Summary
Background
The objective of this study was to assess the concentration of metalloproteinase-2 (MMP-2) and metalloproteinase-9 (MMP-9) in peripheral circulation and their mRNA expression in peripheral blood mononuclear cells (PBMCs) in patients with CAP caused by M. pneumoniae.
Material/Methods
We prospectively analyzed MMPs in 40 hospitalized patients with M. pneumoniae CAP on admission, and in the convalescent phase. Twenty healthy men were used as controls. Quantitative real-time PCR and ELISA tests were used.
Results
MMP-9 mRNA expression in PBMCs was increased in the acute phase of illness compared to the control group as well as in convalescent phase in which case it was statistically significant (Mann-Whitney; p=0.028). The same was found for MMP-9 plasma levels (Mann-Whitney test; p<0.001; p=0.001). Circulating MMP-2 concentration in acute patients was significantly lower than in the control group and convalescent phase (Mann-Whitney test; p=0.012; p=0.001), while no MMP-2 mRNA expression was found in PBMCs. The plasma level of MMP-9 correlated with leukocyte count in peripheral circulation (r=0.67, p<0.001).
Conclusions
We conclude that M. pneumoniae in adult CAP induces activity of MMP-9 in peripheral blood circulation.
Keywords: community-acquired pneumonia, matrix metalloproteinase-2, matrix metalloproteinase-9, Mycoplasma pneumoniae
Background
Mycoplasma pneumoniae is a human pathogen and a frequent cause of community-acquired respiratory infections. Pneumonia is the most severe type of M. pneumoniae respiratory infection and is most common among older children and young adults. Although M. pneumoniae ordinarily causes mild pneumonia (termed “walking pneumonia”), it is a significant cause of pneumonia in adults requiring hospitalization. Marston et al reported that M. pneumoniae was definitely responsible for 5.4% and possibly responsible for 32.5% of 2,776 cases of community-acquired pneumonia (CAP) in hospitalized adults in Ohio [1]. Porath et al showed M. pneumoniae to be second only to Streptococcus pneumoniae, and it was responsible for 29.2% of pneumonias overall [2]. M. pneumoniae is an important cause of CAP sufficiently severe to require hospitalization, especially in elderly persons. Fulminant infections with multiple organ involvement and deaths due to M. pneumoniae, usually in otherwise healthy adults and children, have been reported, but are uncommon [3].
Metalloproteinases (MMPs) are a family of proteolytic enzymes currently comprising at least 24 members [4]. Recently, various studies have shown that MMPs are implicated in the pathogenesis of various pulmonary inflammatory diseases. MMPs are very likely to have a central role in destructive pulmonary diseases where excess proteolytic activity causes aberrant degradation of the lung extracellular matrix (ECM) [5]. The cellular sources of these enzymes include leukocytes and bronchial epithelial cells [6]. Of these, MMP-2 and MMP-9 (also known as gelatinases A and B) have been reported to possess substrate specificity to type IV collagen and can degrade basement membrane structures via collagenolytic actions [7]. Although the substrate specificities of MMP-2 and MMP-9 seem similar, the 2 enzymes are known to be synthesized by different cells in vitro. MMP-2 is synthesized principally by dermal and gingival fibroblasts, endothelial cells, and osteoblasts, whereas MMP-9 is produced mainly by inflammatory cells, including polymorphonuclear leukocytes (PMLs), macrophages, eosinophils and lymphocytes [8–11]. Previous studies showed that activity of MMP-2 and MMP-9 is upregulated in many pulmonary disease [12]. However, the MMP-2 and MMP-9 activity in CAP caused by M. pneumoniae have not yet been studied. The role of various reactive substances in the pathogenesis of M. pneumoniae lung disease has been a topic of considerable interest during the past decade. Numerous clinical studies involving humans, as well as investigations based on animal models, have been reported. However, there is still a lack of knowledge about the pathogenesis of M. pneumoniae pulmonary infection.
The aim of the study was to investigate the gene expression of MMP-2 and MMP-9 in peripheral blood mononuclear cells (PBMCs) and their concentration in peripheral blood circulation in patients with CAP caused by M. pneumoniae. The results were correlated to the laboratory parameters and severity of disease.
Material and Methods
We prospectively followed 40 patients with CAP caused by M. pneumoniae hospitalized at the University Hospital for Infectious Diseases in Zagreb, Croatia. Patients who met the following criteria were included in the study: age between 12–60 years, clinical findings suggestive of pneumonia (cough, fever), evidence of a new pulmonary infiltrate on chest X-ray, and confirmed acute infection of M. pneumoniae. Exclusion criteria included dual infections, nosocomial pneumonia, patients with active tuberculosis and HIV-positive patients. The diagnosis of M. pneumoniae infection was based on serological testing of antibodies. Serum antibodies to M. pneumoniae were assayed by ELISA by using P1 membrane protein as antigen (Savyon Diagnostics LTD, Israel). Evidence of infection was defined as either a single positive serum IgM titre (>10) in any serum sample or a 4-fold increase in IgG titre in paired sera. Paired serum samples were also tested for antibodies to Chlamydophila pneumoniae, Chlamydophila psittaci, Legionella pneumophila and Coxiella burnetii. In addition, urine samples from patients were tested for L. pneumophila serogroup-1 antigen. Blood culture specimens were collected and in selected patients sputum culture was performed prior to antibiotic therapy. All patients were treated with azythromycin 1×500 mg orally for 3 days. On admission the following data were analysed: epidemiological data (age), chest X-ray and laboratory findings: erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), leukocytes and differential blood count.
Wa analyzed the gene expression of MMP-2 and MMP-9 in PBMCs and plasma level of MMP-2 and MMP-9 in male patients with CAP caused by M. pneumoniae on admission, and in the reconvalescent phase, 4 weeks later. Sera from 20 healthy males aged 14–50 years were used as controls. The study was approved by the local Ethics Committee and the examinees and/or their parents signed the informed consent.
Isolation of the peripheral blood mononuclear cells
From each patient and control, 10 ml of sodium-heparinized blood was collected. PBMCs were purified using standard Ficoll-Paque gradient centrifugation according to the instructions of the manufacturer (Pharmacia, Upsala, Sweeden). Briefly, 4 ml of Ficoll-Paque gradient was pipetted into centrifuge tubes. The heparinized blood was diluted 1:1 in phosphate-buffered saline (PBS) and carefully layered over the Ficoll-Paque gradient. The tubes were centrifuged for 15 min at 800 × g. The cell interface layer was harvested carefully, and the cells were washed twice in medium and resuspended in RPMI 1640 before counting. Cell concentration was adjusted to 107/ml.
RNA extraction and quantitative real-time PCR
Total cellular RNA was extracted using TRIzoI/RNA-Bee (Biogenesis, England) followed by phase separation with chloroform, then precipitation with isopropyl alcohol. Measurements of metalloproteinases were determined using quantitative real-time PCR. One μg of total RNA were translated into cDNK by reverse transcription using AMV reverse transcriptase enzyme and random primers. For the synthesis of cDNA, the 1st strand cDNA synthesis kit for RT-PCR (Roche Applied Science) was used according to manufacturer’s instructions. Upon completion of cDNA synthesis, we diluted reaction mixture to a final volume of 300 μl and samples were divided into smaller aliquotes and stored at −20°C until further testing. Levels of transcription of MMP-2 and MMP-9 were analyzed by quantitative real-time PCR. Target sequences were amplified using LightCycler primer set (Search-LC, Heidelberg, Germany) with LightCycler FastStart DNA Master SYBR Green I Kit (Roche Applied Science) according to the manufacturer’s instructions. Analysis of gene expression was performed on the LightCycler real-time, version 1.2 (Roche Diagnostics). GAPDH housekeeping gene was used to normalize samples for their further comparison.
ELISA test
MMP-2 and MMP-9 analyses were performed using a commercially available ELISA kit (Quantikine™, R&D Systems, Oxon, United Kingdom) according to the manufacterer’s instructions.
Statistical analysis
Means and standard deviations were calculated to summarize normal distribution continuous variables, while median and interquartile ranges were calculated for abnormal distribution continous variables. Statistical analysis was performed with the Kruskall-Wallis and the Mann-Whitney tests. A probability level of P<0.05 was considered significant. Correlations were evaluated by the Spearman rank correlation coefficient. The SPSS ver. 13.0 (SPSS Inc., Chicago, Il, USA) statistical software was used for the calculations.
Results
Clinical and laboratory data
The results of epidemiological and laboratory characteristics are shown in Table 1. There were young patients, hospitalized in the first week of disease, with mild elevated (ESR, CRP) or normal (leukocytes) laboratory findings (Table 1). Chest X-ray showed interstitial inflammatory infiltrates in 1 (28 or 70%), or multiple lobes (12 or 30%), and pleural effusion was recorded in 6 or 15% of patients (not shown in Table 1). All patients had positive clinical response to administered antibiotic therapy and no severe disease complications were recorded during hospitalization.
Table 1.
Epidemiological and laboratory characteristics of 40 patients with community acquired pneumonia caused by Mycoplasma pneumoniae.
| Epidemiological and laboratory characteristics of patients (n=40) | Median | Range (interquartile range) |
|---|---|---|
| Age (years) | 19 | 12–54 (8) |
| Duration of symptoms prior admission (days) | 5 | 1–7 (4) |
| Erythrocyte sedimentation rate (mm/h) | 40 | 6–88 (32) |
| C-reactive protein (mg/L) | 74 | 18–266 (82) |
| Leukocytes (×109/L) | 8.9 | 3.9–18.6 (4.3) |
| – neutrophyles (rel.%) | 72 | 53–83 (14) |
| – lymphocytes (rel.%) | 15 | 7–32 (10) |
| – monocytes (rel.%) | 11 | 3–21 (5) |
MMP-2 and MMP-9 gene expression
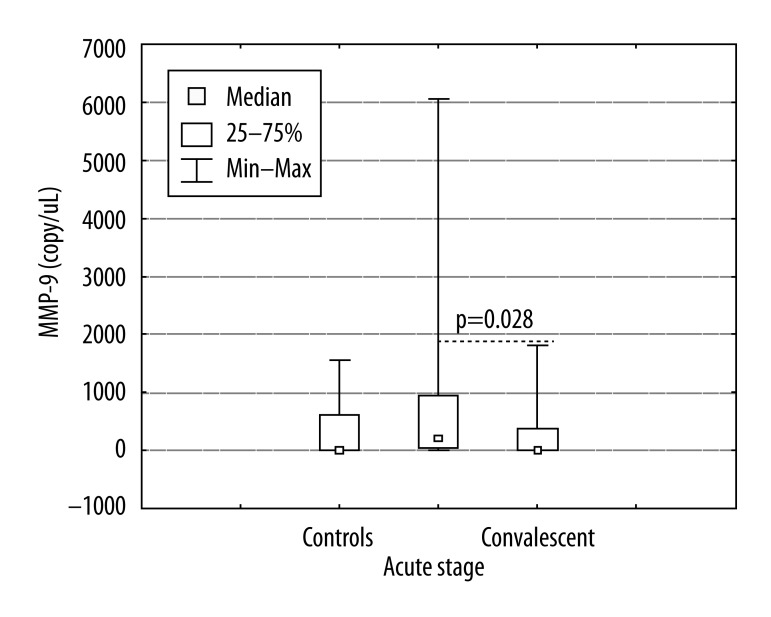
The results of a quantitative RT-PCR analysis from PBMCs for MMP-9 are shown in Figure 1. MMP-9 mRNA expression in PBMCs was significantly increased in the acute phase of illness compared to the control group and convalescent phase (Mann-Whitney; p=0.001; p=0.016). The levels of MMP-2 mRNA were not detectable in patients or controls (data were not shown).
Figure 1.
The gene expression of metalloproteinase 9 (MMP-9) in peripheral blood mononuclear cells (PBMCs) of patient with community acquired pneumonia caused by Mycoplasma pneumoniae, acute stage, convalescent and healthy subjects. Data presented as mean ± standard error of the mean (whiskers). For more information see Patients and methods. * P<0.05 (significant difference).
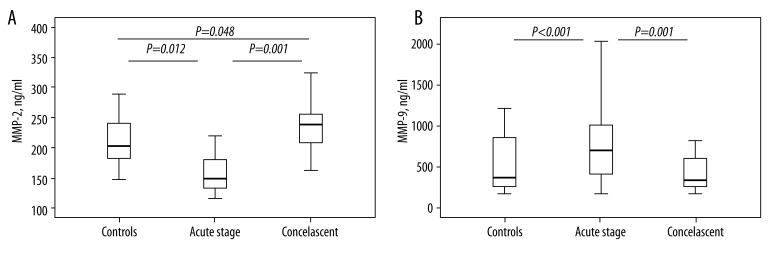
Plasma levels of MMP-2 and MMP-9
Circulating concentrations of MMP-2 and MMP-9 are shown in Figure 2A, B. MMP-2 concentrations in peripheral circulation of acute patients were significantly lower than in the control group and convalescent patients (Mann-Whitney test; p=0.012; p=0.001). However, in convalescent phase, MMP-2 levels were significantly higher than in control subjects (p=0.048). Plasma levels of MMP-9 in acute phase of disease were significantly higher compared to controls and samples from convalescent phase (Mann-Whitney test; p<0.001; p=0.001).
Figure 2.
(A Levels of metalloproteinase 2 (MMP-2) in plasma of patient with community acquired pneumonia caused by Mycoplasma pneumoniae, acute stage, convalescent and healthy subjects. Data presented as mean ± standard error of the mean (whiskers). Data presented as mean ± standard error of the mean (whiskers). For more information see Patients and methods. * P<0.05 (significant difference). (B) Levels of metalloproteinase 9 (MMP-9) in plasma of patient with community acquired pneumonia caused by Mycoplasma pneumoniae, acute stage, convalescent and healthy subjects. Data presented as mean ± standard error of the mean (whiskers). Data presented as mean ± standard error of the mean (whiskers). For more information see Patients and Methods. * P<0.05 (significant difference).
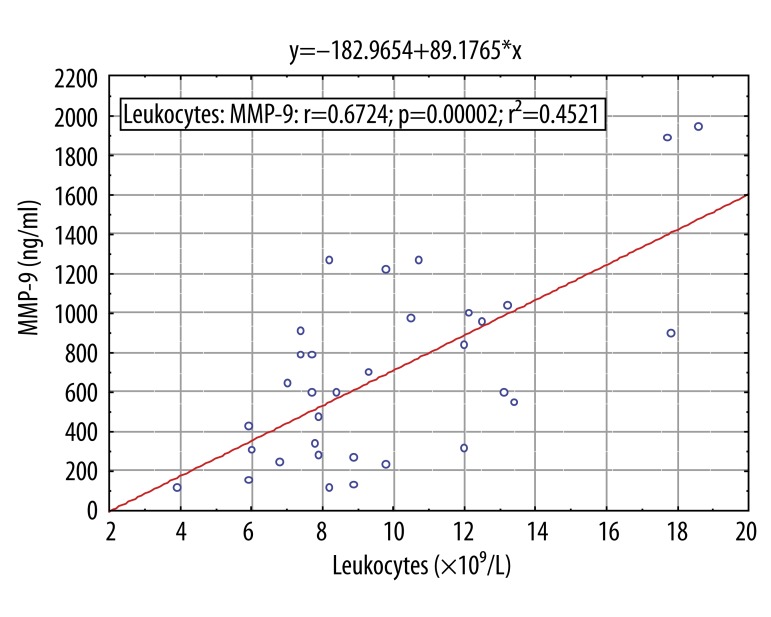
Correlation of metalloproteinases and clinical laboratory parameters
Spearmans correlation test was used to detect possible correlations between mRNA expression of MMPs with CRP, ESR, leukocytes and neutrophils. No correlation was found of MMPs concentration in plasma with laboratory parameters, except the plasma level of MMP-9 slightly correlated with leukocyte count in peripheral circulation (r=0.67, p<0.001) (Figure 3).
Figure 3.
Spearman rank order correlation of metalloproteniase 9 (MMP-9) plasma protein levels against leukocytes count in patient with community acquired pneumonia caused by Mycoplasma pneumoniae. For more information see Patients and methods. * P<0.05 (significant difference).
Discussion
We investigated the role of MMPs in peripheral circulation in CAP caused by M. pneumoniae of previously healthy young male patients. Patients had mild to moderate clinical presentation of CAP. All patients received antibiotic therapy (azithromycin 1×500 mg for 3 days) and were discharged from hospital without complications. We excluded patients with dual infections described in early studies and patients with respiratory infections [13,14].
In this study we demonstrated an increase in MMP-9 gene expression in PBMCs, as well as plasma concentration in patients with CAP caused by M. pneumoniae compared to healthly controls. Moreover, MMP-9 plasma levels positively correlated with the total leukocyte count. However, mRNA and circulating concentration of MMP-9 appeared to decrease quickly.
Two studies showed an increased concentration and activity of MMP-8 and MMP-9 in lungs of patients with HAP, which correlated to systemic signs of inflammation [15,16]. Hartog et al demonstrated a significant increase of MMP-8 and MMP-9 in plasma and in mini-BAL fluids in patients with hospital-acquired pneumonia (HAP) compared with control subjects [16]. The authors suggested that neutrophils are the main source of MMP-8 and MMP-9 in pneumonia. In addition, the authors showed a difference between the MMP-8 and MMP-9 release from PBMC and polymorphonuclear leukocytes in BAL of patients with pneumonia; specifically, in basal condition pulmonary neutrophils were highly activated as opposed to peripheral neutrophils, which are activated after stimulation. Yang et al reported increased activity and plasma level of MMP-9 in patients with CAP compared to control subjects [17]. They also demonstrated a positive correlation of MMP-9 with the number of neutrophils. MMP-9 are normally stored in the intracellular tertiary granules prior to their release by neutrophils [18]. Once released, the activity of MMPs is controlled by regulation of expression and secretion, by proteolytic activation of pro-enzymes, and by the tissue inhibitors of metalloproteinases (TIMPs) [19]. Sakellaropoulou et al founded correlation between leukocyte count, sedimentation rate, CRP, and levels of sodium in children with pneumonia [20].
In our study, plasma levels of MMP-2 were significantly different between acute patients and control subjects. However, circulating concentrations of MMP-2 were statistically significantly higher in convalescent than in acute patients and the control group. MMP-2 mRNA could not be detected by RT-PCR in PBMCs, in patients with CAP, nor in control subjects. Our results suggest that PBMCs are not the source of MMP-2 during M. pneumoniae CAP, but MMP-2 could be involved in the inflammatory process in the lung compartment. Therefore, we found a decreased plasma concentration of MMP-2 in the acute phase, while in the convalescent phase the levels of MMP-2 increased above basal level.
Lichtinghagen et al showed an expression of MMP-2 in liver tissue, but not in white blood cells, in patients with chronic hepatitis C [21]. Baluk et al. found an increased expression of MMP-2 in epithelial cells and MMP-9 in infiltrating neutrophils in infected airways of mice infected by M. pulmoniae (22). Kim et al investigated changes in MMP-2 and MMP-9 in bleomycin-induced pulmonary fibrosis and found temporal change in the major source of the MMPs [23]. In the early phase, both MMPs were mainly expressed in the inflammatory cells – MMP-2 in alveolar macrophage, and MMP-9 in neutrophils. Mid-phase parenchymal cells (bronchial epithelial cells and type II pneumocytes), in addition to the inflammatory cells, present an important cellular source expressing these MMPs. In the later phase, the main cellular sources of the MMPs were again type II pneumocytes. MMPs, especially MMP-2 and MMP-9 have been known to be involved in degradation of extracellular matrix. Torii et al described high MMP-2 and MMP-9 levels in BAL of patients with acute respiratory distress syndrome and suggested that MMPs were implicated in the pathogenesis of ARDS through the disruption of basement membrane structures [24]. Also, Piotrowski et al found elevated messenger RNAs, as well as protein concentration of MMP-2, MMP-7, MMP-9 and TIMP-2 in patients with sarcoidosis [25]. In addition, MMPs may benefit the host because they also are involved in host defense mechanisms. They induce bacterial eradication and might have a protective effect against pulmonary fibrosis following inflammation [26]. Opdenakker et al. showed that MMP-9 truncates chemokines such as interleukin-8 into a more active form, leading to increased MMP-9 levels that again lead to a more potent interleukin-8 [27].
Conclusions
Our study showed that PBMCs are important source of MMP-9 during CAP caused by M. pneumoniae. We also found that MMP-9 plasma level positively correlated to leukocyte count in peripheral circulation. This result indirectly indicates the significance of peripheral neutrophils as one of the major sources of MMP-9 in M. pneumonia CAP. Moreover, we showed that PBMCs are not the source of MMP-2. The MMP-2 decrease in the plasma of patients with acute CAP leads us to speculate that MMP-2 in acute phase of disease is attracted to the pulmonary compartment with other inflammatory mediators. Our results could contribute to further research on immunopathogenic mechanisms in pneumonia caused by M. pneumoniae and the role of MMPs in it.
Abbreviations
- MMP2
matrix metalloproteinase 2
- MMP-9
matrix metalloproteinase 9
- M. pneumoniae
Mycoplasma pneumoniae
- PBMC
peripheral blood mononuclear cells
Footnotes
Source of support: This work was funded by 2 scientific-research projects of the Croatian Ministry of Science, Education and Sports (108-0000000-3491 and 143-1430115-0103)
References
- 1.Marston BJ, Plouffe JF, File TM, Jr, et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. Arch Intern Med. 1997;157:1709–18. [PubMed] [Google Scholar]
- 2.Porath A, Schlaeffer F, Lieberman D. The epidemiology of community-acquired pneumonia among hospitalized adults. J Infect. 1997;34:41–48. doi: 10.1016/s0163-4453(97)80008-4. [DOI] [PubMed] [Google Scholar]
- 3.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wisse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 5.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61:259–66. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohbayashi H. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci. 2002;3:409–21. doi: 10.2174/1389203023380549. [DOI] [PubMed] [Google Scholar]
- 7.Suga M, Iyonaga K, Okamoto T, et al. Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2000;162:1949–56. doi: 10.1164/ajrccm.162.5.9906096. [DOI] [PubMed] [Google Scholar]
- 8.Buisson AC, Zahm JM, Polette M, et al. Gelatinase B is involved in the in vitro wound repair of human respiratory epithelium. J Cell Physiol. 1996;16:413–26. doi: 10.1002/(SICI)1097-4652(199602)166:2<413::AID-JCP20>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 9.D’Ortho MP, Jarreau PH, Delacourt C, et al. Matrix metalloproteinase and elastase activies in LPS-induced acute lung injury in guinea pigs. Am J Physiol. 1994;266:209–16. doi: 10.1152/ajplung.1994.266.3.L209. [DOI] [PubMed] [Google Scholar]
- 10.Takafuji S, Ishida A, Miyakuni Y, Nakagawa T. Matrix metalloproteinase-9 release from human leukocytes. J Investig Allergol Clin Immunol. 2003;13:50–55. [PubMed] [Google Scholar]
- 11.Yao PM, Buhler JM, d’Ortho MP, et al. Expression of matrix metalloproteinase gelatinases A and B by cultured epithelial cells from human bronchial explants. J Biol Chem. 1996;271:15580–89. doi: 10.1074/jbc.271.26.15580. [DOI] [PubMed] [Google Scholar]
- 12.Segura-Valdez L, Pardo A, Gaxiola M, et al. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest. 2000;117:684–94. doi: 10.1378/chest.117.3.684. [DOI] [PubMed] [Google Scholar]
- 13.Puljiz I, Kuzman I, Dakovic-Rode O, Schönwald N, Mise B. Chlamydia pneumonia and Mycoplasma pneumoniae pneumonia: Comparison of clinical, epidemiological characteristics and laboratory profilesm. Epidemiol Infect. 2006;134:548–55. doi: 10.1017/S0950268805005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haidopoulou K, Goutaki M, Damianidou L, et al. Human bocavirus infections in hospitalized Greek children. Arch Med Sci. 2010;1:100–3. doi: 10.5114/aoms.2010.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaaf B, Liebau C, Kurowski V, et al. Hospital acquired pneumonia with high-risk bacteria is associated with increased pulmonary matrix metalloproteniase activity. BMC Pulm Med. 2008;8:12. doi: 10.1186/1471-2466-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartog CM, Wermelt JA, Sommerfeld CO, et al. Pulmonary matrix metalloproteinase excess in hospital-acquired pneumonia. Am J Resp Crit Care Med. 2003;167:593–98. doi: 10.1164/rccm.200203-258OC. [DOI] [PubMed] [Google Scholar]
- 17.Yang SF, Chu SC, Chiang IC, et al. Excessive matrix metalloproteinase-9 in the plasma of community-acquired pneumonia. Clin Chim Acta. 2005;352:209–15. doi: 10.1016/j.cccn.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45:17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- 19.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–22. [PubMed] [Google Scholar]
- 20.Sakellaropoulou A, Hatzistilianou M, Eboriadou M, Athanasiadou-Piperopoulou F. Hyponatraemia in cases of children with pneumonia. Arch Med Sci. 2010;4:578–83. doi: 10.5114/aoms.2010.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichtinghagen R, Huegel O, Seifert T, et al. Expression of matrix metalloproteinase-2 and -9 and their inhibitors in peripheral blood cells of patients with chronic hepatitis C. Clin Chemistry. 2000;46:183–92. [PubMed] [Google Scholar]
- 22.Baluk P, Raymond WW, Ator E, et al. Matrix metalloproteinase -2 and -9 expression increases in Mycoplasma-infected airways but is not required for microvascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2004;287:307–17. doi: 10.1152/ajplung.00404.2003. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, Choeng HC, Ahn C, Cho SH. Early and late changes of MMP-2 and MMP-9 in bleomycin-induced pulmonary fibrosis. Yonsei Med J. 2009;50:68–77. doi: 10.3349/ymj.2009.50.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torii K, Iida K, Miyazaki Y, et al. Higher concentrations of matrix metalloproteinases in bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome. Am J Respir Crit Care Med. 1997;155:43–46. doi: 10.1164/ajrccm.155.1.9001287. [DOI] [PubMed] [Google Scholar]
- 25.Piotrowski WJ, Gorski P, Pietras T, et al. The selected genetic polymorphisms of metalloproteinases MMP2, 7, 9 and MMP inhibitor TIMP2 in sarcoidosis. Med Sci Monit. 2011;17(10):CR598–607. doi: 10.12659/MSM.881987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bottcher T, Spreer A, Azeh I, et al. Matrix metalloproteinase-9 deficiency impairs host defense mechanisms against streptococcus pneumoniae in a mouse model of bacterial meningitis. Neurosci Lett. 2003;338:201–4. doi: 10.1016/s0304-3940(02)01406-4. [DOI] [PubMed] [Google Scholar]
- 27.Opdenakker G, Van den Steen PE, Dubois B, et al. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69:851–59. [PubMed] [Google Scholar]