Summary
We review the current knowledge concerning clinical applications of the advanced technique of magnetic resonance imaging (MRI): diffusion tensor imaging (DTI) of the spinal cord. Due to technical difficulties, DTI has rarely been used in spinal cord diseases. However, in our opinion it is potentially a very useful method in diagnosis of the different pathological processes of the spinal cord and spinal canal. We discuss the physical principles and technical aspects of DTI, as well as current and future applications. DTI seems to be a very promising method for assessment of spinal cord trauma, spinal canal tumors, degenerative myelopathy, as well as demyelinating and infectious diseases of the spinal cord. DTI enables both qualitative and quantitative (by measuring of the fractional anisotropy and apparent diffusion coefficient parameters) assessment of the spinal cord. The particular applications are illustrated by the examples provided in this article.
Keywords: diffusion tensor imaging, spinal cord injury, degenerative myelopathy
Background
Diffusion tensor imaging (DTI) is an advanced non-invasive technique of magnetic resonance (MR) which enables visualization as well as qualitative and quantitative assessment of the integrity of the white matter tracts. DTI originates from the well established MR technique – diffusion-weighted imaging (DWI) – which is based on evaluation of the free diffusion of the water molecules (Brownian motion) in the extracellular space of the live tissue. Disturbances (restriction or increase) of diffusion may be assessed quantitatively by measurements of apparent diffusion coefficient (ADC). The best known applications of DWI are imaging of hyperacute ischemic stroke, as well as cerebral abscesses, epidermoid cysts and brain tumors.
DWI visualizes isotropic diffusion (i.e., occurring in any direction), while DTI reflects anisotropic diffusion (i.e., occurring preferably in 1 direction). DTI is used primarily for assessing white matter of the brain and spinal cord, because movement of the water particles in these structures is limited due to the barrier to diffusion which is formed by myelin sheaths of axons. More precisely, diffusion is limited in directions perpendicular to the white matter fibres, with relatively preferable diffusion in the direction of the given group of axons, which is called anisotropic diffusion (diffusion anisotropy). Diffusion anisotropy can be assessed quantitatively by using the fractional anisotropy (FA) parameter, which may be also visualized on fractional anisotropy maps. FA is considered as a marker of white matter integrity and its decrease indicates damage and degradation of the white matter tracts [1,2].
An additional application of DTI is diffusion tensor tractography (DTT), which provides three-dimensional presentation of white matter’s diffusion in the direction of fibers (axons) and thus visualization of the latter.
DTI is a rapidly developing technique, which is used more and more commonly in the assessment of the focal brain lesions, especially brain tumors, in which it helps in planning surgery by defining the relationship between tumor and strategic white matter tracts. There have also been promising results of the application of DTI in neurodegenerative diseases (eg, Alzheimer’s disease and other dementias) [3,4], as well as multiple sclerosis and lateral amyotrophic sclerosis [5].
DTI is used much less commonly in clinical assessment of the spinal cord due to multiple technical limitations and challenges, which in many cases make it impossible to acquire diagnostically satisfactory images [6–9].
Technical Aspects of DTI of the Spinal Cord
The small size of spinal cord transverse diameters requires acquisition of DTI images with high spatial resolution and small voxel size, which is very difficult to perform with satisfactory signal-to-noise ratio [8,9]. The obtained images may be degraded by artifacts caused by local magnetic inhomogeneity, metal implants, magnetic susceptibility effects (from bony structures of the spinal canal) and chemical shift artifacts (from the fat of the vertebrae and paravertebral soft tissue), as well as the motion artifacts resulting from the pulsatile flow of the cerebro-spinal fluid, breathing, swallowing or physiological motion of the internal organs during examination [8,9]. Therefore the best quality of DTI images are obtained in cervical spinal cord, while DTI of thoracic and upper lumbar segments are more challenging due to even smaller size of the spinal cord and greater number of artifacts.
New techniques, including parallel imaging and cardiac gating, improve the quality of DTI images [8–10]. MR units with field strength of 3 Tesla (3T) are considered to be a better tool for DTI [11]. However, 1.5 T MR units also enable obtaining diagnostically satisfactory results, provided that a carefully planned study protocol has been established. To overcome the difficulties mentioned above, reduction of field of view (FOV) should be used (eg, 12 cm instead for 16cm), as well as decreased reconstructive matrix, smaller slice thickness compared to the brain DTI, appropriate number of diffusion directions (not less than 15), and proper b value (e.g. b=0 and b=1000 mm2/s) [10,12,13].
Sagittal or axial planes may be used for DTI acquisitions and measurements, generating FA maps in the sagittal and axial planes, respectively. Some authors believe that acquisition in the axial plane is a more reliable method for measuring the FA values than in the sagittal plane, because the regions of interest (ROIs) can be placed more precisely in the spinal cord, without cerebrospinal fluid contamination [14].
The presented cases from our material were performed on a 1.5 T MR unit without parallel imaging and cardiac gating. After acquisition, DTI data were post-processed on an Advantage Workstation 4.4 with FuncTool software (General Electric Medical Systems). After an initial correction of geometric distortions, the following parametric maps were generated: color-coded FA maps according to diffusion directions, FA maps and ADC (Apparent Diffusion Coefficient) maps.
Possible clinical applications of DTI
Trauma to the spinal cord
DTI is a sensitive and specific method in evaluation of acute and chronic spinal cord trauma. Significant decrease of FA and ADC values has been found at the site of acute spinal cord trauma, both at the level of direct trauma and adjacent normal-appearing parts of the spinal cord [15]. ADC values seem to be the most sensitive marker of acute cord injury. Detection of abnormalities in regions of the spinal cord that appear normal on plain MRI could be used to assess the true extent of injury and help to better correlate the neurologic deficit with MR imaging [15].
In patients with chronic post-traumatic myelopathy there has been revealed a significant decrease of FA at the site of trauma and its direct vicinity, which seems to be proportional to the severity and extent of the traumatic injury (Figure 1). There was also noted a decrease of ADC in apparently normal-appearing segments of spinal cord, proximally and distally to the trauma level, which may result from reparative processes or secondary degeneration of the axons [16].
Figure 1.
Sagittal T2-weighted image of the thoracolumbar spine in patient after severe spine trauma with severe paraplegia (A) reveals traumatic hyperintense lesion in the spinal cord at T12 level. DTI tractography (B, C) images show functional disruption of the spinal cord at the same level.
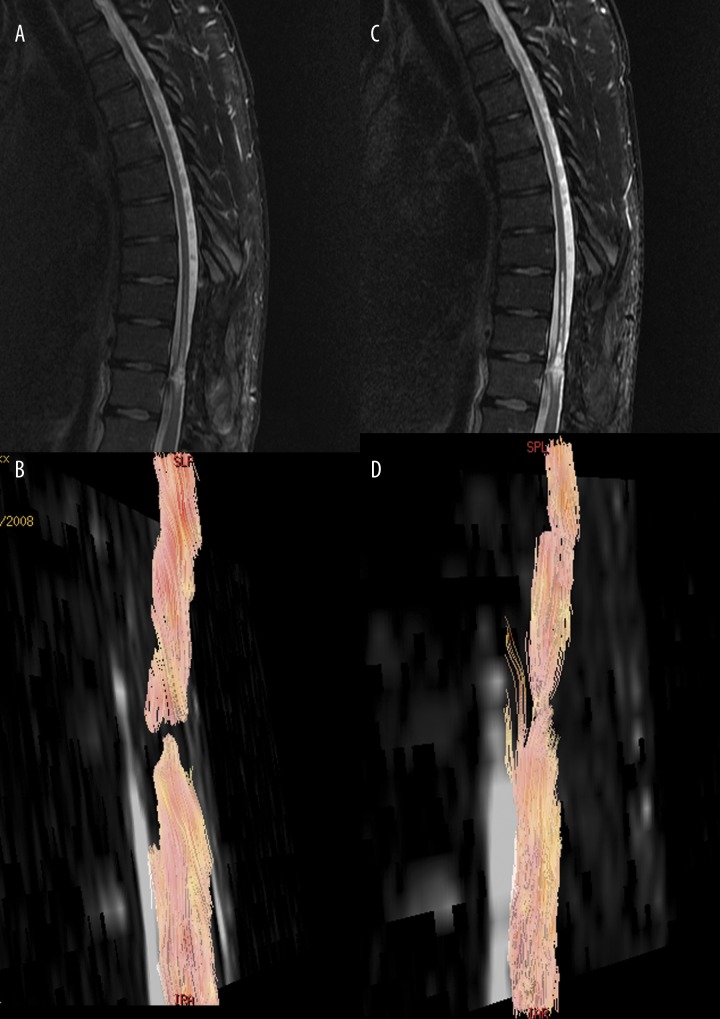
Experimental studies showed that DTI allows for prediction of the outcome of hyperacute spinal cord trauma [17]. DTI and DTT may be applied in the long-term follow-up of the post-traumatic changes and in the evaluation of the effects of therapy with stem cells’ implantation into the spinal cord (Figure 2).
Figure 2.
MR T2-weighted and diffusion tensor tractography images in patient after severe spine trauma with severe paraplegia before (A, B) and after (C, D) stem cells’ implantation. There is functional disruption of the spinal cord, which has been improved after stem cellls’ implantation.
Tumors of the spinal canal
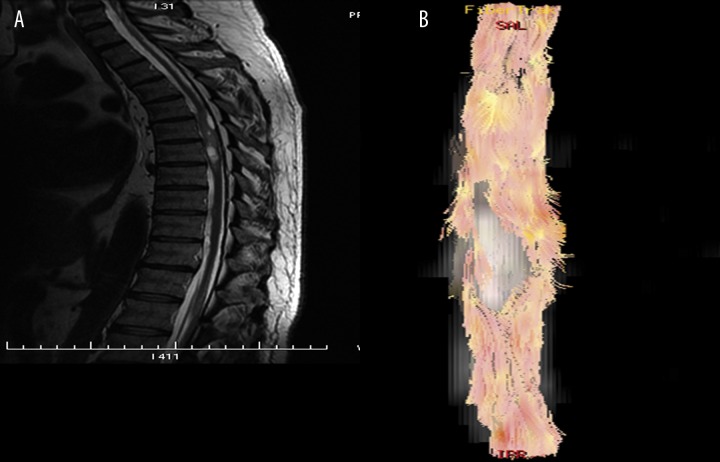
DTI and DTT enable assessment of the changes in the spinal cord caused by neoplastic disease, both in cases of intramedullary and extramedullary tumors. DTI, and especially DTT, may be useful in preoperative evaluation of spinal cord tumors (Figure 3). DTT provides good visualization of the displacement and deformation of the spinal cord tracts at the tumor’s level (in ependymomas or astrocytomas), as well as deformation and disruption of the fibers (in metastatic lesions) [12,18,19]. Thus, DTT may be valuable in the evaluation of the tumors’ resectability [19].
Figure 3.
Sagittal T2-weighted image of the thoracic spine (A) shows partially cystic intramedullary tumor. Tractography (B) reveals deformation and displacement of the spinal cord tracts by tumor.
A significant decrease of FA and variable ADC values has been found in extramedullary tumors compressing the spinal cord. FA measurements proved to have high sensitivity and specificity in the detection of spinal cord pathology in patients with extramedullary spinal canal tumors [20].
Degenerative myelopathy
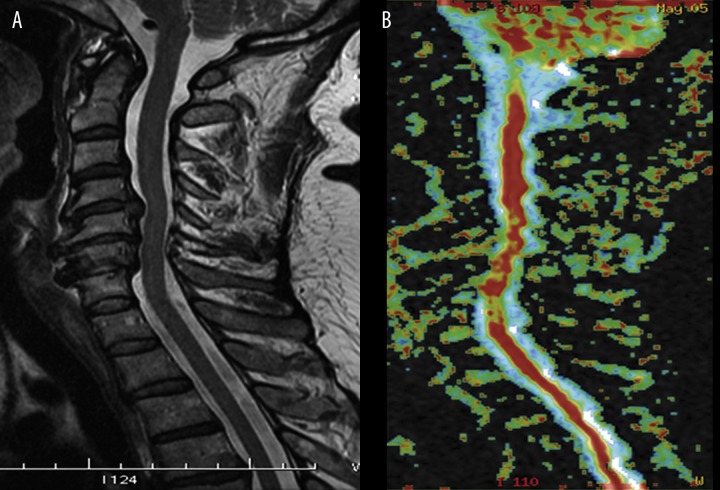
In chronic degenerative myelopathy caused by disk herniation or degenerative spinal canal stenosis, significant decrease of FA and increase of ADC has been found, including cases with no visible changes in the spinal cord on plain MRI (Figure 4) [21–23]. In recently published reports concerning contribution of DTI in cervical myelopathy, the authors have claimed that DTI proved to be more sensitive than conventional T2-weighted images in assessment of cervical degenerative myelopathy [7,14,22,23]. The decrease in the FA values may reflect the degree of microstructural disorganization of the spinal cord, suggesting either local extracellular edema or a smaller number of fibers matching a larger extracellular space, or both. On the other hand, minor lesions and edema with roughly preserved fibrillary microstructure of the spinal cord are not associated with major FA changes, as opposed to demyelination, cavitations and necrotic changes [22]. Thus, the high FA values suggest that the microstructure of the spinal cord is preserved, even in cases with high signal intensity of the spinal cord on T2-weighted images.
Figure 4.
Sagittal T2-weighted image of the cervical spine (A) in patient with myelopathy demonstrates severe spinal canal stenosis with compression of the spinal cord, without visible spinal cord abnormality. FA map of the spinal cord (B) reveals foci of decreased fractional anisotropy at the levels of compression.
Degenerative myelopathy changes, which may be detected on DTI, primarily involve the anterior part of the spinal cord (Figure 5).
Figure 5.
Axial T2-weighted image of the cervical spine (A) in patient with myelopathy shows disk herniation compressing the spinal cord, without visible spinal cord abnormality. Axial FA map of the spinal cord at the same level (B) reveals large area of decreased fractional anisotropy in the region of the spinal cord adjacent to the herniated disk.
In the literature there is still disagreement concerning the correlation between the clinical severity of myelopathy and the DTI parameters. Kara et al. [14] claimed there was no significant difference between the duration of myelopathy and the DTI parameters. Budzik et al. [22] reported that FA values were significantly correlated with clinical scores of the patients. The authors concluded that DTI could become a new tool for the assessment of cervical spondylotic myelopathy. On the other hand, Lee et al. [23] reported that tractography patterns were not related to the severity of myelopathy, although postoperative neurologic improvement was more common in patients with intact fibers on tractography in comparison to those with interrupted fibers on tractography.
It may be concluded that DTI with ADC and FA measurements seems to be an efficient method in assessing the degree of severity of degenerative myelopathy; hence it is potentially helpful in the planning of its treatment.
Demyelinating and infectious diseases
In multiple sclerosis, DTI studies have shown changes in normal-appearing cervical spinal cord. A statistically significant decrease of FA was found in the lateral and posterior parts of the spinal cord, while central gray matter was involved to a lesser degree [24,25]. The decrease of FA in the cervical spinal cord was correlated with the severity of clinical symptoms, what may be helpful in follow-up of the disease and treatment effects [24,26]. This decrease was proved to be significantly more severe in patients with the primary progressive type of multiple sclerosis, but not those with the relapsing-remitting type [26].
In inflammatory diseases of the spinal cord there was detected statistically significant decrease of FA in the focal lesions, suggesting edema or degradation and damage of the white matter fibers. The areas of decreased FA have also been found in normal-appearing areas of the spinal cord in this group of patients, which was correlated with clinical manifestation [27].
Recently, there have been published studies concerning the use of DTI in evaluation of HIV-associated myelopathy (HIVM) [28]. The authors proved that asymptomatic HIV-positive patients demonstrated only slightly decreased mean FA values measured in the cervical spinal cord compared to healthy controls, with the greatest difference in the lateral column of the cervical spinal cord [28]. This initial report does not support the use of DTI for early detection of HIVM in asymptomatic HIV-positive patients; however, the presence of the minor changes mentioned above is encouraging for further DTI studies on HIVM.
Other diseases
DTI also enables evaluation of the degradation of the spinal cord white matter tracts in selected neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) or Hirayama disease [5,8]. DTI and DTT also seem to be useful in assessment of ischemic lesions by qualitative and quantitative evaluation of the severity of damage to the spinal cord in typical anterior locations [12].
Conclusions
Diffusion tensor imaging (DTI) of the spinal cord is a promising advanced technique, which may provide additional diagnostic information in analysis of many pathological changes of the spinal cord. There are many technical limitations of DTI, especially in thoracic and lumbar segments. The wider use of 3T scanners, as well as development of acquisition and postprocessing techniques, should result in the increased role of spinal cord DTI in both research and clinical practice.
Footnotes
Source of support: Self financing
References
- 1.Hasan KM, Walimuni IS, Abid H, Hahn KR. A review of diffusion tensor magnetic resonance imaging computational methods and software tools. Comput Biol Med. 2011;41(12):1062–72. doi: 10.1016/j.compbiomed.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowski R. New diagnostic methods – technology, procedures, measurements. Pol J Radiol. 2010;75(Suppl 1):72. [Google Scholar]
- 3.Zimny A, Szewczyk P, Czarnecka A, et al. Multimodality approach in diagnostics of Alzheimer’s disease and mild cognitive impairment – value of magnetic resonance spectroscopy, perfusion and diffusion tensor imaging of posterior cingulate gyrus. Pol J Radiol. 2010;75(Suppl 1):46. [Google Scholar]
- 4.Szewczyk P, Zimny A, Trypka E, et al. Assessment of degradation of the selected projectile, commissural and association brain fibers in patients with Alzheimer’s disease on diffusion tensor MR imaging. Pol J Radiol. 2010;75(2):7–15. [PMC free article] [PubMed] [Google Scholar]
- 5.Maj E, Kolasa A, Cieszanowski A, et al. Diffusion-Tensor Imaging of Corticospinal Tracts in Patients with Amyotrophic Lateral Sclerosis. Pol J Radiol. 2010;75(Suppl 1):54–55. [Google Scholar]
- 6.Sąsiadek M, Szewczyk P. Imaging of the spine: new possibilities and its role in planning and monitoring therapy. Pol J Radiol. 2009;74(3):52–58. [Google Scholar]
- 7.Song T, Chen WJ, Yang B, et al. Diffusion tensor imaging in the cervical spinal cord. Eur Spine J. 2011;20:422–28. doi: 10.1007/s00586-010-1587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurnher MM, Law M. Diffusion-weighted imaging, diffusion-tensor imaging, and fiber tractography of the spinal cord. Magn Reson Imaging Clin N Am. 2009;17(2):225–44. doi: 10.1016/j.mric.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Maier SE, Mamata H. Diffusion tensor imaging of the spinal cord. Ann NY Acad Sci. 2005;1064:50–60. doi: 10.1196/annals.1340.011. [DOI] [PubMed] [Google Scholar]
- 10.Lee JW, Kim JH, Kang HS, et al. Optimization of acquisition parameters of diffusion-tensor magnetic resonance imaging in the spinal cord. Invest Radiol. 2006;41(7):553–59. doi: 10.1097/01.rli.0000221325.03899.48. [DOI] [PubMed] [Google Scholar]
- 11.Xiangshui M, Xiangjun C, Xiaoming Z, et al. 3T magnetic resonance diffusion tensor imaging and fibre tracking in cervical myelopathy. Clin Radiol. 2010;65:465–73. doi: 10.1016/j.crad.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Vargas MI, Delavelle J, Jlassi H, et al. Clinical applications of diffusion tensor tractography of the spinal cord. Neuroradiology. 2008;50(1):25–29. doi: 10.1007/s00234-007-0309-y. [DOI] [PubMed] [Google Scholar]
- 13.Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging of the neurologically intact human spinal cord. AJNR Am J Neuroradiol. 2008;29(7):1279–84. doi: 10.3174/ajnr.A1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kara B, Celik A, Karadereler S, et al. The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI. Neuroradiology. 2011;53:609–16. doi: 10.1007/s00234-011-0844-4. [DOI] [PubMed] [Google Scholar]
- 15.Shanmuganathan K, Gullapalli RP, Zhuo J, Mirvis SE. Diffusion tensor MR imaging in cervical spine trauma. AJNR Am J Neuroradiol. 2008;29(4):655–59. doi: 10.3174/ajnr.A0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am J Neuroradiol. 2008;29(10):1976–82. doi: 10.3174/ajnr.A1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loy DN, Kim JH, Xie M, et al. Diffusion tensor imaging predicts hyperacute spinal cord injury severity. J Neurotrauma. 2007;24(6):979–90. doi: 10.1089/neu.2006.0253. [DOI] [PubMed] [Google Scholar]
- 18.Ducreux D, Lepeintre JF, Fillard P, et al. MR diffusion tensor imaging and fiber tracking in 5 spinal cord astrocytomas. AJNR Am J Neuroradiol. 2006;27(1):214–16. [PMC free article] [PubMed] [Google Scholar]
- 19.Setzer M, Murtagh RD, Murtagh FR, et al. Diffusion tensor imaging tractography in patients with intramedullary tumors: comparison with intraoperative findings and value for prediction of tumor resectability. J Neurosurg Spine. 2010;13(3):371–80. doi: 10.3171/2010.3.SPINE09399. [DOI] [PubMed] [Google Scholar]
- 20.Facon D, Ozanne A, Fillard P, et al. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol. 2005;26(6):1587–94. [PMC free article] [PubMed] [Google Scholar]
- 21.Demir A, Ries M, Moonen CT, et al. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology. 2003;229(1):37–43. doi: 10.1148/radiol.2291020658. [DOI] [PubMed] [Google Scholar]
- 22.Budzik JF, Balbi V, Le Thuc V, et al. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol. 2011;21:426–33. doi: 10.1007/s00330-010-1927-z. [DOI] [PubMed] [Google Scholar]
- 23.Lee JW, Kim JH, Park JB, et al. Diffusion tensor imaging and fiber tractography in cervical compressive myelopathy: preliminary results. Skeletal Radiol. 2011;40(12):1543–51. doi: 10.1007/s00256-011-1161-z. [DOI] [PubMed] [Google Scholar]
- 24.Hesseltine SM, Law M, Babb J, et al. Diffusion tensor imaging in multiple sclerosis: assessment of regional differences in the axial plane within normal-appearing cervical spinal cord. AJNR Am J Neuroradiol. 2006;27(6):1189–93. [PMC free article] [PubMed] [Google Scholar]
- 25.Ohgiya Y, Oka M, Hiwatashi A, et al. Diffusion tensor MR imaging of the cervical spinal cord in patients with multiple sclerosis. Eur Radiol. 2007;17(10):2499–504. doi: 10.1007/s00330-007-0672-4. [DOI] [PubMed] [Google Scholar]
- 26.Agosta F, Absinta M, Sormani MP, et al. In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain. 2007;130(Pt 8):2211–19. doi: 10.1093/brain/awm110. [DOI] [PubMed] [Google Scholar]
- 27.Renoux J, Facon D, Fillard P, et al. MR diffusion tensor imaging and fiber tracking in inflammatory diseases of the spinal cord. AJNR Am J Neuroradiol. 2006;27(9):1947–51. [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller-Mang Ch, Law M, Mang T, et al. Diffusion tensor MR imaging (DTI) metrics in the cervical spinal cord in asymptomatic HIV-positive patients. Neuroradiology. 2011;53:585–92. doi: 10.1007/s00234-010-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]