Summary
The absence of periodontium causes masticatory load in excess of the self-repairing potential of peri-implant bone; peri-implant bone loss caused by occlusal overload is not uncommon in patients and greatly diminishes chances of long-term success. Regenerative treatments may be useful in inducing peri-implant bone regeneration, but are only stopgap solutions to the aftermaths caused by the imperfect biomechanical compatibility of the dental implant. Despite promising success, the tissue-engineered periodontal ligament still needs a period of time to be perfected before being clinically applied. Hence, we propose a novel design of dental implant that utilizes nano-springs to construct a stress-cushioning structure inside the implant. Many studies have shown that NGF, a neurotrophin, is effective for nerve regeneration in both animal and clinical studies. Moreover, NGF has the potential to accelerate bone healing in patients with fracture and fracture nonunion and improve osseointegration of the implant. The key point of the design is to reduce stress concentrated around peri-implant bone by cushioning masticatory forces and distributing them to all the peri-implant bone through nano-springs, and promote osseoperception and osseointegration by NGF-induced nerve regeneration and new bone formation. This design, which transfers the main biomechanical interface of the implant from outside to inside, if proven to be valid, may to some extent compensate for the functions of lost periodontium in stress cushioning and proprioception.
Keywords: bionic design, dental implant, nano-springs, nerve growth factor, osseointegration, osseoperception
Background
Modern oral implantology began in the mid-20th century, the clinical success of which has led to the widespread use of osseointegrated dental implants as substitutes for missing teeth in partially or completely edentulous patients. However, osseointegration represents a direct connection between the implant and bone tissue without the periodontium. The absence of periodontal fibers and pressoreceptors appears to cause dental implants to be less resistant and sensitive to occlusal overload than natural teeth [1]. Some problems are inevitable, such as peri-implant bone loss caused by overloading [2], which can destroy osseointegration, jeopardize the integrity of the implant, and lead to implant failure [3].
Some regenerative treatments, including autologous bone grafting, augmentation with bone substitutes or growth factors, and guided bone regeneration, were reported in single cases to be effective in inducing peri-implant bone regeneration [4–9]. However, these treatments are only stopgap solutions to the aftermaths caused by the imperfect biomechanical compatibility of the dental implant. Thus, an ideal dental implant should have a periodontium-like architecture, with multiple functions in stress cushioning and proprioception, the same as natural teeth.
Regeneration of lost periodontium is a challenge in that both hard tissues (eg, alveolar bone, cementum) and soft connective tissues (eg, periodontal ligament [PDL]) need to be restored to their original architecture. In particular, to restore functional resistance to masticatory load, PDL fibers must insert perpendicularly to the cementum. Guided tissue regeneration [10] and growth factor application [11] have been utilized to induce periodontal regeneration in patients with periodontal disease. However, these approaches are associated with unpredictable and variable outcomes. A common finding in repaired periodontium is that the new ligament is disorganized and therefore non-functional [12]. In recent years, the development of tissue engineering has opened a new door to periodontal regeneration. Evidence has shown that bone marrow mesenchymal stromal cells (BM-MSCs) are effective in regenerating lost hard and soft periodontal tissues in a rat periodontal defect model, and a greater number of perpendicular and functionally orientated PDL fibers are observed in an experimental group [13]. In another study in dogs, researchers have successfully produced a tissue-engineered PDL by using the cells isolated from PDL and cultured in a bioreactor on pin-like titanium implants. After implantation, new tissue consistent with PDL develops on the surface of the implants. Probing and motility assessments suggest that the implants are well-integrated, with mechanical properties similar to those of teeth [14].
Despite promising success in human patients, the tissue-engineered PDL still needs a period of time to be perfected before being applied clinically. Would it be possible to construct a periodontium-like architecture using existing technology?
In this paper, we propose the hypothesis that utilizing springs as substitutes for lost periodontal fibers to construct a stress cushioning structure inside the implant and applying nerve growth factor to induce peri-implant tissue regeneration may compensate the functions of lost periodontium in stress cushioning and proprioception.
General Information About “Nano-Springs”
The spring is a classic mechanical device for stress cushioning, which is usually made by spring steel and widely used in machines and instruments. However, if we utilize traditional springs to construct a stress-cushioning structure inside the implant, size limitation makes them unable to cushion excessive masticatory loads.
“Nano-springs” were first reported in 2010 [15], denoting a high-efficiency mechanical energy storage and retrieval device through the medium of surface energy. Compared with traditional springs, nano-springs have significantly larger stress-cushioning capacity. For example, nano-springs curved with nanowires 2.3 nm in diameter can cushion 1000 newtons force per square mm, 1600 times as much as a clock spring does. The excellent performance of nano-springs completely matches the demands in our hypothesis, since the maximum masticatory load of normal humans varies with age, tooth site and functional state, from 500 to 700 newtons on average [16].
Influence of NGF on Nerve and Bone Regeneration
The principle of osseoperception was first proposed in the 1990s, and means that osseointegrated dental implants still have certain tactile sensibility to masticatory load without periodontic pressoreceptors, and thus help to coordinate the function of the stomatognathic system. Significant differences in tactile sensibility as a function of different implant surfaces may indicate that receptors in peri-implant hard (alveolar bone) and soft (gingival, periosteum) tissues form the basis of osseoperception [17]. In a dog model, a greater number of neurofilament protein-positive nerve fibers are observed after 3 months loading, which are mainly distributed around the implant and in bone marrow [18]. Based on the principle of osseoperception, using appropriate methods to induce peri-implant nerve regeneration may be effective for promoting proprioception of the dental implant.
First described by Levi-Montalcini and Hamburger in 1953, nerve growth factor (NGF), a member of a family of growth and survival factors known as neurotrophins, promotes the neural differentiation and survival of basal forebrain cholinergic neurons and peripheral sensory neurons [19,20]. Within hours after axonal damage, mRNA levels of NGF and its receptors temporally increase [21], and show a second peak of expression at 2–3 days after injury. Not only increasing the width and length of neuron axons [22], NGF also induces the existing axons to bud, branch, and develop [23,24]. Clinically, NGF is widely used to treat various types of peripheral nerve wounds caused by toxins, traumas, diabetes, etc. [25–27].
Regeneration of neurons is important not only in nerve wound healing but also in the healing process of other tissues. It is well known that areas which are denervated or poorly innervated heal insufficiently. For example, patients with peripheral neuropathies such as diabetes have a reduced capacity to heal wounds. This is strongly supported by experimental findings that intact innervation is important for efficient healing of skin, epithelium, tooth pulp, ligament and bone [28,29].
The influence of NGF on fracture healing was studied by Eppley et al. [30], who found new bone formation around the regenerated neuron axons in a rabbit mandibular nerve defect model. The role of NGF in bone formation after experimental bone fracture has also been investigated in a rodent model [31]. NGF is constitutively expressed in bone tissue, but its expression, together with TrkA expression, is increased during the healing process after fracture. It has been suggested that NGF-mediated autocrine and/or paracrine mechanisms contribute to wound healing of the bone. To support this idea, topical application of NGF by using a mini-osmotic pump to the fracture site for 7 days significantly improves cartilage production, breaking strength and Young’s modulus values of fractured bones.
Overall, NGF stimulates nerve regeneration and bone formation by its biological activities on both neuronal and non-neuronal cells. Topical application of NGF is regarded as an ideal method to promote the long-term success of dental implants.
The Hypothesis
Design of the hypothetical implant
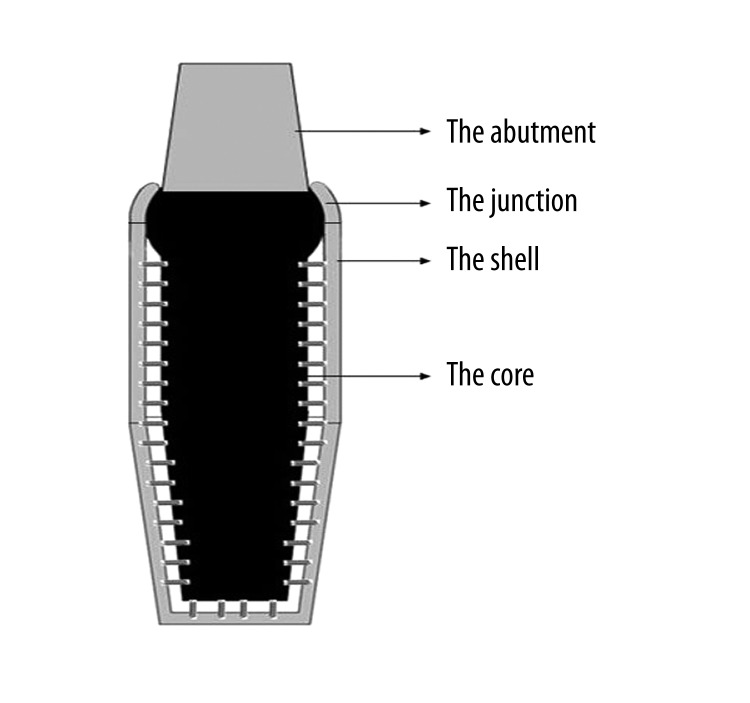
The hypothetical threaded root form implant made of titanium alloy is divided into 2 main parts – the shell, which has direct contact with bone tissue; and the core, which hangs inside the shell by nano-springs and connects with the abutment (Figure 1).
Figure 1.
A longitudinal section of the implant.
The ratio between the shell thickness and the core size is critical for the stability of the hypothetical implant structure, and needs comprehensive biomechanical analysis to confirm. Thus, in some narrow implant sites, the application of the hypothetical implant may have limitations.
Based on the width of natural periodontium [32], the space between the shell and the core is, on average, 0.15 mm wide, in which nano-springs are generally assembled and oriented perpendicularly to both the shell and the core. Nano-springs can be compressed and elongated at most to 0.02 mm in order to simulate the physiological range of motion of natural teeth.
The top of the core is designed to be discoid. Its contour in the longitudinal section is a segment of a circle, the center of which is the midpoint of the opening at the top of the shell. This design makes the core able to tilt or rotate around the center of the circle when loaded by non-axial force.
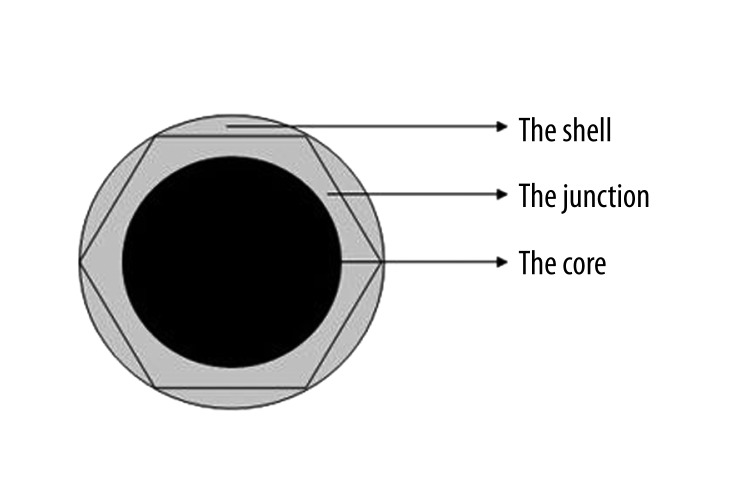
The junction, an arc extension of the shell, has direct contact with the top of the core and prevents the core from dislocation. Its margins are smooth and rounded so as to avoid stress concentration. Looking down from above, the contour design of the junction is hexagonal, which is beneficial to surgical implanting (Figure 2).
Figure 2.
The top design of the implant.
Compared with the tissue engineering method for constructing a periodontium-like architecture, the use of nano-springs can avoid problems in ethics and immunological rejection. This hypothetical implant is suitable for industrial production and could be quickly applied in clinics. But, although the shell has close contact with the core at the top of the implant, saliva would still flow into the space between the shell and the core; bacteria may multiply there and produce toxins.
Application of NGF
An improvement in the osteoconductivity [33] of implants has already been achieved by coating their surfaces with layers of calcium phosphate in various crystalline or amorphous forms [34–38]. Attempts have also been made to endow these coatings with the ability to induce tissue regeneration by the addition of growth factors, such as transforming growth factor beta (TGF-β) [39] or bone morphogenetic proteins (BMPs) [40–45].
After undergoing the treatments of sandblasting, acid-etching and microarc oxidation, the hypothetical implant will be immersed in a 5-fold concentration of simulated body fluid [46] for 24 h at 37°C under high-nucleation conditions to inhibit crystal growth. The fine, dense layer of amorphous calcium phosphate thereby produced [47] serves as a seeding surface for the deposition of a crystalline layer [48]. The crystalline layer will be produced by immersing the implant in a supersaturated solution of calcium phosphate (pH 7.4) containing rat recombinant NGF (10 mg/L), for 48 h at 37°C NGF will be enveloped in the honeycomb-like structure of calcium phosphate. After implanting, layers of calcium phosphate will be slowly degraded, which means that NGF will be released gradually to guide nerve regeneration and new bone formation.
NGF will also be injected into the periosteum and the gingival connective tissue nearby the implant site to promote the generation of nerve endings. We consider that receptors in peri-implant soft tissues may have a greater contribution to osseoperception, because more nerve endings are observed in peri-implant soft tissues compared to peri-implant bone.
Consequences of the Hypothesis and Discussion
After implanting, good osteoconductivity, improved by the layer of calcium phosphate in crystalline form and NGF-induced new bone formation, will lead to a more rapid and finer osseointegration. Since the shell has close contact with peri-implant bone to form a stable combination, the stress-cushioning structure formed by nano-springs transfers the main biomechanical interface of the implant from outside to inside to protect osseointegration from disturbance. When the implant is loaded by either axial forces or non-axial forces, all the nano-springs are activated to cushion the forces and distribute them generously to all the peri-implant bone, so that stress concentration may be reduced.
Meanwhile, NGF-induced regeneration of nerve endings in both hard and soft peri-implant tissues may help to enhance tactile sensibility, so as to minimize occlusal overload on the implant.
Further research needs to be done before this technique is ready for clinical use. The biomechanical property of the implant should be verified first to ensure that its structure is stable enough to resist masticatory forces in humans and its design is able to reduce stress concentration around peri-implant bone in a dog or monkey model. Since the safety and efficacy of NGF in nerve regeneration and new bone formation is confirmed in both animal studies and clinical applications [25–31], clinical trials could thus be carried out to further investigate its efficacy in the enhancement of osseoperception. Our design is only a model. In addition to NGF, there are many other growth factors that also affect the bone healing process, such as TGF-β and BMPs. A reasonable combined application of these factors may have a better result. When these concerns are settled, we believe that the application of the hypothetical implant may have potential advantages over traditional osseointegrated dental implants.
Footnotes
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no potential conflicts of interest regarding employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
Source of support: Nation Nature Science Foundation of China (81170995)
References
- 1.Muhlbradt L, Ulrich R, Mohlmann H, Schmid H. Mechanoperception of natural teeth versus endosseous implants revealed by magnitude estimation. Int J Oral Maxillofac Implants. 1989;4:125–30. [PubMed] [Google Scholar]
- 2.Misch CE, Suzuki JB, Misch-Dietsh FM, Bidez MW. A positive correlation between occlusal trauma and peri-implant bone loss: literature support. Implant Dent. 2005;14:108–16. doi: 10.1097/01.id.0000165033.34294.db. [DOI] [PubMed] [Google Scholar]
- 3.Isidor F. Loss of osseointegration caused by occlusal load of oral implants. A clinical and radiographical study in monkeys. Clin Oral Impl Res. 1996;7:143–52. doi: 10.1034/j.1600-0501.1996.070208.x. [DOI] [PubMed] [Google Scholar]
- 4.Von Arx T, Kurt B, Hardt N. Treatment of severe peri-implant bone loss using autogenous bone and a resorbable membrane. Case report and literature review. Clin Oral Implants Res. 1997;8:517–26. doi: 10.1034/j.1600-0501.1997.080611.x. [DOI] [PubMed] [Google Scholar]
- 5.Büchter A, Kleinheinz J, Meyer U, Joos U. Treatment of severe peri-implant bone loss using autogenous bone and a bioabsorbable polymer that delivered doxycycline (Atridox) Br J Oral Maxillofac Surg. 2004;42(5):454–56. doi: 10.1016/j.bjoms.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Tomson PL, Butterworth CJ, Walmsley AD. Management of peri-implant bone loss using guided bone regeneration: a clinical report. J Prosthet Dent. 2004;92:12–16. doi: 10.1016/j.prosdent.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Rotenberg SA, Tatakis DN. Recombinant Human Bone Morphogenetic Protein-2 for Peri-Implant Bone Regeneration. A Case Report. J Periodontol. 2011;82(8):1212–18. doi: 10.1902/jop.2011.100626. [DOI] [PubMed] [Google Scholar]
- 8.Khoury F, Buchmann R. Surgical therapy of peri-implant disease: a 3-year follow-up study of cases treated with 3 different techniques of bone regeneration. J Periodontol. 2001;72:1498–508. doi: 10.1902/jop.2001.72.11.1498. [DOI] [PubMed] [Google Scholar]
- 9.Athanasiou VT, Papachristou DJ, Panagopoulos A, et al. Histological comparison of autograft, allograft-DBM, xenograft, and synthetic grafts in a trabecular bone defect: an experimental study in rabbits. Med Sci Monit. 2010;16(1):BR24–31. [PubMed] [Google Scholar]
- 10.Karring T, Nyman S, Gottlow J, Laurell L. Development of the biological concept of guided tissue regeneration-animal and human studies. Periodontol 2000. 1993;1:26–35. [PubMed] [Google Scholar]
- 11.Ripamonti U, Reddi AH. Periodontal regeneration: potential role of bone morphogenetic proteins. J Periodont Res. 1994;29:225–35. doi: 10.1111/j.1600-0765.1994.tb01216.x. [DOI] [PubMed] [Google Scholar]
- 12.Shimono M, Ishikawa T, Ishikawa H, et al. Regulatory mechanisms of periodontal regeneration. Microsc Res Tech. 2003;60:491–502. doi: 10.1002/jemt.10290. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Rossi FMV, Putnins EE. Periodontal regeneration using engineered bone marrow mesenchymal stromal cells. Biomaterials. 2010;31(33):8574–82. doi: 10.1016/j.biomaterials.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Gault P, Black A, Romette JL, et al. Tissue-engineered ligament: implant constructs for tooth replacement. J Clin Periodontol. 2010;37(8):750–58. doi: 10.1111/j.1600-051X.2010.01588.x. [DOI] [PubMed] [Google Scholar]
- 15.Li SZ, Ding XD, Li J, et al. High-efficiency mechanical energy storage and retrieval using interfaces in nanowires. Nano Lett. 2010;10(5):1774–79. doi: 10.1021/nl100263p. [DOI] [PubMed] [Google Scholar]
- 16.Scully C. Oxford handbook of applied dental sciences. Oxford University Press; 2002. p. 156. [Google Scholar]
- 17.Enkling N, Utz KH, Bayer S, Stern RM. Osseoperception: active tactile sensibility of osseointegrated dental implants. Int J Oral Maxillofac Implants. 2010;25(6):1159–67. [PubMed] [Google Scholar]
- 18.Wada S, Kojo T, Wang YH, et al. Effect of loading on the development of nerve fibers around oral implants in the dog mandible. Clin Oral Implants Res. 2001;12(3):219–24. doi: 10.1034/j.1600-0501.2001.012003219.x. [DOI] [PubMed] [Google Scholar]
- 19.Korsching S. The role of nerve growth factor in the CNS. Trends Neurosci. 1986;9:570–77. [Google Scholar]
- 20.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–62. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 21.Sebert ME, Shooter EM. Expression of mRNA for neurotrophic factors and their receptors in the rat dorsal root ganglion and sciatic nerve following nerve injury. J Neurosci. 1993;36:357–67. doi: 10.1002/jnr.490360402. [DOI] [PubMed] [Google Scholar]
- 22.Seottek EK, Luo L. How do dendrites take their shape. J Nat Neurosci. 2001;4(4):359–69. doi: 10.1038/86006. [DOI] [PubMed] [Google Scholar]
- 23.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Progr Neurobiol. 2007;82(2):163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Androw PH, Biauca MRK, Freda PM. The localization, trafficking and retrograde transport of BDNF bound to p75NTR sympathetic neurons. Mol Cell Neurosci. 2006;32(7):387–402. doi: 10.1016/j.mcn.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Ebendal T. NGF in CNS: Experimental data and clinical implications. Prog Growth Factor Res. 1989;1(3):143–59. doi: 10.1016/0955-2235(89)90008-2. [DOI] [PubMed] [Google Scholar]
- 26.Olson L. NGF and the treatment of Alzheimer’s disease. Experimental Neurology. 1993;124(1):5–15. doi: 10.1006/exnr.1993.1167. [DOI] [PubMed] [Google Scholar]
- 27.Baringe M. Neurotrophic factors enter the clinic. Science. 1994;264(5160):772–74. doi: 10.1126/science.8171331. [DOI] [PubMed] [Google Scholar]
- 28.Wucherpfennig AL, Chiego DJ, Jr, Avery JK. Tritiated thymidine auto-radiographic study on the influence of sensory and sympathetic innervation on periodontal wound healing in the rat. Arch Oral Biol. 1990;35(6):443–48. doi: 10.1016/0003-9969(90)90207-q. [DOI] [PubMed] [Google Scholar]
- 29.Richards AM, Mitsou J, Floyd DC, et al. Neural innervation and healing. The Lancet. 1997;350(9074):339–40. doi: 10.1016/s0140-6736(05)63391-0. [DOI] [PubMed] [Google Scholar]
- 30.Eppley BL, Snyders RV, Winkelmann TM, et al. Efficacy of nerve growth factor in regeneration of the mandibular nerve: a preliminary report. J Oral Maxillofac Surg. 1999;49(1):61–68. doi: 10.1016/0278-2391(91)90268-q. [DOI] [PubMed] [Google Scholar]
- 31.Grills BL, Schuijers JA, Ward AR. Topical application of nerve growth factor improves fracture healing in rats. J Orthop Res. 1997;15:235–42. doi: 10.1002/jor.1100150212. [DOI] [PubMed] [Google Scholar]
- 32.Cate ART. Oral histology: development, structure, and function. 5th ed. St. Louis: Mosby; 1998. p. 256. [Google Scholar]
- 33.Liu Y, Schoenaers J, de Groot K, et al. Bone healing in porous implants, a histological and histometrical comparative study on sheep. J Mater Sci Mater Med. 2000;1:711–17. doi: 10.1023/a:1008971611885. [DOI] [PubMed] [Google Scholar]
- 34.de Groot K, Geesink R, Klein CP, Serekian P. Plasma sprayed coatings of hydroxylapatite. J Biomed Mater Res. 1987;21:1375–81. doi: 10.1002/jbm.820211203. [DOI] [PubMed] [Google Scholar]
- 35.Freeman MA. Hydroxyapatite coating of prostheses. J Bone Joint Surg Br. 1992;74:933–34. doi: 10.1302/0301-620X.74B6.1447263. [DOI] [PubMed] [Google Scholar]
- 36.Geesink RG. Hydroxyapatite-coated total hip prostheses. Two-year clinical and roentgenographic results of 100 cases. Clin Orthop. 1990:39–58. [PubMed] [Google Scholar]
- 37.Kokubo S, Kushitani H, Ebisawa Y, et al. Apatite formation on bioactive ceramics in body environment. In: Oonishi H, Aoki H, Sawai K, editors. Bioceramics. Vol. 1. Tokyo: Ishiyaku EuroAmerica; 1989. pp. 157–62. [Google Scholar]
- 38.Liu Y, Layrolle P, de Bruijn J, et al. Biomimetic coprecipitation of calcium phosphate and bovine serum albumin on titanium alloy. J Biomed Mater Res. 2001;57:327–35. doi: 10.1002/1097-4636(20011205)57:3<327::aid-jbm1175>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Blom EJ, Klein-Nulend J, Wolke JG, et al. Transforming growth factor-beta1 incorporation in a calcium phosphate bone cement: material properties and release characteristics. J Biomed Mater Res. 2002;59:265–72. doi: 10.1002/jbm.1241. [DOI] [PubMed] [Google Scholar]
- 40.Alam MI, Asahina I, Ohmamiuda K, et al. Evaluation of ceramics composed of different hydroxyapatite to tricalcium phosphate ratios as carriers for rhBMP-2. Biomaterials. 2001;22:1643–51. doi: 10.1016/s0142-9612(00)00322-7. [DOI] [PubMed] [Google Scholar]
- 41.Glass DA, Mellonig JT, Towle HJ. Histologic evaluation of bone inductive proteins complexed with coralline hydroxyapatite in an extraskeletal site of the rat. J Periodontol Mar. 1989;60:121–26. doi: 10.1902/jop.1989.60.3.121. [DOI] [PubMed] [Google Scholar]
- 42.Ono I, Gunji H, Kaneko F, et al. Efficacy of hydroxyapatite ceramic as a carrier for recombinant human bone morphogenetic protein. J Craniofac Surg. 1995;6:238–44. doi: 10.1097/00001665-199505000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Ono T, Tateshita T, Inoue M, Kuboki Y. In vivo strength enhancement of hydroxyapatite combined with rhBMP-2. J Bone Miner Metab. 1998;16:81. [Google Scholar]
- 44.Uludag H, D’Augusta D, Palmer R, et al. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46:193–202. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, de Groot K, Hunziker EB. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone. 2005;36(5):745–57. doi: 10.1016/j.bone.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Layrolle P, van Blitterswijk CA, de Groot K. Biomimetic hydroxyapatite coating on Ti6A14V induced by pre-calcification. In: Le Geros RZ, Le Geros JP, editors. Bioceramics; Proceedings of the 11th International Symposium on Ceramics in Medicine, 11; New York, NY: 1998. pp. 465–68. [Google Scholar]
- 47.Barrere F, van Blitterswijk CA, de Groot K, Layrolle P. Influence of ionic strength and carbonate on the Ca-P coating formation from SBFx5 solution. Biomaterials. 2002;23:1921–30. doi: 10.1016/s0142-9612(01)00318-0. [DOI] [PubMed] [Google Scholar]
- 48.Barrere F, Layrolle P, van Blitterswijk CA, de Groot K. Biomimetic calcium phosphate coatings on Ti6AI4V: a crystal growth study of octacalcium phosphate and inhibition by Mg2+ and HCO3. Bone. 1999;25:107S–11S. doi: 10.1016/s8756-3282(99)00145-3. [DOI] [PubMed] [Google Scholar]