Summary
Background
This study was designed to evaluate maternal levels of leptin and interferon-gamma (IFN-gamma) in pregnancy complicated with hypertension and to assess the role of cytokines in predicting the risk of cesarean section.
Material/Methods
This was a cohort study with a prospective follow-up. After proportional sampling procedure, the study included the follow-up of 40 women with hypertensive disorders of pregnancy (pregnancy-induced hypertension [PIH] or preeclampsia [PE]) and 40 uncomplicated pregnancies. Women were followed from the time of admission to the delivery. Levels of leptin and interferon-gamma were measured in serum samples from all women. A p-value <0.05 was considered as significant.
Results
Significant increase in IFN-gamma and leptin concentration in women with pre-eclampsia was observed. We found a significant 1.4-fold increase in the risk of birth by cesarean section associated with the increase of the IFN-gamma concentration by 0.1 pg/ml and almost 3-fold increase in the risk associated with the increase of the leptin concentration.
Conclusions
IFN-γ and leptin might be risk markers of cesarean section in hypertension disorders of pregnancy, but further studies supporting this evidence are needed.
Keywords: interferon-gamma, leptin, pre-eclampsia, pregnancy induced hypertension, cesarean section
Background
Preeclampsia (PE) is one of the major causes of maternal morbidity and mortality in the developed countries, affecting approximately 2.5–5% of pregnancies [1]. It belongs to a wide group of hypertensive disorders and develops in pregnancy after 20 weeks of gestation in previously normotensive women with development of proteinuria of unknown mechanism of the pathogenesis. One of many theories indicates the failure of the placental vascularization, which causes the placental hypoxia and its dysfunction [2], which finally results in uteroplacental insufficiency. Others postulate a complex polygenetic background [3] with involvement of maternal/fetal genes and possible impact of environmental factors. Early detection is a key strategy for effective management and involves careful monitoring of both mother and fetus. While the symptomatic treatment with antihypertensives is established, the causal treatment is still unknown. That is why there are clinical trials using new medications like L-arginine, which show promising results in decreasing blood pressure by improving endothelial functions [4].
Leptin was initially discovered as a regulator of food intake and energy expenditure, but is now characterized as a pleiotropic molecule involved in a wide range of physiological and pathological functions [5]. It is a product of the Ob gene and its production takes place mostly in adipose tissue, and then is secreted into the circulation. Leptin is also synthesized by the placenta during pregnancy – its levels increase proportionally during the pregnancy and decrease postpartum. It has been determined that leptin concentration in mothers’ blood is significantly higher when pregnancy is complicated by preeclampsia in comparison with gestational age-matched controls [6–8]. It has also been shown that maternal hyperleptinemia indicates the onset of preeclampsia with clinical symptoms by additional rise of its concentration [9].
Apart from food intake and endocrine regulation, the innate immune system also plays a major role in leptin production. In experimental animals, leptin level is acutely increased by inflammatory stimuli, such as the administration of tumor necrosis factor alpha (TNF-α)[5].
While in healthy patients the Th2-activity cytokines promote healthy pregnancy, it has been proved that in PE Th1-type cytokines, like TNF-alpha, IL-2, IL-12, IFN-gamma, are overproduced [10–12]. IFN-gamma is a well known cytokine and is often used to assess the immunological deviations in Th1/Th2 cytokines ratio during pregnancy. IFN-gamma also plays an important role in the remodeling of spiral arteries and angiogenesis at implantation sites [13]. Leptin, on the other hand, is shown to increase the formation of reactive oxygen species (ROS) in endothelial cells and thereby might play a role in the inflammation process through enhanced oxidative stress [14]. These facts suggest that leptin may be considered a proinflammatory adipocyte-derived factor that links immune and inflammatory reactions to the neuroendocrine system [14]. It is postulated that leptin is one of regulators of the Th1/Th2 cytokines balance, in favor of Th1 response. Therefore, this study was designed to evaluate the maternal levels of leptin and IFN-gamma cytokine and to assess their role in predicting the necessity of ending the pregnancy with cesarean section.
Material and Methods
This follow-up study was performed between October 2008 to July 2010 in the Department of Obstetrics and Perinatology in Jagiellonian University – Medical College. The study included women with singleton pregnancies, with maternal ages ranging between 18–38 years old and gestational age between 26–40 weeks. Proportional sampling of pre-term and term pregnancies was applied. Exclusion criteria were chronic diseases such as diabetes mellitus (pre- and gestational), pre-existing chronic hypertension, liver, kidneys or endocrine diseases and fetal abnormalities. All women admitted to hospital were eligible for the study. The response rate was 95%. All recruited subjects were briefed about the nature of the study and written informed consent was obtained. Finally, two groups were created – 40 women with uncomplicated pregnancies considered as control group and 40 women with hypertensive disorders of pregnancy, diagnosed as pregnancy-induced hypertension (PIH) or PE according to ISSHP (International Society for the Study of Hypertension in Pregnancy), analyzed as the study group. Study participants were followed from the time of admission to delivery. Women who develop severe PE were hospitalized until delivery, those with PIH with well-controlled hypertension were discharged and readmitted after a few weeks to give birth. Levels of leptin and interferon-gamma were measured at the time of admission and delivery in serum samples from 80 pregnant women (11 with PE, 29 with PIH, and 40 uncomplicated).
The blood samples from the study groups were collected first at the admission to hospital and next during the labor or before discharge from hospital when still pregnant. The samples from the control group were taken only during labor. Sample of 2.4 ml of venous blood were drawn into tubes and then centrifuged for 10 minutes at 4000g and serum was separated and stored at −25°C until analysis. Serum leptin concentrations and IFN-gamma were measured by ELISA kit according to guidelines of the manufacturer.
The Institutional Ethics Committee approved the study.
Statistical analysis
Categorical variables were described using percentages and analyzed by the chi-squared test if expected frequencies were over 5, otherwise the Fisher’s exact test was used.
All continuous variables were presented by mean, median, standard deviation (SD), and range. Two group differences (between PE and PIH or PE/PIH and controls) were analyzed by the Student’s t-test for normally distributed variables, and by the Mann-Whitney test for skewed data. Simultaneous analysis of differences between PE, PIH and control group at delivery were analyzed by the Kruskal-Wallis ANOVA with multiple comparisons of mean ranks post-hoc test.
Spearman’s rank correlation test was used to analyze the strength of association between leptin and IFN-gamma cytokine and between these cytokines and birth weight or gestational age at delivery.
To reveal the relationship between cytokines, linear regression was used.
Finally, to evaluate possible effect of elevated cytokine level on the risk of the necessity of ending pregnancy with cesarean section, Cox proportional hazard model was used. Two models were created – one evaluated the risk associated with the increase by 0.1 pg/ml in serum concentration of leptin and INF-gamma, while the second model assessed the risk for women whose leptin and IFN-gamma concentration exceeded the median. Every model was adjusted for gestational age as a main confounding variable.
Statistical analysis was performed using Statistica version 10.0 software (Stat Soft, Inc.). A p-value <0.05 was considered as significant.
The sample size and the power of the study
Initially, the average leptin level of 0.60 pg/ml among women with hypertensive disorders and of 0.45 pg/ml among controls with the SD=0.1 was expected. Assuming the alpha level of 0.05 and the power of 0.90, the required sample size was 9 women per group and 40 were recruited for every group in our study to enable more detailed subgroup analysis. For the IFN-gamma level of 8.5 pg/ml among hypertensive and of 5.0 pg/ml in uncomplicated pregnancies, with SD=1.0, alpha=0.05 and the power of 90%, the required sample size was even lower (eg, 3 women/group). Finally, for the IFN-gamma and cesarean section risk analysis (cut off at the median =7.125 pg/ml), we achieved the power of 98.7%.
Results
The basic characteristics of women included into the study are presented in Table 1. No significant differences were found in mother’s age, parity and gestational age at admission between study groups. However, significant difference was observed in the pregnancy age between both study groups and the control group at admission and at delivery. There was no significant difference in cesarean section rates between PIH and the control group (24.1% vs. 32.5%), in comparison to the PE and the control group. Significant difference in birth weight was observed between all groups.
Table 1.
Basic characteristic of study groups.
| Parameters | Study group PE (n=11) | Study group PIH (n=29) | Control group [CG] (n=40) | p |
|---|---|---|---|---|
| Mother’s age (years) [mean±SD] | 26.9±3.1 | 26.8±3.3 | 26.5±2.7 | pK-W=ns |
|
| ||||
| Parity [n,%] | pF = ns | |||
| 1 | 14 (48.3%) | 6 (54.5%) | 20 (50.0%) | |
| 2 | 13 (44.8%) | 4 (36.4%) | 17 (42.5%) | |
| 3 | 2 (6.9%) | 1 (9.1%) | 3 (7.5%) | |
|
| ||||
| Number of IUGR [n,%] | 0 (0.0%) | 6 (54.5%) | 1 (2.5%) | pF <0.001 |
|
| ||||
| Gestational age at admission (weeks) [mean±SD] | 29.09±2.9 | 28.8±2.25 | 39.1±1.1 | PE vs. PIH: pK-W=ns PE vs. CG: pK-W<0.01 PIH vs. CG: pK-W<0.01 |
|
| ||||
| Gestational age at delivery (week) [mean±SD] | 30.6±2.5 | 38.0±0.9 | 39.1±1.1 | PE vs. PIH: pK-W<0.01 PE vs. CG: pK-W<0.01 PIH vs. CG: pK-W<0.01 |
|
| ||||
| Birth weight (g) [mean±SD] | 1467.2±390 | 3218.9±280 | 3500±376 | PE vs. PIH: pK-W<0.01 PE vs. CG: pK-W<0.01 PIH vs. CG: pK-W<0.05 |
|
| ||||
| Cesarean section [n.%] | 11 (100%) | 7 (24.1%) | 13 (32.5%) | df=2 pChi<0.01 |
ns – non significant; pF – Fisher’s exact test; pK-W – Kruskal-Wallis ANOVA with multiple comparisons of mean ranks post-hoc test; df – degrees of freedom; pChi – chi square test.
The concentrations of leptin and interferon-gamma were measured in serum samples obtained from 80 pregnant women (11 with PE, 29 with PIH and 40 uncomplicated). The highest level of leptin was observed in the study group PE at admission as well as at delivery. Moreover, both study groups were characterized by higher level of leptin than observed in the control group. A similar pattern was observed for the concentration of IFN-gamma. Almost all differences observed were statistically significant (Table 2). Interestingly, the level of leptin at admission and at delivery in the study group PIH was similar.
Table 2.
Mean value of leptin and IFN-gamma in groups.
| Parameters | Study group PE (n=11) | Study group PIH (n=29) | p |
|---|---|---|---|
| Leptin at admission [pg/ml] [mean±SD median (range)] | 0.62±0.04 0.62 (0.61–0.62) |
0.46±0.08 0.45 (0.39–0.51) |
pT<0.01 |
| Leptin at delivery [pg/ml] [mean±SD median (range)] | 0.82 ±0.07 0.82 (0.76–0.89) |
0.46±0.07 0.46 (0.41–0.51) |
pK-W<0.01 |
| Control group (n=40) 0.39 ±0.09 (0.32±0.48) |
|||
| pK-W<0.01 | pK-W=0.06 | ||
| IFN at admission [pg/ml] [mean±SD median (range)] | 8.42±0.36 8.33 (8.15–8.5) |
6.94 ±0.68 (6.55±7.38) | pM-W<0.01 |
| IFN at delivery [pg/ml] [mean±SD median (range)] | 10.88±0.86 10.87 (10.01–11.56) |
7.02±0.69 (6.69±7.5) | pK-W<0.05 |
| Control group (n=40) 4.85±0.93 (4.07±5.66) |
|||
| pK-W<0.01 | pK-W<0.01 | ||
pT – t-Student test; pK-W – Kruskal-Wallis ANOVA with multiple comparisons of mean ranks post-hoc test; pM-W – U Mann-Whitney test.
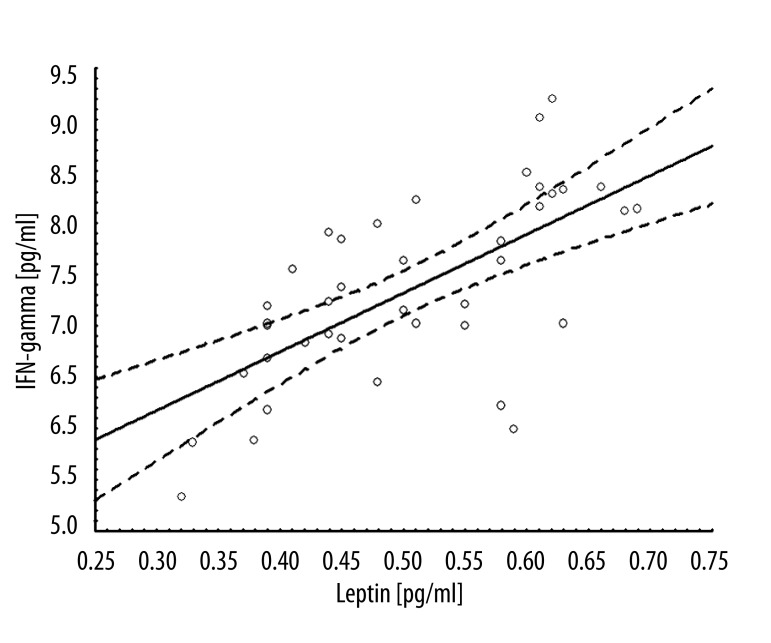
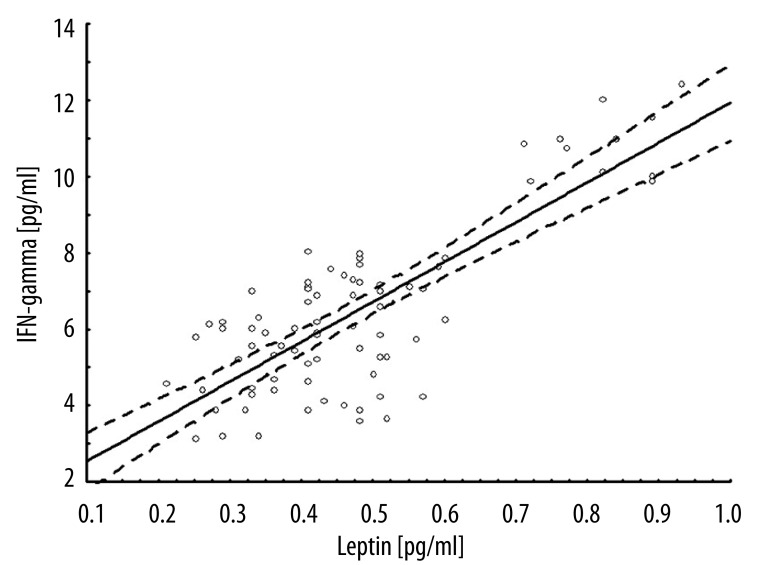
The relationship between IFN-gamma and leptin concentration was also analyzed. In the linear regression model, the regression coefficient at admission was 5.7 (r=0.66; p<0.001), and at delivery 10.4 (r=0.79; p<0.001) (Figures 1, 2). After exclusion of controls, the regression coefficient for INF-gamma and leptin was 9.5 (r=0.90; p<0.001). Among controls, the relationship was not observed. Moreover, the strength of association between leptin or IFN-gamma and perinatal outcomes were analyzed. The correlation coefficients were negative and ranged from 39% to 79% (Table 3). Additionally, leptin and IFN-gamma concentration were evaluated within groups defined on the basis of the Apgar score at 1 minute. For every cytokine measured, level observed among women having Apgar <8 children was significantly higher than those having children with Apgar score of 8 and higher (Table 4).
Figure 1.
Linear regression model IFN-gamma and leptin at admission.
Figure 2.
Linear regression model IFN-gamma and leptin at labor.
Table 3.
Relationship (collelation coefficients) between leptin or INF-gamma levels and perinatal outcomes.
| Perinatal outcome | Leptin at admission (n=40) | Leptin at delivery (n=80) | IFN at admission (n=40) | IFN at delivery (n=80) |
|---|---|---|---|---|
| r | ||||
| Birth weight | −0.50* | −0.39* | −0.59* | −0.53* |
| Gestational age at delivery | – | −0.44* | – | −0.60* |
| Apgar | −0.61* | −0.40* | −0.79* | −0.55* |
r and p value counted by Spearman’s rank correlation test;
p<0.001.
Table 4.
Mean value of leptin and IFN-gamma in groups according to Apgar score at 1 minute.
| Cytokines | Apgar <8 | Apgar >7 | p |
|---|---|---|---|
| Leptin at admission (pg/ml) | |||
| mean±SD | 0.62 (0.05) | 0.47 (0.09) | pM-W<0.001 |
| median (range) | 0.62 (0.51–0.68) | 0.45 (0.32–0.68) | |
|
| |||
| Leptin at delivery | |||
| mean±SD | 0.81 (0.08) | 0.43 (0.12) | pM-W<0.001 |
| median (range) | 0.82 (0.71–0.93) | 0.42 (0.21–0.89) | |
|
| |||
| IFN at admission | |||
| mean±SD | 8.46 (0.39) | 7.02 (0.73) | pM-W<0.001 |
| median (range) | 8.33 (8.13–9.21) | 7.01 (5.34–8.34) | |
|
| |||
| IFN at delivery | |||
| mean±SD | 10.96 (0.90) | 5.88 (1.57) | pM-W<0.001 |
| median (range) | 10.87 (9.91–12.44) | 5.89 (3.15–11.01) | |
pM-W – U Mann-Whitney test.
Finally, we tried to verify if the IFN-gamma and leptin concentration at admission were possible indicators of the necessity of ending the pregnancy with cesarean section in women with hypertensive disorders. For this purpose, two models were used. One analyzed the risk of cesarean section associated with increase in IFN-gamma or leptin concentration by 0.1 pg/ml. In these models significant increase in the risk associated with IFN-gamma (HR=1.37; 95%CI: 1.19–1.57) as well as with leptin (HR=2.75, 95%CI: 1.52–4.99) was observed.
The second group of models evaluated the risk of cesarean section related to the higher level of IFN-gamma and leptin. The medians for cytokine level at admission were used as thresholds. Similarly, in the latter models increased risk of the necessity of ending the pregnancy with cesarean section for both, IFN-gamma >7.125 pg/ml (HR=15.3, 95% CI: 3.44–68.4) and for leptin >0.50 pg/ml (HR=2.84, 95% CI: 1.08–7.43) was noticed.
Discussion
Previous published data have demonstrated increased leptin levels in pre-eclampsia [6–8]. Our findings revealed a significant increase in serum levels of leptin in preterm PE and term PIH when compared to controls, which is in agreement with the findings of Bartha et al. [3]. The theory of the placental hypoxia causing overproduction of leptin in human trophoblastic cells under hypoxic conditions, as showed in Mise et al., indicates the unclear role of high levels of leptin in PE hypoxic placentas [2].
One mechanism promoting fetal-placental survival in normal pregnancy is trophoblast insensitivity to interferons (IFNs) [16]. There are studies showing that IFN-gamma inhibits the migration of human cytotrophoblasts in a natural killer cells dose-dependent manner and blocks trophoblast invasion, like in the early alterations in the placentation process, which may be related to the origin of the pre-eclampsia [17].
Several lines of evidence support the proinflammatory cytokine hypothesis in pre-eclampsia. In this study, we have found increased concentrations of cytokines in pre-eclamptic women as compared to healthy pregnant women. The serum level of IFN-gamma was significantly higher in PE than in PIH and the control group, probably because of the Th1 activation in the immune system postulated in pre-eclampsia etiology. Abnormal cytokine responses in the mother may be involved in the pathogenesis of this maternal syndrome. Supporting our findings, few studies have demonstrated an increase in IFN-gamma in pre-eclampsia [10–12]. Our data showed the association between raised levels of IFN-gamma or leptin and PE, which is why both IFN- gamma and leptin might be used as markers of inflammation and immunological dysfunction leading to PE.
Hypertensive subjects frequently have higher leptin levels than normotensive subjects. A positive relationship between serum leptin level and blood pressure has been reported [18], but information concerning relations between serum leptin concentration and the levels of serum IFN-gamma are limited [15]. Lord et al proposed a new important role of leptin in the regulation of the Th1/Th2 balance, showing that leptin increases IL-2 and IFN-gamma production and decreases IL-4. Our study showed the significant positive correlation between levels of leptin and IFN-gamma. These alterations may be the result of immunological dysfunction and affecting abnormal Th1-like cytokine and leptin production.
The negative correlation between leptin, IFN-gamma and neonatal birth weight, as well as gestational age at delivery, were shown, which seems to be in agreement with the theory of the placental hypoxia resulting in poor perinatal outcome, but without the IUGR complication [19]. The present study suggests that higher maternal serum levels of leptin and IFN-γ might be used as markers of preterm delivery in pre-eclampsia.
Some other possible mechanisms also support the use of serum leptin as a risk marker of preterm delivery, as has been suggested to be involved in the proatherogenic process by increasing oxidative stress [20], and leptin has been reported to induce oxidative stress in cultured endothelial cells [15].
Leptin levels are elevated in an obese populations [21]. Some studies have shown the influence of maternal body mass index (BMI) on leptin level in pregnancy, when others conclude that leptin in pregnancy complicated with PE is not related to maternal adipose tissue [3,15,22]. Bertha et al. showed also lack of correlation between leptin level and maternal BMI, both pre-gestational and gestational. Nevertheless, one of the limitations of our study is lack of BMI measurements as we focused on IFN-gamma and leptin levels without any other suspected markers of PE.
Conclusions
We conclude that both IFN-gamma and leptin might be risk markers of necessity of cesarean section in pregnancies with hypertensive disorders. However, to use these markers for daily clinical practice, further studies supporting our evidence are needed.
Acknowledgments
We thank the Department of Obstetrics and Perinatology and University Hospital in Cracow for supporting our study. We would also like to thank all of the women who participated in this research.
Footnotes
Disclosure of interests
All authors of this article state that they have no competing interests to declare.
Source of support: This study was supported by grant K/ZDS/001075 from Jagiellonian University Medical College
References
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–94. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Mise H, Sagawa N, Matsumoto T, et al. Augmented placental production of leptin in pre-eclampsia: L Possible involvement of placental hypoxia. J Clin Endocrinol Metab. 1998;83:3225–29. doi: 10.1210/jcem.83.9.5117. [DOI] [PubMed] [Google Scholar]
- 3.Bartha JL, Romero-Carmona R, Escobar-Llompart M, Comino-Delgado R. The relationships between leptin and inflammatory cytokines in women with pre-eclampsia. BJOG. 2001;108:1272–76. doi: 10.1111/j.1471-0528.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 4.Rytlewski K, Olszanecki R, Korbut R, Zdebski Z. Effects of prolonged oral supplementation with l-arginine on blood pressure and nitric oxide synthesis in preeclampsia. Eur J Clin Invest. 2005;35(1):32–37. doi: 10.1111/j.1365-2362.2005.01445.x. [DOI] [PubMed] [Google Scholar]
- 5.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–46. [PubMed] [Google Scholar]
- 6.Molvarec A, Szarka A, Walentin S, et al. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod Biol Endocrinol. 2011;9:124. doi: 10.1186/1477-7827-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teppa RJ, Ness RB, Eromble Holm WR, Roberts JM. Free leptin is increased in normal pregnancy and further increased in pre-eclampsia. Metabolism. 2000;49:1043. doi: 10.1053/meta.2000.7707. [DOI] [PubMed] [Google Scholar]
- 8.Iftikhar U, Iqbal A, Shakoor S. Relationship between leptin and lipids during pre-eclampsia. JPMA. 2010;60:432–35. [PubMed] [Google Scholar]
- 9.Anim-Nyame N, Sooranna SR, Steer PJ, Johnson MR. Longitudinal analysis of maternal plasma leptin concentrations during normal pregnancy and pre-eclampsia. Hum Reprod. 2000;15:2033–36. doi: 10.1093/humrep/15.9.2033. [DOI] [PubMed] [Google Scholar]
- 10.Szarka A, Rigó J, Jr, Lázár L, et al. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilczyński JR, Tchórzewski H, Głowacka E, et al. Cytokine secretion by decidual lymphocytes in transient hypertension of pregnancy and pre-eclampsia. Mediators Inflamm. 2002;11:105–11. doi: 10.1080/09629350220131962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tinsley JH, Chiasson VL, South S, et al. Immunosuppression improves blood pressure and endothelial function in a rat model of pregnancy-induced hypertension. Am J Hypertens. 2009;22:1107–14. doi: 10.1038/ajh.2009.125. [DOI] [PubMed] [Google Scholar]
- 13.Murphy SP, Tayade C, Ashkar AA, et al. Interferon Gamma in Successful Pregnancies. Biol Reprod. 2009;80:848–59. doi: 10.1095/biolreprod.108.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231–38. [PubMed] [Google Scholar]
- 15.Lord GM, Matarese G, Howard JK, et al. Lepin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 16.Laresgoiti-Servitje E, Gomez-Lopez N, Olsen DM. An immunological insight into the origins of pre-eclampsia. Hum Reprod Update. 2010;16(5):510–24. doi: 10.1093/humupd/dmq007. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Dutz JP, MacCalman CD, et al. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-{gamma} J Immunol. 2006;177:8522–30. doi: 10.4049/jimmunol.177.12.8522. [DOI] [PubMed] [Google Scholar]
- 18.Mumtaz F, Memon AR, Yousfani S, et al. Role of serum leptin level as a marker of severity of Pre-eclampsia. Med Coll Abbottabad. 2008;20(1):13–15. [PubMed] [Google Scholar]
- 19.Laivuori H, Gallaher MJ, Collura L, et al. Relationship between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre-eclampsia and intrauterine growth restriction without pre-eclampsia. Mol Hum Reprod. 2006;12(9):551–56. doi: 10.1093/molehr/gal064. [DOI] [PubMed] [Google Scholar]
- 20.Shin MJ, Park E. Plasma level of leptin are associated with the plasma level of LDL conjugated dienes in children. Ann Nutr Metab. 2007;51:1–6. doi: 10.1159/000099010. [DOI] [PubMed] [Google Scholar]
- 21.Zirlik S, Hauck T, Fuchs FS, et al. Leptin, obestatin and apelin levels in patients with obstructive sleep apnoea syndrome. Med Sci Monit. 2011;17(3):CR159–64. doi: 10.12659/MSM.881450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kokot F, Wiecek A, Adamczak M, et al. Pathophysiological role of leptin in patients with chronic renal failure, in kidney transplant patients, in patients with essential hypertension, and in pregnant women with preeclampsia. Artif Organs. 1999;23:70–74. doi: 10.1046/j.1525-1594.1999.06279.x. [DOI] [PubMed] [Google Scholar]