP was accomplished over a 1–2 hour time frame. A first approximation of the rate of conversion of administered L-DOPA to THP yielded a value of approximately 0.1%. Of equal importance, a compartmental model emerges whereby THP is rapidly synthesized at peripheral sites, followed by rapid blood brain barrier transport into the CNS [45].
At this time, a cogent mechanism of peripheral THP biosynthesis in the presence or absence of ethanol has not been elucidated. Retrospectively, the contention of prominent scientists in alcohol addiction research that THP represents an aberrant and biologically deleterious DA derivative [40,42,46–52] that is markedly enhanced by ethanol, an ethanol metabolite such as acetaldehyde, or an enzyme involved in ethanol metabolism, i.e., acetaldehyde dehydrogenase appears to be critically flawed by the presence of THP in the urine of healthy, alcohol naïve, subjects [38]. Furthermore, the reluctance of alcoholism researchers to embrace THP as a naturally occuring morphine precursor is saliently at odds with preclinical studies demonstrating marked reductions of alcohol intake by opiate antagonists such as naloxone and naltrexone [53,54] and widespread clinical employment of naltrexone as a frontline pharmacotherapy for treatment of alcohol dependence [55].
In contrast to alcoholism research, there appeared to be a greater depth of critical thinking among PD researchers that pertained to positive and negative biological effects of THP and related tetrahydroisoquinoline alkaloids subsequent to L-DOPA administration. Despite a series of preclinical studies drawing a functional association between aberrant DA metabolism, cellular expression of THP and related tetrahydroisoquinoline alkaloids, and the etiology of PD [38,41,52,56–76], select clinical studies were supportive of positive therapeutic effects of pharmacologically administered morphine for treatment of PD dyskinesias [75,76]. Of potentially greater significance, a small body of biochemical and pharmacological studies demonstrated normative expression of THP and related tetrahydroisoquinoline alkaloids within the adrenal medulla and their associated regulatory activities on catecholamine synthetic and metabolic enzymes [57,68].
Spector’s laboratory was the first to quantify relatively high concentrations of chemically authentic morphine and codeine in rat adrenal gland [77]. Interestingly, levels of the penultimate morphine precursor codeine were found to be greater than those of morphine, suggesting a precursor to product biosynthetic relationship of the two opiate alkaloids in this glandular tissue. Relatively recently, our group has provided extensive empirical evidence supporting the role of the adrenal medulla as a major pheripheral site of endogenous morphine expression and physiological “hot spot” for opiate regulation of adrenergic sympethetic activities [2,4–7,9].
Based on the collective complementary lines of evidence presented above, we propose that restorative L-DOPA therapy for chronic management of PD patients represents an in vivo substrate loading model of rapid THP synthesis within peripheral sites, notably the adrenal medulla. Consistent with previous biochemical analyses [78,79], THP is further converted to key intermediate precursors within the morphine biosynthetic scheme, i.e., reticuline and salutaridine, at additional peripheral sites such as the liver, or is rapidly transported into the CNS. In support of these contentions, a prior clinical report has monitored relatively high concentrations of morphine and codeine in the cerebrospinal fluid (CSF) of healthy, opiate naïve, human volunteers [80] and implicates a regulatory role for endogenous morphine in normative CNS DA neurotransmission and as a potent restorative agent expressed from pharmacological administration of L-DOPA to PD patients.
Conclusions
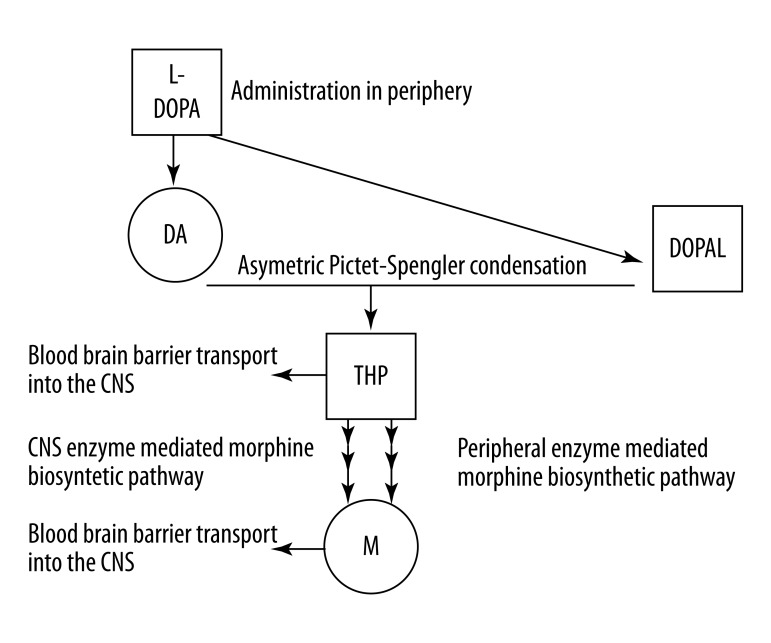
Historically, the identification of THP as a biologically active Pictet-Spengler condensation product of DA and 3,4-dihydroxyphenylacetaldehyde (DOPAL) preceded the identification of low steady-state levels of immunologically detectable morphine in several species of mammalian brain [81,82]. A similar enzymatic step in Papaver somniferum is mediated by the biosynthetic enzyme nococlaurine synthase that catalyzes an asymmetric Pictet-Spengler condensation of DA and 4-hydroxyphenylacetaldehyde to yield (S)-norcoclaurine, the plant equivalent of THP [83] (Figure 1).
Figure 1.
Biosynthesis of the putative morphine (M) intermediate precursor tetrahydropapaveroline (THP) proceeds via an asymmetric Pictet-Spengler condensation of dopamine (DA) and 3,4 dihydroxyphenylacetaldehyde (DOPAL) following peripheral administration of L- 3,4-dihydroxyphenylalanine (L-DOPA). Endogenous morphine is synthesized within peripheral sites via conversion of THP in an enzyme mediated biosynthetic pathway with striking similarities to that elucidated in Papaver somniferum. Conversely, THP may be directly transported into CNS and converted to endogenous morphine within a similar biosynthetic pathway.
De novo biosynthesis and utilization of endogenous morphine by animal systems is governed by a complex set of regulatory controls that reflect both evolutionary conservation and divergent adaptation of biochemical, molecular, and cellular processes required for the emergence, elaboration, and maintenance of DA-ergic and related catecholaminergic signaling systems [76,84].
Morphine, DA, and catecholamine synthesis and metabolism share a similar set of L-Tyrosine-related substrates and enzymes activities. The role of endogenous morphine as an evolutionary model in the adaptation and maintenance of DA and catecholamines as predominant signaling molecules in relatively simple and complex nervous/CNS structures defines its biological presence as an autocrine/paracrine regulator of cellular homeostasis [36,37,84–86]. The biological activities underlying endogenous morphine expression and its interaction with its major precursor DA strongly suggest that endogenous morphine systems are reciprocally dysregulated in PD [87,88].
Footnotes
Source of support: Self financing
References
- 1.Boettcher C, Fellermeier M, Boettcher C, et al. How human neuroblastoma cells make morphine. Proc Natl Acad Sci USA. 2005;102:8495–500. doi: 10.1073/pnas.0503244102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goumon Y, Bouret S, Casares F, et al. Lipopolysaccharide increases endogenous morphine levels in rat brain. Neuroscience Letters. 2000;293:135–38. doi: 10.1016/s0304-3940(00)01507-x. [DOI] [PubMed] [Google Scholar]
- 3.Goumon Y, Casares F, Pryor S, et al. Ascaris suum, an internal parasite, produces morphine. J Immunol. 2000;165:339–43. doi: 10.4049/jimmunol.165.1.339. [DOI] [PubMed] [Google Scholar]
- 4.Goumon Y, Weeks BS, Cadet P, Stefano GB. Identification of morphine in the adrenal medullary chromaffin PC-12 cell line. Mol Brain Res. 2000;81:177–80. doi: 10.1016/s0169-328x(00)00141-8. [DOI] [PubMed] [Google Scholar]
- 5.Goumon Y, Casares F, Zhu W, Stefano GB. The presence of morphine in ganglionic tissues of Modiolus deminissus: A highly sensitive method of quantitation for morphine and its derivatives. Mol Brain Res. 2001;86:184–88. doi: 10.1016/s0169-328x(00)00132-7. [DOI] [PubMed] [Google Scholar]
- 6.Goumon Y, Strub JM, Stefano GB, et al. Characterization of a morphine-like molecule in secretory granules of chromaffin cells. Med Sci Monit. 2005;11(5):MS31–34. [PubMed] [Google Scholar]
- 7.Goumon Y, Stefano GB. Identification of morphine in the rat adrenal gland. Mol Brain Res. 2000;77:267–69. doi: 10.1016/s0169-328x(00)00056-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhu W, Baggerman G, Goumon Y, et al. Presence of morphine and morphine-6-glucuronide in the marine mollusk Mytilus edulis ganglia determined by GC/MS and Q-TOF-MS. Starvation increases opiate alkaloid levels. Brain Res Mol Brain Res. 2001;88:155–60. doi: 10.1016/s0169-328x(01)00048-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhu W, Baggerman G, Goumon Y, et al. Identification of morphine and morphine-6-glucuronide in the adrenal medullary chromaffin PC-12 cell line by nano electrospray ionization double quadrupole orthogonal acceleration time of flight mass spectrometry. Eur J of Mass Spect. 2001;7:25–28. [Google Scholar]
- 10.Zhu W, Baggerman G, Secor WE, et al. Dracunculus medinensis and Schistosoma mansoni contain opiate alkaloids. Ann Trop Med Parasitol. 2002;96:309–16. doi: 10.1179/000349802125000808. [DOI] [PubMed] [Google Scholar]
- 11.Zhu W, Bilfinger TV, Baggerman G, et al. Presence of endogenous morphine and morphine 6 glucuronide in human heart tissue. Int J Mol Med. 2001;7:419–22. [PubMed] [Google Scholar]
- 12.Zhu W, Ma Y, Stefano GB. Presence of isoquinoline alkaloids in molluscan ganglia. Neuroendocrinol Lett. 2002;23:329–34. [PubMed] [Google Scholar]
- 13.Stefano GB, Digenis A, Spector S, et al. Opiate-like substances in an invertebrate, an opiate receptor on invertebrate and human immunocytes, and a role in immunosuppression. Proc Natl Acad Sci USA. 1993;90:11099–103. doi: 10.1073/pnas.90.23.11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brix-Christensen V, Goumon Y, Tonnesen E, et al. Endogenous morphine is produced in response to cardiopulmonary bypass in neonatal pigs. Acta Anaesthesiol Scand. 2000;44:1204–8. doi: 10.1034/j.1399-6576.2000.441004.x. [DOI] [PubMed] [Google Scholar]
- 15.Guarna M, Bianchi E, Bartolini A, et al. Endogenous morphine modulates acute thermonociception in mice. J Neurochem. 2002;80:271–77. doi: 10.1046/j.0022-3042.2001.00708.x. [DOI] [PubMed] [Google Scholar]
- 16.Stefano GB, Zhu W, Cadet P, et al. A hormonal role for endogenous opiate alkaloids: Vascular tissues. Neuro Endocrinol Lett. 2002;23:21–26. [PubMed] [Google Scholar]
- 17.Guarna M, Bartolini A, Ghelardini C, et al. Anti-mu opioid antiserum against the third external loop of the cloned mu opioid receptor acts a mu receptor neutral antagonist. Mol Brain Res. 2003;119:100–10. doi: 10.1016/j.molbrainres.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Cadet P, Mantione KJ, Stefano GB. Molecular identification and functional expression of mu3, a novel alternatively spliced variant of the human mu opiate receptor gene. J Immunol. 2003;170:5118–23. doi: 10.4049/jimmunol.170.10.5118. [DOI] [PubMed] [Google Scholar]
- 19.Stefano GB, Cadet P, Rialas CM, et al. Invertebrate Opiate Immune and Neural Signaling. In: Machelska H, Stein C, editors. Immune Mechanisms of Pain and Analgesia. New York, NY: Plenum Publ; 2003. pp. 126–47. [PubMed] [Google Scholar]
- 20.Zhu W, Ma Y, Cadet P, et al. Presence of reticuline in rat brain: A pathway for morphine biosynthesis. Mol Brain Res. 2003;117:83–90. doi: 10.1016/s0169-328x(03)00323-1. [DOI] [PubMed] [Google Scholar]
- 21.Cadet P, Rasmussen M, Zhu W, et al. Endogenous morphinergic signaling and tumor growth. Front Biosci. 2004;9:3176–86. doi: 10.2741/1471. [DOI] [PubMed] [Google Scholar]
- 22.Stefano GB, Zhu W, Cadet P, Mantione K. Morphine enhances nitric oxide release in the mammalian gastrointestinal tract via the m3 opiate receptor subtype: A hormonal role for endogenous morphine. J Physiol Pharmacol. 2004;55:279–88. [PubMed] [Google Scholar]
- 23.Stefano GB, Zhu W, Cadet P, et al. Music alters constitutively expressed opiate and cytokine processes in listeners. Med Sci Monit. 2004;10(6):MS18–27. [PubMed] [Google Scholar]
- 24.Zhu W, Pryor SC, Putnam J, et al. Opiate alkaloids and nitric oxide production in the nematode Ascaris suum. J Parasitol. 2004;90:15–22. doi: 10.1645/GE-3208. [DOI] [PubMed] [Google Scholar]
- 25.Zhu W, Ma Y, Bell A, et al. Presence of morphine in rat amygdala: Evidence for the mu3 opiate receptor subtype via nitric oxide release in limbic structures. Med Sci Monit. 2004;10(12):BR433–39. [PubMed] [Google Scholar]
- 26.Zhu W, Stefano GB. Reticuline exposure to invertebrate ganglia increases endogenous morphine levels. Neuro Endocrinol Lett. 2004;25:323–30. [PubMed] [Google Scholar]
- 27.Casares FM, McElroy A, Mantione KJ, et al. The American lobster, Homarus americanus, contains morphine that is coupled to nitric oxide release in its nervous and immune tissues: Evidence for neurotransmitter and hormonal signaling. Neuro Endocrinol Lett. 2005;26:89–97. [PubMed] [Google Scholar]
- 28.Zhu W, Mantione KJ, Shen L, Stefano GB. In vivo and in vitro L-DOPA exposure increases ganglionic morphine levels. Med Sci Monit. 2005;11(5):MS1–5. [PubMed] [Google Scholar]
- 29.Zhu W, Mantione KJ, Shen L, et al. Tyrosine and tyramine increase endogenous ganglionic morphine and dopamine levels in vitro and in vivo: CYP2D6 and tyrosine hydroxylase modulation demonstrates a dopamine coupling. Med Sci Monit. 2005;11(11):BR397–404. [PubMed] [Google Scholar]
- 30.Zhu W, Cadet P, Baggerman G, et al. Human white blood cells synthesize morphine: CYP2D6 modulation. J Immunol. 2005;175:7357–62. doi: 10.4049/jimmunol.175.11.7357. [DOI] [PubMed] [Google Scholar]
- 31.Dusek JA, Chang BH, Zaki J, et al. Association between oxygen consumption and nitric oxide production during the relaxation response. Med Sci Monit. 2006;12(1):CR1–10. [PubMed] [Google Scholar]
- 32.Zhu W, Mantione K, Kream RM, Stefano GB. Alcohol-, Nicotine-, and Cocaine-Evoked Release of Morphine from Human White Blood Cells: Substances of Abuse Actions Converge on. Endogenous Morphine Release Med Sci Monit. 2006;12(11):BR350–54. [PubMed] [Google Scholar]
- 33.Zhu W, Mantione KJ, Casares FM, et al. Alcohol-, nicotine-, and cocaine-evoked release of morphine from invertebrate ganglia: Model system for screening drugs of abuse. Med Sci Monit. 2006;12(5):BR155–61. [PubMed] [Google Scholar]
- 34.Zhu W, Cadet P, Mantione KJ, et al. Response to Comment on “Human White Blood Cells Synthesize Morphine: CYP2D6 Modulation”. J Immunol. 2006;176:5704. doi: 10.4049/jimmunol.175.11.7357. [DOI] [PubMed] [Google Scholar]
- 35.Zhu W, Mantione KJ, Casares FM, et al. Cholinergic regulation of endogenous morphine release from lobster nerve cord. Med Sci Monit. 2006;12(9):BR295–301. [PubMed] [Google Scholar]
- 36.Cadet P, Mantione KJ, Zhu W, et al. A functionally coupled mu3-like opiate receptor/nitric oxide regulatory pathway in human multi-lineage progenitor cells. J Immunol. 2007;179:5839–44. doi: 10.4049/jimmunol.179.9.5839. [DOI] [PubMed] [Google Scholar]
- 37.Kream RM, Sheehan M, Cadet P, et al. Persistence of evolutionary memory: Primordial six-transmembrane helical domain mu opiate receptors selectively linked to endogenous morphine signaling. Med Sci Monit. 2007;13(12):SC5–6. [PubMed] [Google Scholar]
- 38.Matsubara K, Fukushima S, Akane A, et al. Increased urinary morphine, codeine and tetrahydropapaveroline in parkinsonian patient undergoing L-3,4-dihydroxyphenylalanine therapy: a possible biosynthetic pathway of morphine from L-3,4-dihydroxyphenylalanine in humans. J Pharmacol Exp Ther. 1992;260:974–78. [PubMed] [Google Scholar]
- 39.Cashaw JL. Determination of tetrahydropapaveroline in the urine of parkinsonian patients receiving L-dopa-carbidopa (Sinemet) therapy by high-performance liquid chromatography. J Chromatogr. 1993;613:267–73. doi: 10.1016/0378-4347(93)80141-p. [DOI] [PubMed] [Google Scholar]
- 40.Walsh MJ, Davis VE, Yamanaka Y. Tetrahydropapaveroline: an alkaloid metabolite of dopamine in vitro. J Pharmacol Exp Ther. 1970;174:388–400. [PubMed] [Google Scholar]
- 41.Sandler M, Carter SB, Hunter KR, Stern GM. Tetrahydroisoquinoline alkaloids: in vivo metabolites of L-dopa in man. Nature. 1973;241:439–43. doi: 10.1038/241439a0. [DOI] [PubMed] [Google Scholar]
- 42.Weiner H. Relationship between 3,4-dihydroxyphenylacetaldehyde levels and tetrahydropapaveroline formation. Alcohol Clin Exp Res. 1978;2:127–31. doi: 10.1111/j.1530-0277.1978.tb04712.x. [DOI] [PubMed] [Google Scholar]
- 43.Sango K, Maruyama W, Matsubara K, et al. Enantio-selective occurrence of (S)-tetrahydropapaveroline in human brain. Neurosci Lett. 2000;283:224–26. doi: 10.1016/s0304-3940(00)00963-0. [DOI] [PubMed] [Google Scholar]
- 44.Cashaw JL, Geraghty CA, McLaughlin BR, Davis VE. Effect of acute ethanol administration on brain levels of tetrahydropapaveroline in L-dopa-treated rats. J Neurosci Res. 1987;18:497–503. doi: 10.1002/jnr.490180318. [DOI] [PubMed] [Google Scholar]
- 45.Cashaw JL, Geraghty CA. Tetrahydropapaveroline and the blood-brain barrier in rats. Alcohol. 1991;8:317–19. doi: 10.1016/0741-8329(91)90481-b. [DOI] [PubMed] [Google Scholar]
- 46.Clow A, Stolerman IP, Murray RM, Sandler M. Ethanol preference in rats: increased consumption after intraventricular administration of tetrahydropapaveroline. Neuropharmacology. 1983;22:563–65. doi: 10.1016/0028-3908(83)90181-8. [DOI] [PubMed] [Google Scholar]
- 47.Duncan CC, Fernando PW. Effects of tetrahydropapaveroline in the nucleus accumbens and the ventral tegmental area on ethanol preference in the rat. Alcohol. 1991;8:87–90. doi: 10.1016/0741-8329(91)91314-r. [DOI] [PubMed] [Google Scholar]
- 48.Halushka PV, Hoffmann PC, Davis VE, Walsh MJ. Alcohol addiction and tetrahydropapaveroline. Science. 1970;169:1104–6. doi: 10.1126/science.169.3950.1104. [DOI] [PubMed] [Google Scholar]
- 49.Myers RD. Anatomical “circuitry” in the brain mediating alcohol drinking revealed by THP-reactive sites in the limbic system. Alcohol. 1990;7:449–59. doi: 10.1016/0741-8329(90)90031-7. [DOI] [PubMed] [Google Scholar]
- 50.Myers RD, Robinson DE. Tetrahydropapaveroline injected in the ventral tegmental area shifts dopamine efflux differentially in the shell and core of nucleus accumbens in high-ethanol-preferring (HEP) rats. Alcohol. 1999;18:83–90. doi: 10.1016/s0741-8329(99)00008-7. [DOI] [PubMed] [Google Scholar]
- 51.Sallstrom BS, Hill R, Kiianmaa K, Rommelspacher H. Effect of ethanol on (R)- and (S)-salsolinol, salsoline, and THP in the nucleus accumbens of AA and ANA rats. Alcohol. 1999;18:165–69. doi: 10.1016/s0741-8329(98)00080-9. [DOI] [PubMed] [Google Scholar]
- 52.Sandler M, Glover V, Armando I, Clow A. Pictet-Spengler condensation products, stress and alcoholism: some clinical overtones. Prog Clin Biol Res. 1982;90:215–26. [PubMed] [Google Scholar]
- 53.Myers RD, Critcher EC. Naloxone alters alcohol drinking induced in the rat by tetrahydropapaveroline (THP) infused ICV. Pharmacol Biochem Behav. 1982;16:827–36. doi: 10.1016/0091-3057(82)90243-x. [DOI] [PubMed] [Google Scholar]
- 54.Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 55.Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets. 2010;9:13–22. doi: 10.2174/187152710790966704. [DOI] [PubMed] [Google Scholar]
- 56.Heikkila R, Cohen G, Dembiec D. Tetrahydroisoquinoline alkaloids: uptake by rat brain homogenates and inhibition of catecholamine uptake. J Pharmacol Exp Ther. 1971;179:250–58. [PubMed] [Google Scholar]
- 57.Greenberg RS, Cohen G. Tetrahydroisoquinoline alkaloids: stimulated secretion from the adrenal medulla. J Pharmacol Exp Ther. 1973;184:119–28. [PubMed] [Google Scholar]
- 58.Katz S, Cohen G. A comparison of 6,7-dihydroxytetrahydroisoquinoline, salsolinol and tetrahydropapaveroline as inhibitors of monoamine oxidase within the adrenergic nerve plexus of the isolated mouse atrium. Res Commun Chem Pathol Pharmacol. 1976;13:217–24. [PubMed] [Google Scholar]
- 59.Britton DR. A convergent approach to the pharmacology of tetrahydroisoquinolines. Prog Clin Biol Res. 1982;90:321–26. [PubMed] [Google Scholar]
- 60.Suzuki K, Mizuno Y, Yoshida M. Inhibition of mitochondrial respiration by 1,2,3,4-tetrahydroisoquinoline-like endogenous alkaloids in mouse brain. Neurochem Res. 1990;15:705–10. doi: 10.1007/BF00973651. [DOI] [PubMed] [Google Scholar]
- 61.Niwa T, Maruyama W, Nakahara D, et al. Endogenous synthesis of N-methylsalsolinol, an analogue of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, in rat brain during in vivo microdialysis with salsolinol, as demonstrated by gas chromatography-mass spectrometry. J Chromatogr. 1992;578:109–15. doi: 10.1016/0378-4347(92)80231-e. [DOI] [PubMed] [Google Scholar]
- 62.Naoi M, Maruyama W, Kasamatsu T, Dostert P. Oxidation of N-methyl(R)salsolinol: involvement to neurotoxicity and neuroprotection by endogenous catechol isoquinolines. J Neural Transm Suppl. 1998;52:125–38. doi: 10.1007/978-3-7091-6499-0_14. [DOI] [PubMed] [Google Scholar]
- 63.Collins MA. Tetrahydropapaveroline in Parkinson’s disease and alcoholism: a look back in honor of Merton Sandler. Neurotoxicology. 2004;25:117–20. doi: 10.1016/S0161-813X(03)00145-1. [DOI] [PubMed] [Google Scholar]
- 64.Johnston GA. L-dopa and pyridoxal 5′-phosphate: tetrahydroisoquinoline formation. Lancet. 1971;1:1068. doi: 10.1016/s0140-6736(71)91629-1. [DOI] [PubMed] [Google Scholar]
- 65.Davis VE, Cashaw JL, McMurtrey KD. Disposition of catecholamine-derived alkaloids in mammalian systems. Adv Exp Med Biol. 1975;59:65–78. doi: 10.1007/978-1-4757-0632-1_6. [DOI] [PubMed] [Google Scholar]
- 66.Coscia CJ, Burke W, Jamroz G, et al. Occurrence of a new class of tetrahydroisoquinoline alkaloids in L-dopa-treated parkinsonian patients. Nature. 1977;269:617–19. doi: 10.1038/269617a0. [DOI] [PubMed] [Google Scholar]
- 67.Cadet P, Zhu W, Mantione K, et al. Cyclic exercise induces anti-inflammatory signal molecule increases in the plasma of Parkinson’s patients. Int J Mol Med. 2003;12:485–92. [PubMed] [Google Scholar]
- 68.Galloway MP, Burke WJ, Coscia CJ. Tetrahydroisoquinolinecarboxylic acids and catecholamine metabolism in adrenal medulla explants. Biochem Pharmacol. 1982;31:3251–56. doi: 10.1016/0006-2952(82)90558-5. [DOI] [PubMed] [Google Scholar]
- 69.Nimit Y, Schulze I, Cashaw JL, et al. Interaction of catecholamine-derived alkaloids with central neurotransmitter receptors. J Neurosci Res. 1983;10:175–89. doi: 10.1002/jnr.490100207. [DOI] [PubMed] [Google Scholar]
- 70.Okada T, Shimada S, Sato K, et al. Tetrahydropapaveroline and its derivatives inhibit dopamine uptake through dopamine transporter expressed in HEK293 cells. Neurosci Res. 1998;30:87–90. doi: 10.1016/s0168-0102(97)00121-1. [DOI] [PubMed] [Google Scholar]
- 71.Soh Y, Shin MH, Lee JS, et al. Oxidative DNA damage and glioma cell death induced by tetrahydropapaveroline. Mutat Res. 2003;544:129–42. doi: 10.1016/j.mrrev.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 72.Shin MH, Jang JH, Surh YJ. Potential roles of NF-kappaB and ERK1/2 in cytoprotection against oxidative cell death induced by tetrahydropapaveroline. Free Radic Biol Med. 2004;36:1185–94. doi: 10.1016/j.freeradbiomed.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 73.Kim YM, Kim MN, Lee JJ, Lee MK. Inhibition of dopamine biosynthesis by tetrahydropapaveroline. Neurosci Lett. 2005;386:1–4. doi: 10.1016/j.neulet.2005.04.105. [DOI] [PubMed] [Google Scholar]
- 74.Fricchione GL, Stefano GB. Placebo neural systems: Nitric oxide, morphine and the dopamine brain reward and motivation circuitries. Med Sci Monit. 2005;11(5):MS54–65. [PubMed] [Google Scholar]
- 75.Berg D, Becker G, Reiners K. Reduction of dyskinesia and induction of akinesia induced by morphine in two parkinsonian patients with severe sciatica. J Neural Transm. 1999;106:725–28. doi: 10.1007/s007020050192. [DOI] [PubMed] [Google Scholar]
- 76.Berg D, Becker G, Naumann M, Reiners K. Morphine in tardive and idiopathic dystonia (short communication) J Neural Transm. 2001;108:1035–41. doi: 10.1007/s007020170022. [DOI] [PubMed] [Google Scholar]
- 77.Donnerer J, Cardinale G, Coffey J, et al. Chemical characterization and regulation of endogenous morphine and codeine in the rat. J Pharmacol Exp Ther. 1987;242:583–87. [PubMed] [Google Scholar]
- 78.Donnerer J, Oka K, Brossi A, et al. Presence and formation of codeine and morphine in the rat. Proc Natl Acad Sci USA. 1986;83:4566–67. doi: 10.1073/pnas.83.12.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weitz CJ, Faull KF, Goldstein A. Synthesis of the skeleton of the morphine molecule by mammalian liver. Nature. 1987;330:674–77. doi: 10.1038/330674a0. [DOI] [PubMed] [Google Scholar]
- 80.Cardinale GJ, Donnerer J, Finck AD, et al. Morphine and codeine are endogenous components of human cerebrospinal fluid. Life Sci. 1987;40:301–6. doi: 10.1016/0024-3205(87)90347-x. [DOI] [PubMed] [Google Scholar]
- 81.Gintzler AR, Levy A, Spector S. Antibodies as a means of isolating and characterizing biologically active substances: Presence of a non-peptide morphine-like compound in the central nervous system. Proc Natl Acad Sci USA. 1976;73:2132–36. doi: 10.1073/pnas.73.6.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gintzler AR, Gershon MD, Spector S. A nonpeptide morphine-like compound: immunocytochemical localization in the mouse brain. Science. 1978;199:447–48. doi: 10.1126/science.339350. [DOI] [PubMed] [Google Scholar]
- 83.Luk LY, Bunn S, Liscombe DK, et al. Mechanistic studies on norcoclaurine synthase of benzylisoquinoline alkaloid biosynthesis: an enzymatic Pictet-Spengler reaction. Biochemistry. 2007;46:10153–61. doi: 10.1021/bi700752n. [DOI] [PubMed] [Google Scholar]
- 84.Stefano GB, Kream RM. Endogenous morphine synthetic pathway preceded and gave rise to catecholamine synthesis in evolution (Review) Int J Mol Med. 2007;20:837–41. [PubMed] [Google Scholar]
- 85.Stefano GB, Bianchi E, Guarna M, et al. Nicotine, alcohol and cocaine coupling to reward processes via endogenous morphine signaling: The dopamine-morphine hypothesis. Med Sci Monit. 2007;13(6):RA91–102. [PubMed] [Google Scholar]
- 86.Nasuti C, Gabbianelli R, Falcioni ML, et al. Dopaminergic system modulation, behavioral changes, and oxidative stress after neonatal administration of pyrethroids. Toxicology. 2007;229:194–205. doi: 10.1016/j.tox.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 87.Stefano GB, Kream RM, Mantione KJ, Ptacek R. Endogenous morphine, stress and psychiatric disorders – review of actual findings. Journal of Czech Psychology. 2012 In Press. [Google Scholar]
- 88.Kream RM, Stefano GB. Schizophrenia: Comorbidity and/or Self Medication. Biomedical Papers. 2012 In Press. [Google Scholar]