Summary
Background
This study aimed to explore the effects of progressive resistance exercise training (PRET) on hemorheology.
Material/Methods
Exercise sessions included 1–3 sets of 8–12 repetitions at 40–60% of 1-repetition maximum (1-RM) for 3 weeks and at 75–80% of 1-RM during weeks 4–12. Red blood cell (RBC) deformability and aggregation were determined by ektacytometry, plasma and whole blood viscosities (WBV) by rotational viscometry. Lactate concentration was evaluated by an analyzer and fibrinogen was evaluated by coagulometry. Plasma total oxidant/antioxidant status was measured by colorimetry.
Results
Following an acute increase after exercise on the first day, RBC deformability was elevated during weeks 3 and 4 (p=0.028; p=0.034, respectively). The last exercise protocol applied in week 12 again caused an acute increase in this parameter (p=0.034). RBC aggregation was increased acutely on the first day, but decreased after that throughout the protocol (p<0.05). At weeks 4 and 12 pre-exercise measurements of WBV at standard hematocrit and plasma viscosity were decreased (p=0.05; p=0.041, respectively), while post-exercise values were increased (p=0.005; p=0.04, respectively). Post-exercise WBV at autologous hematocrit measured at week 12 was increased (p=0.01). Lactate was elevated after each exercise session (p<0.05). Fibrinogen was decreased on the third week (p<0.01), while it was increased on the 4th week (p=0.005). Plasma antioxidant status was increased at week 3 (p=0.034) and oxidative stress index was decreased at week 4 (p=0.013) after exercise.
Conclusions
The results of this study indicate that PRET may have positive effects on hemorheological parameters.
Keywords: sports, hemodynamics, oxidant-antioxidant status, cardiovascular risk
Background
Progressive resistance exercise training (PRET), which is a method of increasing the ability of muscles to generate force, is widely used in clinics, in athletic conditioning, maintaining health and preventing disease [1,2]. PRET, when incorporated into a comprehensive fitness program, is known to improve cardiovascular functions [3], reduce the risk factors associated with coronary heart disease [4], preserve functional capacity [5] and thus improve quality of life [6].
The principles of PRET are to systematically increase the intensity of load, and to deliver maximum performance by increasing muscle strength and endurance. These principles are detailed in the guidelines of the American College of Sports Medicine (ACSM) [1,2]. Intensity of an exercise in PRET can be estimated as a percentage of 1-repetition maximum (1 RM). It is suggested that for novice individuals circuit training loads should be ~50–60% of 1 RM or less for 8–12 repetitions and for advanced individuals 80–100% of 1 RM is recommended. While training at a specific RM load, a 2–10% increase in load is recommended to be applied when the individual can perform the current workload for 1–2 repetitions over the desired number on 2 consecutive training sessions. Additionally, training of 1–3 sets, for 2 or 3 days a week and rest periods of at least 2–3 min are also suggested [1,2].
Blood flow, deformability and aggregability of red blood cells (RBC) are the main components of hemorheology. In large blood vessels, a basic component is the flow. In microcirculation, where cells must deform to pass through narrow capillaries, deformability and aggregation of RBCs are the major determinants of resistance to flow [7]. Increases in plasma fibrinogen concentrations in response to exercise are known to cause enhancements in RBC aggregation [8]. Similarly, exercise-induced alterations in oxidative stress and plasma lactic acid concentrations have been shown to affect rheological properties of blood [9,10].
The short- and long-term effects of various exercise types on hemorheology have been well-defined and reviewed [8–12]. Exercise-induced alterations in blood rheology depend on the type, duration and intensity of exercise, and the athletic capacity of the individual also plays a significant role [11,12].
Previous studies have also presented some evidence of hemorheological alterations induced by resistance exercise training (RET). Plasma viscosity (PV) and fibrinogen levels were found to be increased after RET performed at an intensity corresponding to 80% of 1RM [13]. A recent study was carried out in our laboratory to investigate the acute and long-term (6 weeks) effects of RET, performed at 2 different intensities (corresponding to 70% and 85% of 1RM) on hemorheological and hematological parameters. RET-induced post-exercise increases in RBC aggregation and deformability on the first and last day of both programs were demonstrated in this study. On the other hand, WBC, RBC, Hb and Hct values were significantly increased immediately after the RET on the first and last day of the program only in the moderate intensity group of subjects [14].
Although previous studies have already presented some evidence of hemorheological and hematological alterations induced by RET [10,13,14], no information is available about the effects of PRET, which is widely used in clinics for taking highest level of advantage from RET, on hemorheological parameters. Therefore, the present study was designed to explore the acute and chronic effects of PRET performed at 12 weeks on hemorheological parameters in healthy, young, sedentary males. Hematological parameters, blood lactate and plasma fibrinogen concentrations, plasma total oxidant (TOS) and antioxidant (TAS) status were also determined to examine the contribution of these parameters to the possible PRET-induced alterations in hemorheological parameters.
Material and Methods
Study population
Twelve male student volunteers from Pamukkale University (mean age 22.08±0.86 years) with no apparent health problems, who had not regularly performed resistance exercise training before, participated to the study. They were all nonsmokers and were instructed not to use alcohol or any medication during the study. Female students were excluded to avoid the effects of ovarian hormones, which are known to influence hemorheology and blood flow in healthy women. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Additionally, the approval of experimental procedures was provided by the Pamukkale University Ethics Committee. Written consent forms were obtained from all subjects, who were completely informed about the study. The subjects’ body fat was estimated by bioimpedance method by use of the TANITA body composition analyzer (TANITA Corporation of America).
Progressive Resistance Exercise Training (PRET) protocols
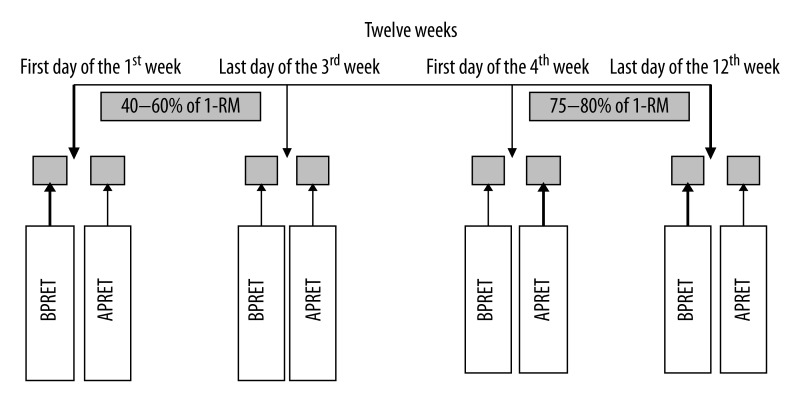
The full exercise program was completed in the Sports Rehabilitation Unit of the Physical Medicine and Rehabilitation Department of Pamukkale University. Before the beginning of the study, the subjects were familiarized with PRET upper and lower body exercises. All subjects performed PRET 3 times a week for 12 weeks under staff supervision. Seven stations were used to exercise upper and lower body large muscle groups: leg extension (quadriceps), leg curl (hamstring), chest press (pectoralis major), arm flexion (biceps), arm extension (triceps), abdominal crunch (abdominal), twisting oblique (external and internal oblique) and outer thigh pull (hip abduction, gluteus medius). After the familiarization session, subject’s 1-repetition maximum (1-RM) strength for each exercise was evaluated as the heaviest maximum weight that a subject could lift in a single repetition while maintaining appropriate exercise technique. Training protocol was applied as recommended by ACSM [1,2]. Exercise sessions included the performance of 1 set of 8–12 repetitions at 40–60% of 1-RM during the first week, 2 sets during the second week and 3 sets during the third week. Three sets of 8–12 repetitions at 75–80% of 1-RM were applied during weeks 4–12 (Figure 1). Repeated 1-RM measurements were performed at baseline, once a week during the first 3 weeks and every 2 week afterwards. The exercise intensity was adjusted according to these measurements.
Figure 1.
Blood sampling protocol; BPRET: before progressive resistance exercise training, APRET: after progressive resistance exercise training.
The warm-up procedure consisted of 5–7 min walking on a treadmill and 5–7 min light stretching exercises. After each exercise session a 5-min cool-down period of light stretching was applied. The speed execution of the exercise was 3:3 with a 2-minute rest interval. Anticoagulated blood samples (Heparin was used for TOS/TAS measurements, sodium citrate for fibrinogen and EDTA for the other parameters) were collected in Vacutainers at weeks 1, 3, 4 and 12 before and after the exercise sessions (Figure 1) and measurements from week 1 before the exercise session was used as the baseline value. Hemorheological parameters were carried out within 3 hours after blood collection.
Assessment of RBC deformability
RBC deformability (ie, the ability of the entire cell to adopt a new configuration when subjected to applied mechanical forces) was determined by laser diffraction analysis using an ektacytometer (LORCA, RR Mechatronics; Hoorn, The Netherlands). The system has been described elsewhere in detail [15]. Briefly, a low Hct suspension of RBC in 4% polyvinylpyrrolidone 360 solution (MW 360 kD, Sigma P 5288, St. Louis, MO) (4% in PBS, viscosity: 23.2 cP) was sheared in a Couette system composed of a glass cup and a precisely fitting bob with a gap of 0.3 mm between the cylinders. A laser beam was directed through the sheared sample, and the diffraction pattern produced by the deformed cells was analyzed by a microcomputer. On the basis of the geometry of the elliptical diffraction pattern, an elongation index (EI) was calculated for 9 shear stresses between 0.3 and 30 Pascal (Pa) as: EI=(L−W)/(L+W), where L and W are the length and width of the diffraction pattern, respectively. An increased EI at a given shear stress indicates greater cell deformation and hence greater RBC deformability. All measurements were carried out at 37°C.
Measurements of RBC aggregation
RBC aggregation was also determined by LORCA as described elsewhere [16]. The measurement is based on the detection of laser back-scattering from the sheared (disaggregated), then unsheared (aggregating) blood, performed in a computer-assisted system at 37°C. Back-scattering data were evaluated by the computer and the aggregation index (AI), aggregation half-time (t1/2) which shows the kinetics of aggregation and the amplitude (AMP), which is a measure for the total extent of aggregation, were calculated on the basis that there is less light back-scattered from aggregating red cells. The hematocrit (Hct) of the samples used for aggregation measurements was adjusted to 40% and blood was fully oxygenated.
Determination of the whole blood and plasma viscosity
Whole blood viscosities (WBV) were determined with a Wells-Brookfield cone-plate rotational viscometer (model DV-II + Pro, Brookfield engineering Labs, Middleboro, MA) at shear rates between 75 and 375 s−1 at 37°C at both native Hct and standard (40%) Hct. The Hct of blood samples was adjusted to 0.4 l/l by adding or removing a calculated amount of autologous plasma. PV was determined using the same viscometer at 375 s−1 at 37°C.
Determination of hematological parameters
RBC counts, Hb, Hct, mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) were determined using an electronic hematology analyzer (Cell-Dyn Sapphire, Abbott Diagnostic Division, USA).
Measurement of blood lactate concentration
Venous blood samples were obtained just before and immediately after the PRET protocol on weeks 1, 3, 4, and 12 from subjects’ earlobes and lactate concentration was measured by a lactate analyzer (YSI 1500 Yellow Spring Inst., USA) [17].
Measurement of plasma fibrinogen concentration
Sodium citrated blood samples were analyzed for plasma fibrinogen concentration using a fully automated coagulometer (Dade Behring, BCS XP).
Determination of plasma total oxidant status (TOS)
The total oxidant status (TOS) of plasma was measured using a novel automated colorimetric measurement method for TOS developed by Erel [18]. In this method, oxidants present in the sample oxidize the ferrous ion O-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules (eg, lipids, proteins) present in the sample. The assay is calibrated with hydrogen peroxide, and the results are expressed in terms of micromolar hydrogen peroxide equivalent per liter (μmol H2O2 equiv/L).
Measurement of plasma total antioxidant status (TAS)
The total antioxidant status (TAS) of plasma was measured using a novel automated colorimetric measurement method for TAS developed by Erel [19]. In this method the hydroxyl radical, the most potent biological radical, is produced by the Fenton reaction and reacts with the colorless substrate O-dianisidine to produce the dianisyl radical, which is bright yellowish-brown in color. Upon the addition of a plasma sample, the oxidative reactions initiated by the hydroxyl radicals present in the reaction mix are suppressed by the antioxidant components of the plasma, preventing the color change and thereby providing an effective measure of the TAS of the plasma. The assay results are expressed as mmol Trolox equiv/L.
Calculation of oxidative stress index
The ratio of TOS to TAS is referred to as the oxidative stress index (OSI). The OSI is calculated according to the following formula:
| [ 20] |
Measurement of maximal oxygen uptake (VO2 max)
Subjects were exercised until being exhausted on a motorized treadmill according to Bruce protocol [21]. The treadmill grade was increased 3% every 3 min, starting from a 2.7 km/h speed and grade 10%. Oxygen consumption was measured indirectly according to the following formula: (VO2max (ml/kg/min)=14.76−(1.379 × Time) + (0.451 × Time2) − (0.012 × Time3).
Statistical analyses
Results are expressed as means ± standard error (SE). The data were not normally distributed and non-parametric tests were used. Statistical comparisons between groups were done by Tests for Several Related Samples (Friedman Variance Analysis) followed by Wilcoxon Signed Rank Test, with p values ≤0.05 accepted as statistically significant. Correlations were assessed with Spearman’s procedure. All analyses were carried out with the computerized SPSS 10.0 program (Statistical Package for Social Sciences, SPSS, Inc).
Results
Physical characteristics and maximal oxygen uptake variables
All subjects completed both exercise protocols without problems. The PRET protocol applied did not induce statistically significant alterations in physical characteristics and maximal oxygen uptake (VO2 max) of the subjects. On the other hand, 1-RM of each muscle group measured on week 12 after the exercise protocol was statistically significantly increased compared to basal values obtained on the first day of the exercise (Table 1).
Table 1.
Physical and training characteristic data of the subjects.
| BPRET on first week | APRET on twelfth week | p change (%) | ||||
|---|---|---|---|---|---|---|
| Mean ± SE | Min–max | Mean ± SE | Min–max | |||
| Weight (kg) | 71.21±3.74 | 51.50–95.40 | 71.96±3.71 | 53.10–97.50 | 0.054 | 1 |
| Body Mass Index (kg/m2) | 22.29±1.09 | 16.60–29.40 | 22.46±1.09 | 17.10–30.10 | 0.343 | 0.76 |
| Fat mass (%/kg) | 11.36±1.66 | 1.70–21.00 | 11.55±1.63 | 2.10–21.30 | 0.638 | 1.7 |
| VO2max (lt/min) | 47.42±1.63 | 38.00–58.40 | 47.06±1.44 | 39.50–55.01 | 0.784 | −1 |
| 1RM-Leg extension (quadriceps)(kg) | 65.83±5.60 | 40–90 | 75.83±4.72 | 50–100 | 0.002 | 15 |
| 1RM-Leg curl (hamstring) (kg) | 59.16±4.39 | 40–90 | 70.41±2.64 | 50–95 | 0.002 | 19 |
| 1RM-Chest press (pectoralis major) (kg) | 64.17±5.80 | 30–90 | 71.25±6.10 | 35–100 | 0.004 | 11 |
| 1RM-Arm flexion (biceps) (kg) | 24.17±1.72 | 15–35 | 30.00±1.74 | 20–40 | 0.001 | 24 |
| 1RM-Arm extension (triceps) (kg) | 27.50±2.42 | 15–50 | 33.33±2.64 | 20–55 | 0.002 | 21 |
| 1RM-Abdominal crunch (abdominal) (kg) | 40.42±4.06 | 20–65 | 49.17±3.68 | 30–70 | 0.003 | 22 |
| 1RM-Twisting oblique (external and internal oblique) (kg) | 37.08±2.92 | 25–55 | 48.75±4.97 | 30–95 | 0.002 | 31 |
| 1RM-Right Outer thigh pull (hip abduction, gluteus medius) (kg) | 15.00±1.07 | 10–20 | 20.00±1.07 | 15–25 | 0.001 | 33 |
| 1RM-Left Outer thigh pull (hip abduction, gluteus medius) (kg) | 15.11±0.74 | 10–20 | 20.00±1.07 | 15–25 | 0.002 | 32 |
BPRET – before progressive resistance exercise training; APRET – after progressive resistance exercise training; VO2 max – maximal oxygen uptake; 1-RM – one-repetition maximum.
RBC deformability measurements
RBC deformability (ie, the elongation index, EI) for the RBCs of all experimental groups was measured at 9 shear stresses between 0.3 and 30.0 pascal (Pa). Effects of PRET on RBC deformability were mostly observed at low and moderate stress levels (0.95–5.33 Pa). Alterations in EI values measured at 0.53 Pa were more prominent and are shown in Table 2 as a representative. EI values of week 3 and pre-exercise EI value of week 4 were increased compared to the basal value (p<0.05). The last exercise session on week 12 induced increases in RBC deformability compared to the basal value and pre-exercise value of the same week (p=0.028, p=0.034, respectively). Additionally, a positive correlation between the percent change in EI and 1 RM of arm extension (triceps) after 12 weeks of training was observed (r=0.739; p=0.006, data not shown).
Table 2.
RBC elongation index (EI), aggregation amplitude (AMP), aggregation index (AI) and aggregation half time (t½) of the subjects.
| 1st week | 3rd week | 4th week | 12th week | |||||
|---|---|---|---|---|---|---|---|---|
| BPRET on first week | APRET on first week | BPRET on third week | APRET on third week | BPRET on fourth week | APRET on fourth week | BPERT on twelfth week | APRET on twelfth week | |
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |
| EI (0.53 Pa) | 0.061±0.004 | 0.076±0.008 | 0.074±0.005** | 0.075±0.007** | 0.074±0.007** | 0.069±0.005 | 0.068±0.004 | 0.080±0.005**,≠≠ |
| AMP | 25.14±0.76 | 27.13±1.15** | 27.26±0.75 | 26.83±1.46 | 25.25±0.74≠ | 25.48±0.71≠ | 25.26±0.78 | 26.24±0.90 |
| AI (%) | 56.64±1.55 | 61.85±2.34* | 53.21±1.91**,ø | 55.13±1.64ø | 55.67±1.70øø | 54.87±2.03øø | 53.41±1.79øø | 54.37±1.81øø |
| t 1/2 (s) | 3.08±0.21 | 2.45±0.26* | 3.59±0.31ø | 3.20±0.21ø | 3.03±0.25≠,øø | 3.37±0.29øø | 3.57±0.28ø | 3.40±0.25øø |
Values are expressed as mean ± SE. BPRET – before progressive resistance exercise training; APRET – after progressive resistance exercise training.
p≤0.01, difference from first week BPRET;
p<0.05, difference from first week BPRET values;
p≤0.01, difference from first week APRET;
p<0.05, difference from first week APRET values;
p≤0.05, difference from third week BPRET;
p<0.05, difference from twelfth week BPRET values.
RBC aggregation measurements
Table 2 also shows that 1 set of exercise applied at 40–60% of 1-RM induced a statistically significant acute increase in RBC aggregation amplitude (AMP) (p=0.05), aggregation index (AI) (p=0.01) and decrease in aggregation half-time (t1/2) (p=0.01). Although the alteration in t½ was not statistically significant, the pre-exercise AI values measured on week 3 were decreased and t½ increased compared to the basal value. Additionally, AI values measured on weeks 3, 4 and 12 were lower (p=0.006, p=0.003, p=0.015, p=0.023, p=0.023, p=0.019, respectively) and t½ higher than the values measured on the 1st week after the exercise session (p=0.01, p=0.003, p=0.034, p=0.023, p=0.01, p=0.019, respectively). Taken together, the decreases in AMP and AI of aggregation and the increase in t1/2 indicate an exercise-induced decrease in RBC aggregation. Positive correlations between the percent change in AI and 1 RM of chest press (m. pectoralis major) after 12 weeks of training were observed (r=0.642; p=0.024, data not shown).
Whole blood and plasma viscosity measurements
Whole blood viscosities (WBV) were determined at shear rates between 75 and 375 s−1 at 37°C at both native and standard (40%) Hct. Table 3 shows results of 75 s−1 as an example. Although the alterations were not statistically significant, the exercise protocol applied resulted in decreases in WBV measured at native Hct, except for the post-exercise increases on weeks 4 and 12. On the contrary, WBV measured at standard (40%) Hct after the exercise session on the 4th week was low, indicating that the increase observed at native Hct was due to the alterations in Hct value. WBV at standard (40%) Hct was still low on the 12th week, in which the last exercise session resulted in an increase in this parameter. Additionally, PRET protocol applied herein caused decrease in PV at a shear rate of 375 s−1 which were statistically significant on 4th (BPRET) and 12th (APRET) week measurements (Table 3). There was a positive correlation between the percent change in PV and 1 RM of twisting oblique (external and internal oblique) after 12 weeks of training (r=0.688; p=0.003, data not shown).
Table 3.
Whole blood and plasma viscosity of the subjects.
| 1st week | 3rd week | 4th week | 12th week | |||||
|---|---|---|---|---|---|---|---|---|
| BPRET on first week | APRET on first week | BPRET on third week | APRET on third week | BPRET on fourth week | APRET on fourth week | BPERT on twelfth week | APRET on twelfth week | |
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |
| WBV at native Hct (75 s−1) | 6.14±0.23 | 5.92±0.21 | 5.77±0.17 | 5.99±0.09 | 5.73±0.15 | 6.23±0.23** | 5.86±0.15 | 6.39±0.22≠,≠≠,& |
| WBV at standard Hct (%40) (75−1) | 5.51±0.31 | 5.92±0.21 | 5.41±0.22 | 5.43±0.15 | 5.33±0.24 | 5.04±0.14≠ | 4.71±0.16≠,*,≠≠,øø | 5.58±0.23& |
| Plasma viscosity (375 s−1) | 1.84±0.06 | 1.92±0.13 | 1.78±0.07 | 1.68±0.06 | 1.63±0.07**,ø | 1.75±0.06 | 1.68±0.05 | 1.68±0.05* |
BPRET – before progressive resistance exercise training; APRET – after progressive resistance exercise training. Values are expressed as mean ± SE.
p≤0.05, difference from first week BPRET values;
p<0.05, difference from first week APRET values;
p<0.05, difference from third week BPRET values;
p<0.01, difference from third week BPRET values;
p≤0.05, difference from third week APRET;
p<0.05, difference from fourth week BPRET values;
p≤0.01, difference from twelfth week BPRET values.
Measurements of hematological parameters
Alterations in hematological parameters in response to PRET applied in our study are presented in Table 4. Although exercising for 3 weeks resulted in a decrease in RBC count (p<0.05), it increased again on the 4th week. One set of exercise applied at 40–60% of 1-RM induced a statistically significant decrease in Hb value (p=0.014). Hb values proceeded at a low level for 4 weeks and increased again at the 12th week. Pre-exercise Hct values measured at week 3 was significantly decreased compared to week 1 (p<0.05). Three sets of exercise applied at 75–80% of 1-RM (4th–12th weeks) induced increases at Hct value. PRET applied in our study did not induce any alteration in MCV until the 12th week, whereas MCV measured at this week was significantly higher than all the previous measurements. The post-exercise MCHC value measured on week 4 was lower compared to the basal value.
Table 4.
Hematological parameters of the subjects.
| 1st week | 3rd week | 4th week | 12th week | |||||
|---|---|---|---|---|---|---|---|---|
| BPRET on first week | APRET on first week | BPRET on third week | APRET on third week | BPRET on fourth week | APRET on fourth week | BPERT on twelfth week | APRET on twelfth week | |
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |
| RBC count (106/μl) | 5.11±0.15 | 5.09±0.12 | 4.90±0.14*,≠ | 4.89±0.10*,≠≠ | 5.08±0.11& | 5.10±0.15 Ø | 5.00±0.12 | 4.97±0.12 |
| Hb (g/dl) | 15.40±0.30 | 14.93±0.33* | 14.92±0.24* | 14.80±0.29** | 15.20±0.20& | 14.78±0.29**,$ | 15.62±0.23 Ø,&,$,§ | 15.37±0.27&,§§ |
| Hct(%) | 43.45±1.12 | 43.09±0.79 | 41.86±0.83*,≠ | 42.19±0.59 | 43.48±0.79 | 43.74±1.03 Ø | 44.25±0.81 Ø,& | 43.55±0.74& |
| MCV (fl) | 85.14±1.15 | 85.05±1.16 | 85.60±1.10 | 85.95±1.13 | 85.64±1.01 | 86.00±1.12 | 88.10±1.11*,≠, ØØ,&,$$,§§ | 87.76±1.10*,≠, Ø,&,$,§§, ß |
| MCHC (g/dl) | 35.53±0.41 | 34.79±0.55 | 35.70±0.50 | 35.33±0.58 | 35.10±0.56 | 33.87±0.63*,Ø | 35.24±0.49 | 35.30±0.51 |
BPRET – before progressive resistance exercise training; APRET – after progressive resistance exercise training; RBC – red blood cell; Hb – hemoglobin; Hct – hematocrit; MCV – mean corpuscular volume; MCHC – mean corpuscular hemoglobin concentration. Values are expressed as mean ± SE.
p≤0.05, difference from first week BPRET values;
p<0.01, difference from first week BPRET values;
p≤0.05, difference from first week APRET values;
p<0.01, difference from first week APRET values;
p<0.05, difference from third week BPRET values;
p≤0.01, difference from third week BPRET values;
p<0.05, difference from third week APRET values;
p<0.05, difference from fourth week BPRET values;
p≤0.01, difference from fourth week BPRET values;
p<0.01, difference from fourth week APRET values;
p<0.05, difference from fourth week APRET values;
p<0.05, difference from twelfth week BPRET values.
Blood lactate concentration measurements
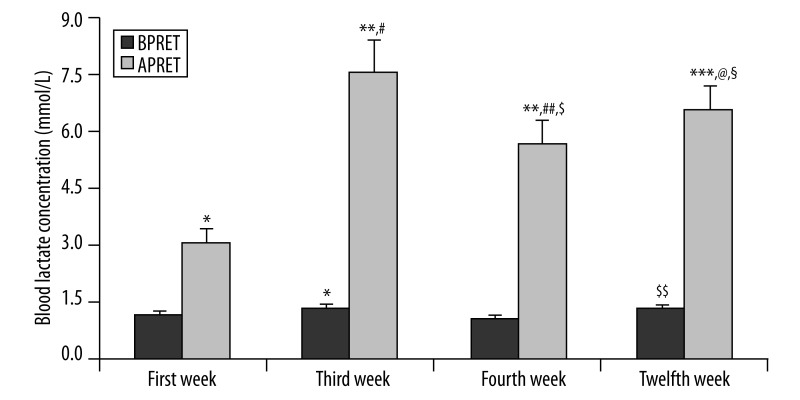
Figure 2 demonstrates that each exercise session resulted in acute statistically significant enhancements in blood lactate concentration. When pre-exercise measurements were compared with each other, it was observed that pre-exercise measurements of week 3 were elevated compared to that of the 1st week (p=0.043) and blood lactate concentration measured on the 12th week was higher than on week 4 (p=0.043). On the other hand, when post-exercise measurements were compared with each other, it was found that post-exercise values of all weeks were significantly higher than that of the 1st week. Additionally, post-exercise blood lactate concentration measured on the 4th week was lower compared to post-exercise value of weeks 3 (p=0.015) and 12 (p=0.028).
Figure 2.
Blood lactate concentration values of the subjects before (BPRET) and after (APRET) progressive resistance exercise training on the first, third, fourth and twelfth week of the program. Values are expressed as mean ± SE. * p<0.05, difference from first week BPRET values; ** p<0.01, difference from first week APRET; *** p<0.05, difference from first week APRET; #p<0.01, difference from third week BPRET values; ##p<0.05, difference from third week APRET values; $p<0.01, difference from fourth week BPRET values; $$p<0.05, difference from fourth week BPRET values; @p<0.05, difference from fourth week APRET values; §p<0.05, difference from twelfth week BPRET values.
Plasma fibrinogen concentration measurements
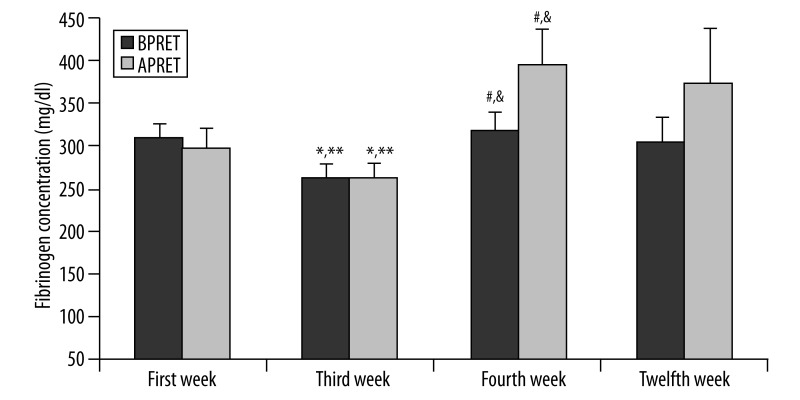
Figure 3 shows that plasma fibrinogen concentrations were decreased on the 3rd week but increased again on the 4th week. The alterations are statistically significant.
Figure 3.
Plasma fibrinogen concentrations of the subjects before and after progressive resistance exercise training (BPRET- APRET) on the first, third, fourth and twelfth week of the program. Values are expressed as mean ± SE. * p<0.01, difference from first week BPRET values; ** p<0.01, difference from first week APRET; #p<0.01, difference from third week BPRET values; &p<0.01, difference from third week APRET values.
Plasma total oxidant (TOS) and antioxidant status (TAS) measurements
The parameters showing the oxidant (TOS) and antioxidant status (TAS) as well as the oxidative stress index of the groups are presented in Table 5. Although acute exercise induced increases in both TOS and TAS levels at every exercise session, this augmentation was only statistically significant for TAS on the 3rd week (p=0.034). Similarly, the oxidative stress index (OSI) calculated for each exercise session as TOS/TAS ×100 was decreased acutely after each exercise session except on week 1. However, only 3 sets of exercise applied at 75–80% of 1-RM on the 4th week induced a statistically significant decrease in this parameter (p=0.013).
Table 5.
Plasma total oxidant (TOS), antioxidant (TAS) status and oxidative stress index (OSI) values of the subjects.
| 1st week | 3rd week | 4th week | 12th week | |||||
|---|---|---|---|---|---|---|---|---|
| BPRET on first week | APRET on first week | BPRET on third week | APRET on third week | BPRET on fourth week | APRET on fourth week | BPERT on twelfth week | APRET on twelfth week | |
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |
| TOS (μmol H2O2 Eqv/L) | 8.82±0.70 | 9.61±1.55 | 9.10±0.49 | 10.56±1.32 | 9.05±0.40 | 9.23±0.94 | 8.34±0.74 | 9.01±0.31 |
| TAS (mmol Trolox Eqv/L) | 0.76±0.08 | 0.91±0.14 | 0.77±0.08 | 0.94±0.11* | 0.90±0.14 | 1.01±0.13 | 0.8±0.1 | 0.93±0.12 |
| OSI (arbitrary unit) | 1.39±0.26 | 1.60±0.52 | 1.62±0.43 | 1.38±0.27 | 1.55±0.45 | 1.51±0.60≠ | 1.35±0.43 | 1.27±0.24 |
BPRET – before progressive resistance exercise training; APRET – after progressive resistance exercise training. Values are expressed as mean ± SE.
p<0.05, difference from third week BPRET values;
p<0.05, difference from fourth week BPRET values.
Discussion
Effects of PRET on hemorheological parameters were investigated in this study. To clarify the mechanisms of these effects, hematological parameters, blood lactate and plasma fibrinogen concentrations, plasma total oxidant (TOS) and antioxidant (TAS) status were also determined. The PRET protocol applied herein did not induce any statistically significant alteration in physical and training characteristics of the subjects. This finding is consistent with other findings in the literature which report no effect of resistance exercises on these parameters [22]. As expected, 1-RM of each muscle group measured on week 12 after the exercise session was increased, indicating improvement in muscle strength.
The ability of the entire RBC to deform is of crucial importance for performing its function in oxygen delivery and it is also a determinant of the cell survival time in the circulation [7]. Following an acute increase after the exercise on the first day, RBC deformability measured at 0.53 Pa was elevated throughout the experimental protocol. This higher RBC deformability may be an advantageous factor during exercise by increasing O2 diffusion from alveoli to pulmonary capillaries, resulting in higher blood and thus muscle oxygen content. Additionally, more deformable RBCs may contribute to physical performance, thus increasing muscle strength observed herein by carrying O2 to smaller muscle capillaries.
The findings of the studies investigating effects of exercise on RBC deformability in the literature differ depending on: (a) the type, duration, intensity of the exercise, (b) athletic capacity of the individuals, and (c) the method used to measure RBC deformability [23]. Exercise-induced decreases in RBC deformability were described in subjects with low physical fitness [12]. On the other hand, a 3-week training program was reported to increase RBC deformability in sedentary people [24]. The participants of our current study had not regularly performed resistance exercise or weight training before, but they were physically active (football, running) young people. The short-term and chronic effects of RET performed at 2 different intensities (70% and 85% of 1-RM) on RBC deformability and aggregation were studied in our laboratory in physically active subjects who were not engaged in resistance training. RBC deformability was found to be increased immediately after the RET on the first and the last day of the program, but this augmentation was statistically significant only for the moderate intensity group on the first day [14]. The deformability results of our current study, which is the first one in the literature exploring the effects of PRET on hemorheology, are consistent with the findings of the above-mentioned study.
Physical activity was demonstrated to cause oxidative stress via increasing metabolic processes, which can be reversed by the antioxidant defence system [25,26]. Although statistically insignificant, our results indicating acute exercise-induced increases in both TOS and TAS levels at every exercise session are in concordance with the above explanation. OSI was decreased acutely after each exercise session except on week 1. However, only the exercise of 3 sets applied at 75–80% of 1-RM induced a statistically significant decrease in this parameter, which can be explained as a more pronounced increase in TAS induced by this severe exercise applied on the 4th week. Alterations in oxidative stress affect RBC deformability during exercise [9,10]. In addition, exercise-induced hemolysis is a well-known phenomenon leading to an increased number of young erythrocytes in the circulation which have higher antioxidant system levels and are more deformable [27,28]. Although age distribution of RBCs was not analyzed in the current study, when our data are evaluated together it can be speculated that the increase of the antioxidant defence systems may at least partially have contributed to the elevation of RBC deformability.
Increase of lactate during exercise is another factor affecting RBC deformability [29]. Elevated post-exercise blood lactate concentrations of the current study are in agreement with results in the literature [30,31]. Results of some studies have indicated that lactate impairs RBC deformability in untrained subjects but improves it in trained individuals [29]. As we mentioned before, subjects of our study group were physically active young students. On the other hand, divergent literature data are found concerning lactate as a potential antioxidant agent acting by preventing lipid peroxidation [32]. These factors may explain the increase of RBC deformability in spite of the elevation of blood lactate concentration.
Decreased MCV and increased MCHC are known to impair RBC deformability via increase of internal viscosity [33,34]. Young erythrocytes, which increase as a result of hemolysis during exercise, have less MCHC and higher MCV and deformability [28,34]. Although the current study does not include any parameter directly showing degree of hemolysis, the decrease of RBC count and Hct on the 3rd week and increases observed in these parameters on the 4th week may indicate hemolysis and augmented RBC turn-over. Ernst et al. reported a significant decrease in MCHC and increase in MCV, which coincided with increase of RBC deformability after 3 weeks of training [24]. The alterations observed in MCV and MCHC in our study are in agreement with the results of the studies summarized above and may explain the PRET-induced alterations in RBC deformability, at least to some extent. Additionally, although neither plasma volume nor the total RBC mass were measured, the decreases observed in RBC count, Hct and Hb in our study may be related to possible exercise-induced increases in plasma volume.
Another hemorheological parameter determined in this study is the RBC aggregation, which is the reversible adhesion of adjacent erythrocytes. The influence of exercise on erythrocyte aggregation is controversial, for some authors have found it increased [8], while others found that it decreased [12,35] or was unchanged [36]. These discrepant results may be due to the type of exercise applied, physical condition of the subjects and methods used to measure RBC aggregation. Cakir-Atabek et al. performed the first study investigating effects of resistance exercise training (RET) on RBC aggregation. Their findings indicating acute RET-induced increases in RBC aggregation, followed by slight decreases in the moderate intensity group after 6 weeks of RET are in agreement with the results of the current study [14]. The long-term decrease in RBC aggregation during PRET may be beneficial for tissue perfusion by decreasing resistance to flow and thus may also be related to the improvements observed in muscle strength after 12 weeks of training.
Plasma fibrinogen concentration is a major determinant of RBC aggregation [8,9]. Several reports indicate exercise induced enhancements in fibrinogen concentrations [8,37]. Ahmadizad et al. reported that plasma fibrinogen was significantly increased immediately after 3 sets of 5–7 repetitions of 6 exercises at an intensity corresponding to 80% of 1-RM, but the rise in plasma fibrinogen was transient and returned to pre-exercise values after 30 min of recovery [13]. In our study, 3 sets of exercise at an intensity corresponding to 75–80% of 1-RM (4th and 12th weeks) caused acute slight increases in plasma fibrinogen concentrations, similar to the study summarized above. Additionally, the results of our study also demonstrate that 3 weeks of PRET causes decrease in plasma fibrinogen concentrations. The reduction in RBC aggregation observed in our study at the same period may at least partly depend on decreased plasma fibrinogen concentration.
Exercise-induced oxidative stress is known to increase RBC aggregation by reducing the amount of erythrocyte membrane sialic acid and thus creating negativity [33]. On the contrary, oxidative stress may decrease RBC aggregation via echinocyte formation [38]. When the results of the current study are evaluated together, it can be seen that the alterations in RBC aggregation are far from explained by the changes in oxidative stress. Alterations in lactic acid concentrations are also known to affect RBC aggregation during exercise [39]. Brun et al. have shown that erythrocyte aggregation is positively correlated with lactate accumulation into blood during exercise [40]. The post-exercise increases in RBC aggregation, which are in concordance with the literature, may be related to the post-exercise rises observed in blood lactate concentration. On the other hand, it can be speculated that the general decrease observed in RBC aggregation after 12 weeks of training may partly be due to the increased RBC turnover, since young erythrocytes are known to aggregate less [27].
Generally, exercise is known to cause acute decreases in blood fluidity (hemoconcentration) in sedentary individuals [10,35]. Following exercise training, increase in plasma volume results in autohemodilution, thus a decrease in WBV was reported [36]. However, no information is available about effects of resistance exercises on blood viscosity. Hct is a main determinant of blood viscosity [10,31,41], which is why we measured WBV at both native and standard (40%) Hct. PRET applied in the current study induced slight decreases in WBV until week 4. On the 4th week, a post-exercise rise in WBV values, measured at native Hct, and decline in standard Hct were observed. This difference may be explained by the possible acute hemoconcentration and thus the rise in Hct induced by the severe exercise (3 sets of 8–12 repetitions at 75–80% of 1-RM) applied on the 4th week. The general decrease in WBV and the decrease measured in the 12th week before the exercise session are in concordance with the autohemodilution process described in the literature for other exercise types. On the other hand, WBV increased acutely again after the last exercise session.
The results of a study exploring the effects of RET on PV have shown that although 3 sets of 5–7 repetitions of 6 exercises (80% of 1-RM) induced elevation in PV, this alteration returned to pre-exercise values 30 min later [13]. The rise obtained in PV on the 4th week after PRET applied at 75–80% of 1-RM is similar to the findings of the above- mentioned study. This post-exercise rise may also be explained by acute plasma loss, as mentioned above. Additionally, the decrease observed in PV after 12 weeks of training may contribute to the increase observed in 1 RM.
Conclusions
In conclusion, the results of this study in young, healthy subjects indicate that the PRET protocol applied herein may have beneficial effects on the regulation of circulation and tissue oxygenation, and thus contribute to cardiovascular well-being by increasing RBC deformability, general decreases in viscosities and an acute enhancement, then decrease, in RBC aggregation. The changes observed in muscle strength (1 RM) after 12 weeks of training may be related to these improvements in hemodynamics. It is worth emphasizing that effects of different resistance training models (isokinetic, isometric) as well as different training systems (eg, different combinations of resistance, sets, repetitions, speed, rest period) on hemorheological parameters may also be studied in order to observe which of them are more effective and can be suggested for healthy exercise. Additionally, to get more relevant information about the risks and health aspects of PRET, the alterations in hemorheological parameters in response to PRET of a group of patients with cardiovascular pathologies may also be investigated.
Acknowledgements
This study is the MS thesis of Emine Kilic-Toprak which was presented to the Health Sciences Institute, Pamukkale University, Denizli, Turkey. We are thankful to Rıdvan Colak Uskup Primary School, Bagcilar, Istanbul, Turkey and Yusuf Koklu Pamukkale University, School of Sport Science and Technology, Kinikli, 20070 Denizli, Turkey for helping measurements of blood lactate concentration.
Footnotes
This study was presented orally at the 36th National Congress of the Turkish Physiological Sciences Society 14–17 September, 2010 at Edirne, Turkey and printed in the abstract book
Source of support: This study was supported by Pamukkale University Research Fund through Project No. 2009SBE005
References
- 1.Kraemer WJ, Adams K, Cafarelli E, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–80. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Ratamess NA, Avlar BA, Evetoch TK, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 3.Kelley GA, Kelley KS. Progressive resistance exercise and resting blood pressure: A meta-analysis of randomized controlled trials. Hypertension. 2000;35:838–43. doi: 10.1161/01.hyp.35.3.838. [DOI] [PubMed] [Google Scholar]
- 4.Pu CT, Johnson MT, Forman DE, et al. Randomized trial of progressive resistance training to counteract the myopathy of chronic heart failure. J Appl Physiol. 2001;90:2341–50. doi: 10.1152/jappl.2001.90.6.2341. [DOI] [PubMed] [Google Scholar]
- 5.Levinger I, Goodman C, Hare DL, et al. The effect of resistance training on functional capacity and quality of life in individuals with high and low numbers of metabolic risk factors. Diabetes Care. 2007;30:2205–10. doi: 10.2337/dc07-0841. [DOI] [PubMed] [Google Scholar]
- 6.Levinger I, Goodman C, Hare DL, et al. Psychological responses to acute resistance exercise in men and women who are obese. J Strength Cond Res. 2009;23:1548–52. doi: 10.1519/JSC.0b013e3181a026e5. [DOI] [PubMed] [Google Scholar]
- 7.Stuart J, Nash GB. Red cell deformability and haematological disorders. Blood Rev. 1990;4:141–47. doi: 10.1016/0268-960x(90)90041-p. [DOI] [PubMed] [Google Scholar]
- 8.Varlet-Marie E, Gaudard A, Monnier JF, et al. Reduction of red blood cell disaggregability during submaximal exercise: relationship with fibrinogen levels. Clin Hemorheol Microcirc. 2003;28:139–49. [PubMed] [Google Scholar]
- 9.Brun JF. Exercise hemorheology as a three acts play with metabolic actors: is it of clinical relevance? Clin Hemorheol Microcirc. 2002;26:155–74. [PubMed] [Google Scholar]
- 10.El-Sayed MS, Ali, El-Sayed Ali Z. Haemorheology in exercise and training. Sports Med. 2005;35:649–70. doi: 10.2165/00007256-200535080-00001. [DOI] [PubMed] [Google Scholar]
- 11.Brun JF, Monnier JF, Raynaud E, et al. Erythrocyte disaggregability is reduced during submaximal exercise. (abstr) Haemostasis. 1996;26:603. [Google Scholar]
- 12.Yalcin O, Erman A, Muratli S, et al. Time course of hemorheological alterations after heavy anaerobic exercise in untrained human subjects. J Appl Physiol. 2003;94:997–1002. doi: 10.1152/japplphysiol.00368.2002. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadizad S, El-Sayed MS. The acute effects of resistance exercise on the main determinants of blood rheology. J Sports Sci. 2003;23:243–49. doi: 10.1080/02640410410001730151. [DOI] [PubMed] [Google Scholar]
- 14.Cakir-Atabek H, Atsak P, Gunduz N, Bor-Kucukatay M. Effects of resistance training intensity on deformability and aggregation of red blood cells. Clin Hemorheol Microcirc. 2009;41:251–61. doi: 10.3233/CH-2009-1176. [DOI] [PubMed] [Google Scholar]
- 15.Hardeman MR, Goedhart PT, Dobbe JGG, Lettinga KP. Laser assisted optical rotational cell analyzer (LORCA): a new instrument for measurement of various structural hemorhelogical parameters. Clin Hemorheol. 1994;14:605–18. [Google Scholar]
- 16.Hardeman MR, Dobbe JGG, Ince C. The Laser-assisted Optical Rotational Cell Analyzer (LORCA) as red blood cell aggregometer. Clin Hemorheol Microcirc. 2001;25:1–11. [PubMed] [Google Scholar]
- 17.Jahr JS, Osgood S, Rothenberg SJ, et al. Lactate measurement interference by hemoglobin-based oxygen carriers (Oxyglobin, Hemopure, and Hemolink) Anesth Analg. 2005;100:431–36. doi: 10.1213/01.ANE.0000142116.42938.82. [DOI] [PubMed] [Google Scholar]
- 18.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–19. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Kosecik M, Erel O, Sevinc E, Selek S. Increased oxidative stress in children exposed to passive smoking. Int J Cardiol. 2005;100:61–64. doi: 10.1016/j.ijcard.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 21.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assesment of functional aerobic impairtment in cardiovascular disease. American Heart Journal. 1973;85:546–62. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 22.Kraemer WJ, Patton JF, Gordon SE, et al. Compatibility of high intensity strength and endurance training on hormonal and skeletal muscle adaptations. J Appl Physiol. 1995;78:976–89. doi: 10.1152/jappl.1995.78.3.976. [DOI] [PubMed] [Google Scholar]
- 23.Gurcan N, Erbas D, Ergen E, et al. Changes in blood haemorheological parameters after submaximal exercise in trained and untrained subjects. Physiol Res. 1998;47:23–27. [PubMed] [Google Scholar]
- 24.Ernst E, Schmid M, Matrai A. Inter-individual changes of hemorheological and other variables by regular exercise. J Sports Cardiol. 1985;2:50–54. [Google Scholar]
- 25.Senturk UK, Gunduz F, Kuru O, et al. Exercise-induced oxidative stress affects erythrocytes in sedentary rats but not exercise-trained rats. J Appl Physiol. 2001;91:1999–2004. doi: 10.1152/jappl.2001.91.5.1999. [DOI] [PubMed] [Google Scholar]
- 26.Senturk UK, Yalcin O, Gunduz F, et al. Effect of antioxidant vitamin treatment on the time course of hematological and hemorheological alterations after an exhausting exercise episode in human subjects. J Appl Physiol. 2005;98:1272–79. doi: 10.1152/japplphysiol.00875.2004. [DOI] [PubMed] [Google Scholar]
- 27.Muravyov AV, Draygin SV, Eremin NN, Muravyov AA. The microrheological behavior of young and old red blood cells in athletes. Clin Hemorheol Microcirc. 2002;26:183–88. [PubMed] [Google Scholar]
- 28.Smith JA. Exercise, training and red blood cell turnover. Sports Med. 1995;19:9–31. doi: 10.2165/00007256-199519010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Connes P, Bouix D, Py G, et al. Opposite effects of in vitro lactate on erythrocyte deformability in athletes and untrained subjects. Clin Hemorheol Microcirc. 2004b;31:311–18. [PubMed] [Google Scholar]
- 30.Brun JF, Fons C, Supparo I, et al. Could exercise-induced increase in blood viscosity at high shear rate be entirely explained by hematocrit and plasma viscosity changes? Clin Hemorheol. 1993;13:187–99. [Google Scholar]
- 31.Brun JF, Micallef JP, Orsetti A. Hemorheologic effects of light prolonged exercise. Clin Hemorheol. 1994;14:807–18. [Google Scholar]
- 32.Groussard C, Morel I, Chevanne M, et al. Free radical scavenging and antioxidant effects of lactate ion: an in vitro study. J Appl Physiol. 2000;89:169–75. doi: 10.1152/jappl.2000.89.1.169. [DOI] [PubMed] [Google Scholar]
- 33.Shiga T, Maeda N, Kon K. Erythrocyte rheology. Crit Rev Oncol Hematol. 1990;10:9–48. doi: 10.1016/1040-8428(90)90020-s. [DOI] [PubMed] [Google Scholar]
- 34.Temiz A, Baskurt OK, Pekcetin C, et al. Leukocyte activation, oxidant stress and red blood cell properties after acute, exhausting exercise in rats. Clin Hemorheol Microcirc. 2000;22:253–59. [PubMed] [Google Scholar]
- 35.Yalcin O, Bor-Kucukatay M, Senturk UK, Baskurt OK. Effects of swimming exercise on red blood cell rheology in trained and untrained rats. J Appl Physiol. 2000;88:2074–80. doi: 10.1152/jappl.2000.88.6.2074. [DOI] [PubMed] [Google Scholar]
- 36.Ernst E, Daburger L, Saradeth T. The kinetics of blood rheology during and after prolonged standardized exercise. Clin Hemorheol. 1991;11:429–39. [Google Scholar]
- 37.Rampling MW. Red cell aggregation and yield stres. In: Lowe GDO, editor. Clinical Blood Rheology. Boca Raton: FL CRC Press; 1988. pp. 45–64. [Google Scholar]
- 38.Reinhart WH, Singh A. Erythrocyte aggregation: the roles of cell deformability and geometry. Eur J Clin Invest. 1990;20:458–62. doi: 10.1111/j.1365-2362.1990.tb01884.x. [DOI] [PubMed] [Google Scholar]
- 39.Varlet-Marie E, Brun JF. Reciprocal relationships between blood lactate and hemorheology in athletes: another hemorheologic paradox? Clin Hemorheol Microcirc. 2004;30:31–37. [PubMed] [Google Scholar]
- 40.Brun JF, Khaled S, Raynaud E, et al. The triphasic effects of exercise on blood rheology: which relevance to physiology and pathophysiology? Clin Hemorheol Microcirc. 1998;19:89–104. [PubMed] [Google Scholar]
- 41.Lowe GDO, Barbenel JC. Plasma and blood viscosity. In: Lowe GDO, editor. Clinical Blood Rheology. Boca Raton: FL CRC Press; 1998. pp. 11–44. [Google Scholar]