Summary
Background
Several HIV protease mutations, which are resistant to clinical HIV protease inhibitors (PIs), have been identified. There is a great need for second-generation PIs with different chemical structures and/or with an alternative mode of inhibition. Ginkgolic acid is a natural herbal substance and a major component of the lipid fraction in the nutshells of the Ginkgo biloba tree. The objective of this study was to determine whether ginkgolic acid could inhibit HIV protease activity in a cell free system and HIV infection in human cells.
Material/Methods
Purified ginkgolic acid and recombinant HIV-1 HXB2 KIIA protease were used for the HIV protease activity assay. Human peripheral blood mononuclear cells (PBMCs) were used for HIV infection (HIV-1SF162 virus), determined by a p24gag ELISA. Cytotoxicity was also determined.
Results
Ginkgolic acid (31.2 μg/ml) inhibited HIV protease activity by 60%, compared with the negative control, and the effect was concentration-dependent. In addition, ginkgolic acid treatment (50 and 100 μg/ml) effectively inhibited the HIV infection at day 7 in a concentration-dependent manner. Ginkgolic acid at a concentration of up to 150 μg/ml demonstrated very limited cytotoxicity.
Conclusions
Ginkgolic acid effectively inhibits HIV protease activity in a cell free system and HIV infection in PBMCs without significant cytotoxicity. Ginkgolic acid may inhibit HIV protease through different mechanisms than current FDA-approved HIV PI drugs. These properties of ginkgolic acid make it a promising therapy for HIV infection, especially as the clinical problem of viral resistance to HIV PIs continues to grow.
Keywords: ginkgolic acid, HIV protease inhibitor, cytotoxicity, HIV infection
Background
Human immunodeficiency virus-1 (HIV-1) infection represents an ongoing global health crisis; over 1.3 million Americans and approximately 33 million people worldwide are infected [1]. Approximately 2.5 million new cases are diagnosed each year, and half of these infections occur in individuals under the age of 25 years [1]. Fortunately, since the advent of highly active antiretroviral therapy (HAART) nearly two decades ago, the morbidity and mortality associated with HIV-1 infection have become markedly reduced. However, there are important challenges now associated with HAART therapy including cardiovascular disease, cancer and rapid development of drug-resistant HIV viruses [2,3]. Potential targets for anti-HIV drugs are developed based on the HIV viral life cycle. Various drugs can inhibit virus adsorption, viral fusion, viral uncoating, or specific viral replicative enzymes such as reverse transcriptase (RT), integrase (IN) and HIV protease (PR) [4,5]. Most anti-HIV drugs are HIV reverse transcriptase inhibitors (RTIs) including the nucleotide RTIs (NRTIs) and non-nucleotide RTIs (NNRTIs). At present, there are several FDA approved HIV protease inhibitors (PIs) including saquinavir (SQV), ritonavir (RTV), indinavir (IDV), nelfinavir (NFV), lopinavir (LPV), atazanavir (ATZ), tipranavir (TPV), amprevavir (APV), and fosamprevnir (FPV) [5–7]. Clinical application of RTIs and PIs in various combinations has dramatically reduced the morbidity and mortality of AIDS and has significantly improved life expectancy for HIV positive patients. Meanwhile, widespread use of PIs has led to the emergence of drug-resistant HIV proteases [2], leading to substantial numbers of HIV-infected patients on HAART regimens that are unresponsive to currently available PIs. In these patients, mutations have been found in the coding sequence of 49 of the 99 amino acids of HIV protease. Substitutions at 18 or more positions directly correlates with loss of responsiveness to PI treatment. Since the existing PIs target the active site of HIV protease and have similar structures, most of the drug-resistant HIV proteases confer cross-resistance to multiple PIs. There is a great need for second-generation PIs with different chemical structures and/or with an alternative mode of inhibition [8,9]. In addition, reduced general toxicity may be achieved. Natural herbal substances represent a great opportunity for the development of new PIs.
Chinese herbal medicines have been used for thousands of years based largely on anecdotal observations by practitioners. However, it is quite possible that certain herbal remedies may have specific therapeutic action with respect to HIV infection [10]. For example, some natural herbal substances have been shown to be effective at inhibiting the growth of HIV in vitro at different stages in HIV-1 replication [10,11]. Collins et al. [8] reported that 6 out of 19 aqueous herbal extracts significantly inhibited the interaction between HIV-1 gp120 and immobilized CD4 receptors. Several extracts have also been shown to be capable of inhibiting the activity of recombinant HIV-1 protease [12,13]. Extract from Ginkgo leaves is one of the most widely used herbal supplements and has become increasingly popular in recent years. Ginkgo contains two groups of active substances: flavonoid glycosides including quercetin and rutin, and terpene lactones including ginkgolides A, B, C and ginkgolic acid. The antioxidative activity of ginkgo compounds contributes to the protective effects seen in humans in multiple organ systems including visual, cardiovascular, pulmonary, and central nervous systems [14]. However, it is not known whether ginkgo compounds can affect HIV infection.
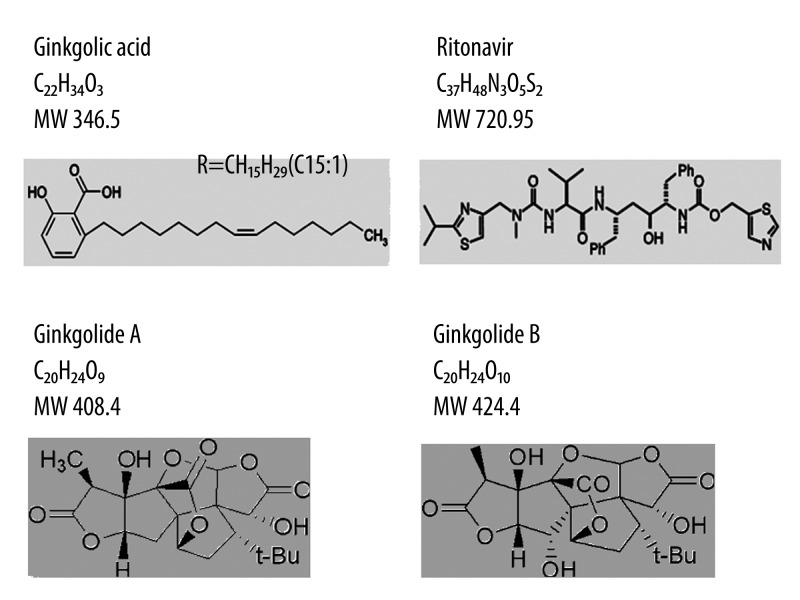
Ginkgolic acids have low cytotoxicity [15]. They are 2-hydroxy-6-alkylbenzoic acids (also known as 6-alkyl salicylic acids) with saturated or up to triple unsaturated n-C13- to n-C19- alkyl residues; the most common residues are monounsaturated C15H29 and C17H33. A total of 9 derivatives have been identified and exist as a mixture in ginkgo extracts. Ginkgolic acids are found in the lipid fraction of the nutshells of Ginkgo biloba and are also present in Ginkgo leaves. In this study, we focused on one specific compound, a simple unsaturated (R=C15:1) ginkgolic acid, which is the main component of the nutshells and leaves [16–18]. The chemical structure of ginkgolic acid is different than that of current HIV PIs and ginkgolides, though it has some similarity with aspirin (Figure 1). The objective of this study was to determine whether ginkgolic acid could inhibit HIV protease activity in the cell-free system and control HIV infection in a cell culture model.
Figure 1.
Chemical structures of ginkgolic acid (used in this study), the commonly used HIV protease inhibitor ritonavir, ginkgolide A, and ginkgolide B.
Material and Methods
Reagents
Purified single compound ginkgolic acid (C22H34O3) with 90% purity by HPLC was purchased from AXXORA, LLC (San Diego, CA). Recombinant HIV-1 HXB2 KIIA protease was generously provided by Dr. David Davis from the HIV and AIDS Malignancy Branch, National Cancer Institute at the National Institutes of Health. HIV-1 HXB2 KIIA protease was produced from E. Coli and had >95% purity by HPLC analysis. The specific activity was 6.3 μM/minute/mg when assayed for 5 minutes against this substrate. The molecular weight was 10,746 by mass spectrometry. One μg/ml of HIV protease was the optimal concentration for this study. Laboratory adapted HIV-1SF162, originally isolated from the cerebrospinal fluid of patient with AIDS, was obtained from the NIH AIDS Research and Reference Reagent Program. Human peripheral blood mononuclear cells (PBMCs) of seronegative donors obtained from a local blood bank. The EnzoLyte™ 520 HIV-1 protease assay kit was from AnaSpec Co., (Fremont, CA). All other reagents were from VWR.
Determination of the kinetics of the inhibition of HIV protease activity by ginkgolic acid
The EnzoLyte™ 520 HIV-1 protease assay kit (AnaSpec Co.) was used to measure HIV protease enzyme activity and inhibition by ginkgolic acid. This protease assay kit provides a convenient assay for measuring HIV protease enzyme activity and screening HIV-1 PIs using a HiLyte Fluor™488/QXL™520 fluorescence resonance energy transfer (FRET) peptide. The sequence of this FRET peptide is derived from the native p17/p24 cleavage site on Prgag for HIV-1 protease. In the FRET peptide, the fluorescence of HiLyte Fluor™488 is quenched by QXL™520 until this peptide is cleaved into two separate fragments by HIV-1 protease. Upon cleavage, the fluorescence of HiLyte Fluor™488 is recovered, and can be monitored at excitation/emission (490 nm/520 nm). Recombinant HIV-1 HXB2 KIIA protease was used in this assay [19].
Determination of the effect of ginkgolic acid on the inhibition of HIV infection in cell culture
Ficoll/Hypaque-isolated human PBMCs were stimulated for 3 days in RPMI/FCS containing phytohemagglutinin (5 μg/ml). PBMCs were suspended at 107 cells/ml in RPMI medium only or RPMI with varying concentrations of ginkgolic acid; then, they were mixed with 105 TCID50 of HIV-1SF162 cell free virus at the multiplicity of infection (m.o.i.) of 0.005 TCID50 per cell. After a 30 min adsorption period, sequential 2-fold dilutions of the solutions were added to cultures of PBMCs (2×105 PBMCs with 0.5 m.o.i of HIV-1SF162 virus). After that, the cells were incubated with HIV-1SF162 virus in a 5% CO2 humidified incubator at 37°C for 7 days. The supernatants were harvested and analyzed for HIV-1 p24. The levels of HIV p24 antigen in the supernatant samples were assayed by a p24gag enzyme-linked immunosorbent assay according to the manufacturer’s instructions (NEN Life Science Products).
Cytotoxicity of ginkgolic acid in cell culture
Jurkat cells are derived from human T-cell leukemia cells. Jurkat cells (106 cells/ml) were cultured in the RPMI medium with or without different concentrations of ginkgolic acid for 48 hours to test the cytotoxicity of ginkgolic acid. The cytotoxicity of ginkgolic acid was determined with the CellTiter 96® AQueous Assay (Promega) which uses a tetrazolium compound (MTS) and an electron coupling reagent (PMS). MTS is chemically reduced by cells into formazan, which is soluble in the tissue culture medium. The measurement of the absorbance of the formazan can be carried out using 96 well microplates at 492 nm. Since the production of formazan is proportional to the number of living cells, the intensity of the produced color is a good indication of the viability of the cells.
Statistical analysis
All experiments were performed at least 3 times. Differences between the treated and control groups were analyzed using the Student t-test for paired data with a significance level of P<0.05. The results are reported as a mean with standard error.
Results
The effect of ginkgolic acid on the inhibition of HIV protease activity in a cell-free system
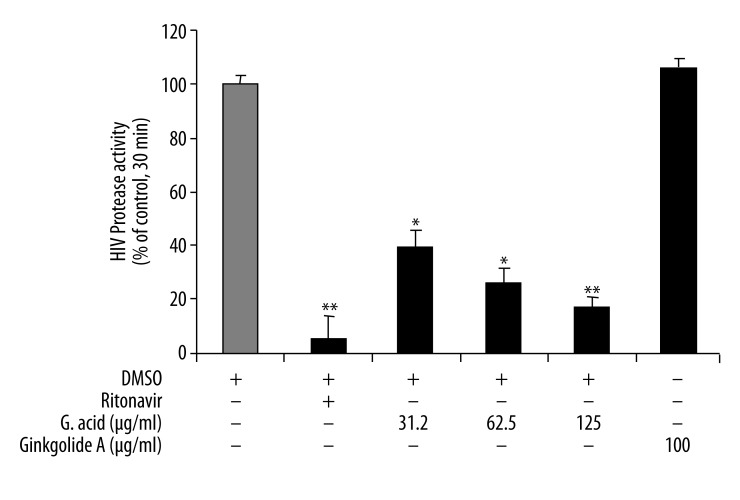
HIV protease enzyme activity and inhibition by ginkgolic acid was assayed with the EnzoLyte™ 520 HIV-1 protease assay kit (AnaSpec Co.) in a cell free enzyme system. HIV protease inhibitor ritonavir (10.8 μg/ml or 15 μM) was used as a positive control. Ginkgolic acid showed a potent effect on the inhibition of HIV protease activity in the cell free system (Figure 2). Ginkgolic acid (31.2 μg/ml) inhibited HIV protease activity by 60%, compared with the negative control, and the effect was concentration-dependent. In addition, the effect of ginkgolic acid on the inhibition of HIV protease activity was also compared with that of other ginkgo compounds such as ginkgolide A, which did not have any effect on HIV protease activity. Furthermore, relatively small amounts of ginkgolic acid did not change the pH in the reaction system.
Figure 2.
Ginkgolic acid inhibits HIV protease activity in a concentration-dependent manner. Recombinant HIV-1 HXB2 KIIA protease and the EnzoLyte™ 520 HIV-1 protease assay kit (AnaSpec Co.) were used in this study. HIV protease inhibitor ritonavir (10.8 μg/ml or 15 μM) was used as a positive control. Ginkgolic acid (32.2, 62.6 and 125 μg/ml) and ginkgolide A (100 μg/ml) were used. ** P<0.01 and * P<0.05 as compared with the negative control (DMSO).
The effect of ginkgolic acid on the inhibition of HIV infection in vitro
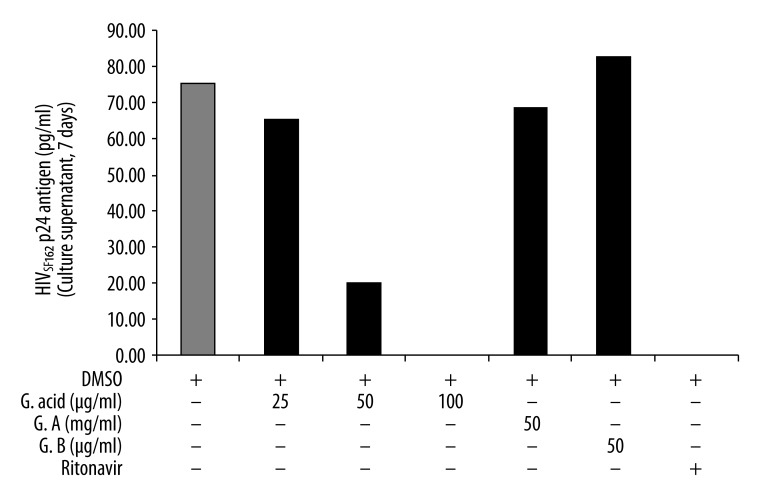
HIV-1SF162 cell free virus combined with varying concentrations of ginkgolic acid in RPMI medium were added to cultures of human PBMCs with 0.5 m.o.i of HIV-1SF162 virus. HIV p24 antigen in the supernatant was measured by quantitative ELISA. Ginkgolic acid treatment (50 and 100 μg/ml) effectively inhibited the HIV infection at day 7 in a concentration-dependent manner (Figure 3). However, Ginkgolides A and B at 50 μg/ml did not inhibit HIV-1SF162 infection in HBPCs (Figure 3). Ritonavir was used as a positive control in the experiment.
Figure 3.
Ginkgolic acid, but not ginkgolide A and B, inhibits HIV infection in human PBMC cells. Human PBMCs were stimulated for 3 days in RPMI/FCS containing phytohemagglutinin (5 μg/ml). PBMCs (2×105 per well) were infected with HIV-1SF162 (0.5 m.o.i.) for 7 days. Ritonavir (15 μM), ginkgolic acid (25, 50 and 100 μg/ml), Ginkgolide A (50 μg/ml) or Ginkgolide B (50 μg/ml) was included in the culture. The levels of HIV p24 antigen in the supernatant samples were assayed by a p24gag enzyme-linked immunosorbent assay.
The cytotoxicity of ginkgolic acid on Jurkat cells in vitro
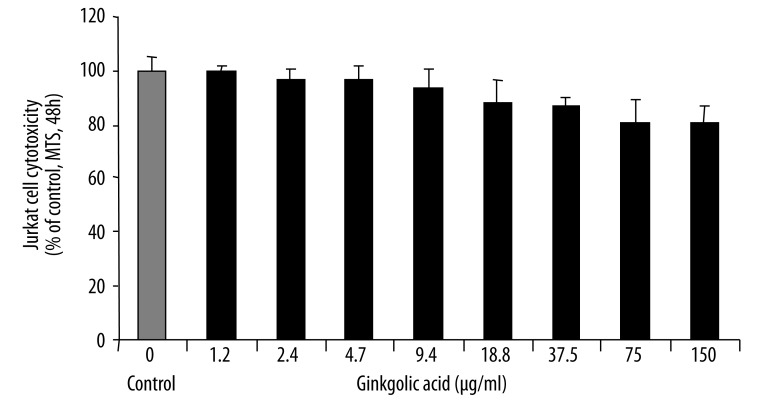
The cytotoxicity of ginkgolic acid on Jurkat cells was determined in vitro with the CellTiter 96® AQueous Assay (Promega) which uses a novel tetrazolium compound (MTS) and an electron coupling reagent (PMS). The Jurkat cells were cultured for 48 hours with various concentrations of ginkgolic acid and the number of viable cells were analyzed by MTS assay. The cytotoxicity of ginkgolic acid was minimal in Jurkat cells tested in vitro (Figure 4). PBMCs treated with ginkgolic acid did not show any cytotoxicity characteristics (data not known).
Figure 4.
Ginkgolic acid at the concentrations up to 150 μg/ml did not cause any significant cytotoxicity in Jurkat cells. The Jurkat cells were cultured for 48 hours with various concentrations of ginkgolic acid. The cytotoxicity of ginkgolic acid on Jurkat cells was determined in vitro with the CellTiter 96® AQueous Assay.
Discussion
HIV-1 protease plays an essential role in the life cycle of HIV because it cleaves the newly synthesized polyproteins to yield viral structural and functional proteins necessary for maturation. Thus, inhibitors of HIV-1 protease are very effective antiviral drugs that can significantly prolong the life of patients with AIDS [20]. The current PIs, however, have several unwanted side effects. Ritonavir, for example, may cause asthenia, malaise, diarrhea, nausea and vomiting, abdominal pain, dizziness, insomnia, sweating, taste abnormalities, and problems related to metabolism [21]. As such, the development of new inhibitors of HIV-1 protease is an urgent task.
In a previous paper, Lee et al. [22] reported that ginkgolic acid (C15: 1) and several other compounds from Ginkgo biloba exhibited potent dose-dependent inhibitory activities on HIV-1 protease with an IC50 24.9 μM, which was assayed in a cell-free system by HPLC with the synthetic heptapeptide [His-Lys-Ala-Arg-Val-Leu-(pNO2-Phe)-Glu-Ala-Nle-Ser-NH2] as the substrate. In this study, we assayed the HIV-1 inhibition activity of ginkgolic acid with a convenient HIV-1 protease assay kit in a cell free system; furthermore, we investigated the inhibitory action of ginkgolic acid on HIV-1 infection and its cytotoxicity in Jurkat cells. Our data demonstrate that ginkgolic acid effectively inhibits in vitro HIV infection in PBMC cells with limited cytotoxicity. Ginkgolic acid showed a potent effect on the inhibition of HIV protease activity in a concentration-dependent manner in the cell free system. Importantly, ginkgolic acid at a concentration up to 150 μg/ml had very limited cytotoxicity in Jurkat cells tested in vitro.
In this study we demonstrate that ginkgolic acid is able to inhibit HIV protease activity in a concentration-dependent manner, with an estimated IC50 of less than 30 μg/ml in the cell free system. This effect was specific; other ginkgo compounds such as ginkgolide A did not have any effect on HIV protease activity. Furthermore, the effect was not caused by pH change from the addition of ginkgolic acid. Therefore, the strong inhibitory effect of ginkgolic acid is possibly related directly to its unique chemical structure. Ginkgolic acid has a structure distinct from that of ritonavir and other current PIs, which all target the active site of HIV protease and can confer cross-resistance to multiple PIs.
Furthermore, ginkgolic acid treatment effectively inhibited the HIV-1SF162 infection in human PBMCs in a concentration-dependent manner; ginkgolide A and ginkgolide B (50 μg/ml) had no effects on HIV infection. This confirmed that the HIV inhibition is related to the unique structure of the ginkgolic acid.
Although some reports indicate that ginkgolic acid may have allergenic, cytotoxic, mutagenic and carcinogenic effects [15,23], systematic and detailed investigations of these events in animal studies and human observations have not been performed. In general, all compounds may have both therapeutic effects and side effects, which must be weighed in the development of clinically useful drugs. Clinical studies using Ginkgo extract with less than 5 ppm ginkgolic acid have shown Ginkgo to be remarkably free of side effects. However, the side effect profile for Ginkgo exceeding this standard is not known. Our data showed ginkgolic acid was an effective HIV protease inhibitor in both a cell free system and cell culture model, with limited cytotoxicity even at concentration up to 150 μg/ml.
Conclusions
In conclusion, our preliminary data indicate that ginkgolic acid is a HIV protease inhibitor. This is a good start in the investigation of new PIs, especially in light of the growing problem of PI-resistant HIV infection, and further investigation is warranted. Several critical questions must be addressed, such as the kinetics of inhibition and the time course of the effect of ginkgolic acid on HIV infection in cell culture. Also, inhibition of several HIV strains including lab adapted strains and clinical isolates of HIV should be assessed, as well as cytotoxicity in other cell types. Bioavailability, pharmacokinetics and toxicity should be studied in animal models. Finally, a strategy to modify the chemical structure of ginkgolic acid to enhance its therapeutic effects and reduce toxic effects should be examined.
Acknowledgements
We thank the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, for providing RTV and HIV-1SF162. The authors confirm that there are no conflicts of interest.
Footnotes
Source of support: This work is partially supported by research grants from the National Institutes of Health (R01 HL065916 and R01 HL083471 to C.C.). S.M.W. and Z.L. was supported by a training grant from NIH (T32HL083774)
References
- 1.UNAIDS/WHO. AIDS Epidemic Update. Dec, 2010. http://www.unaids.org.
- 2.Erickson JW. HIV-1 protease as a target for AIDS therapy. In: Ogden RC, Flexner CW, editors. Protease inhibitors in AIDS therapy. Marcel Dekker, Inc; New York, N Y: 2001. pp. 1–25. [Google Scholar]
- 3.Reisler RB, Han C, Burman WJ, et al. Grade 4 events are as important as AIDS events in the era of HAART. J Acquir Immune Defic Syndr. 2003;34:379–86. doi: 10.1097/00126334-200312010-00004. [DOI] [PubMed] [Google Scholar]
- 4.De Clercq E. Highlights in the development of new antiviral agents. Mini Rev Med Chem. 2002;2:163–75. doi: 10.2174/1389557024605474. [DOI] [PubMed] [Google Scholar]
- 5.OARAC. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. 2008 Nov 3;2008 [cited 2010 Aug. 18]; Available from: http://aidsinfo.nih.gov. [Google Scholar]
- 6.De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115–33. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Moore JP, Doms RW. The entry of entry inhibitors: a fusion of science and medicine. Proc Natl Acad Sci USA. 2003;100:10598–602. doi: 10.1073/pnas.1932511100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorsey BD, McDonough C, McDaniel SL, et al. Identification of MK-944a: a second clinical candidate from the hydroxylaminepentanamide isostere series of HIV protease inhibitors. J Med Chem. 2000;43:3386–99. doi: 10.1021/jm9903848. [DOI] [PubMed] [Google Scholar]
- 9.Navia MA, Fitzgerald PM, McKeever BM, et al. Three-dimensional structure of aspartyl protease from human immunodeficiency virus HIV-1. Nature. 1989;337:615–20. doi: 10.1038/337615a0. [DOI] [PubMed] [Google Scholar]
- 10.Chang RS, Yeung HW. Inhibition of growth of human immunodeficiency virus in vitro by crude extracts of Chinese medicinal herbs. Antiviral Res. 1988;9:163–75. doi: 10.1016/0166-3542(88)90001-0. [DOI] [PubMed] [Google Scholar]
- 11.Lu W. Prospect for study on treatment of AIDS with traditional Chinese medicine. J Tradit Chin Med. 1995;15:3–9. [PubMed] [Google Scholar]
- 12.Collins RA, Ng TB, Fong WP, et al. A comparison of human immunodeficiency virus type 1 inhibition by partially purified aqueous extracts of Chinese medicinal herbs. Life Sci. 1997;60:PL345–51. doi: 10.1016/s0024-3205(97)00227-0. [DOI] [PubMed] [Google Scholar]
- 13.Lam TL, Lam ML, Au TK, et al. A comparison of human immunodeficiency virus type-1 protease inhibition activities by the aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2000;67:2889–96. doi: 10.1016/s0024-3205(00)00864-x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou W, Chai H, Lin PH, et al. Clinical use and molecular mechanisms of action of extract of Ginkgo biloba leaves in cardiovascular diseases. Cardiovasc Drug Rev. 2004;22:309–19. doi: 10.1111/j.1527-3466.2004.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu ZH, Zeng S. Cytotoxicity of ginkgolic acid in HepG2 cells and primary rat hepatocytes. Toxicol Lett. 2009;187:131–36. doi: 10.1016/j.toxlet.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Jaggy H, Koch E. Chemistry and biology of alkylphenols from Ginkgo biloba L. Pharmazie. 1997;52:735–38. [PubMed] [Google Scholar]
- 17.Fuzzati N, Pace R, Villa F. A simple HPLC-UV method for the assay of ginkgolic acids in Ginkgo biloba extracts. Fitoterapia. 2003;74:247–56. doi: 10.1016/s0367-326x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 18.Choi YH, Choi HK, Peltenburg-Looman AM, et al. Quantitative analysis of ginkgolic acids from Ginkgo leaves and products using 1H-NMR. Phytochem Anal. 2004;15:325–30. doi: 10.1002/pca.786. [DOI] [PubMed] [Google Scholar]
- 19.The EnzoLyte™ 520 HIV-1 protease assay kit. [cited 2010 Aug. 18]; Available from: http://www.anaspec.com/products/product.asp?id=30170.
- 20.Deeks SG, Smith M, Holodniy M, Kahn JO. HIV-1 protease inhibitors. A review for clinicians. JAMA. 1997;277:145–53. [PubMed] [Google Scholar]
- 21.http://www.rxlist.com/cgi/generic/ritonavirsol_ad.htm [cited 2010 Aug. 18]
- 22.Lee JS, Hattori M, Kim J. Inhibition of HIV-1 protease and RNase H of HIV-1 reverse transcriptase activities by long chain phenols from the sarcotestas of Ginkgo biloba. Planta Med. 2008;74:532–34. doi: 10.1055/s-2008-1074497. [DOI] [PubMed] [Google Scholar]
- 23.Chao JC, Chu CC. Effects of Ginkgo biloba extract on cell proliferation and cytotoxicity in human hepatocellular carcinoma cells. World J Gastroenterol. 2004;10:37–41. doi: 10.3748/wjg.v10.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]