Abstract
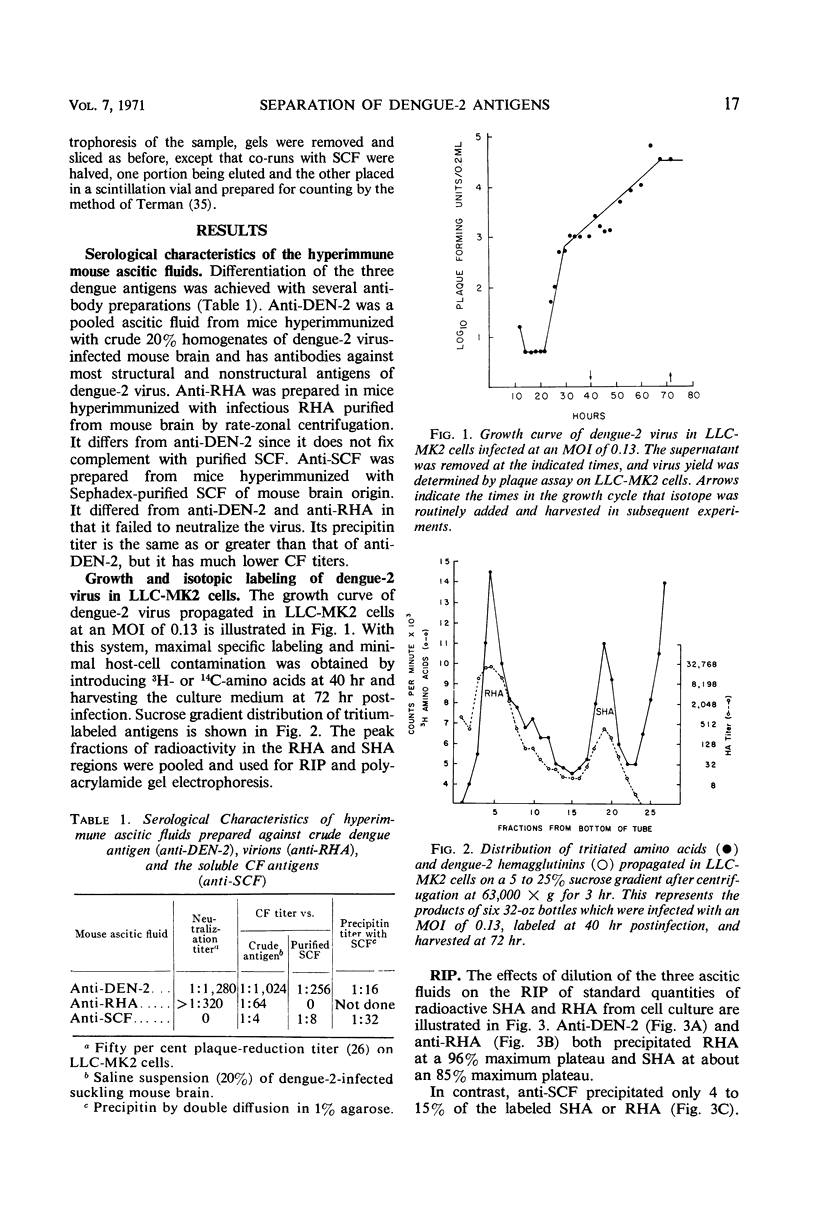
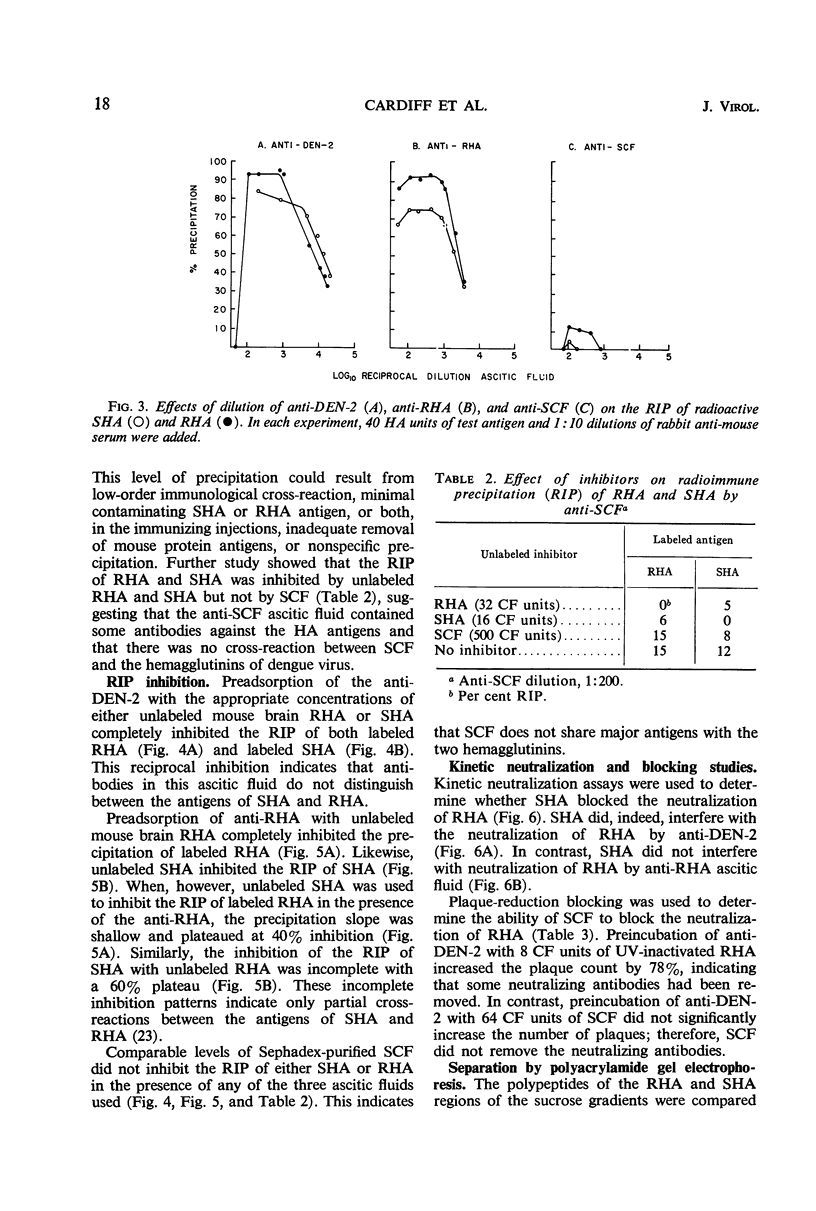
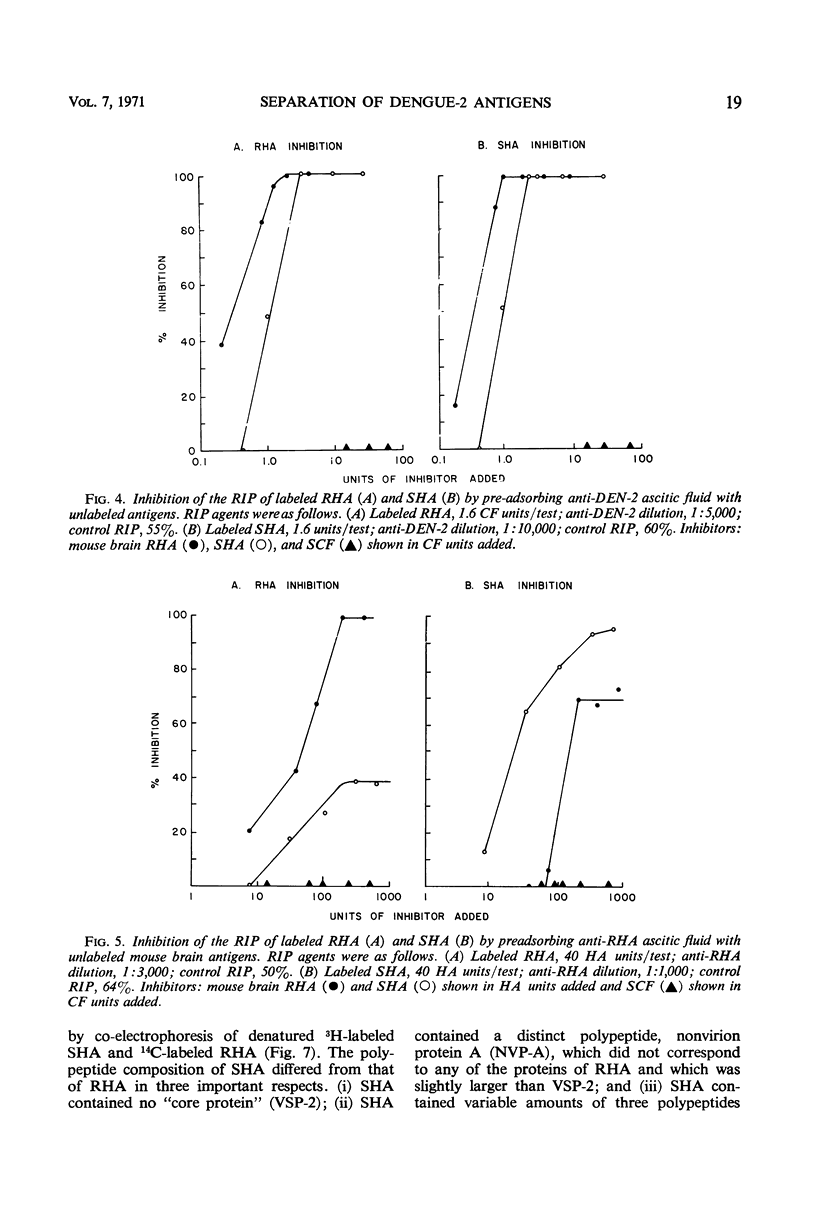
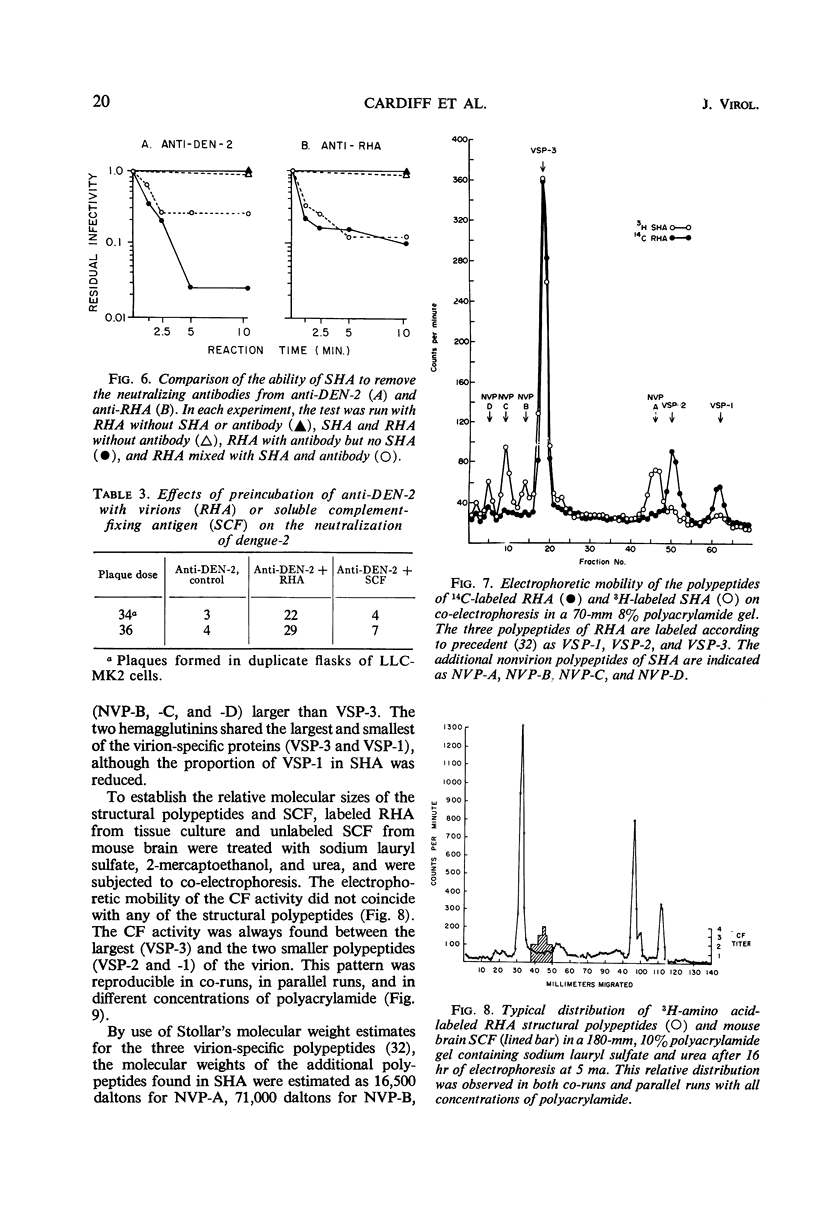
Antigenic compositions of slowly sedimenting dengue-2 hemagglutinin (SHA) and soluble complement-fixing antigen (SCF) were compared with the virion (rapidly sedimenting hemagglutinin, RHA) by radioimmune precipitation (RIP), RIP inhibition, kinetic neutralization, and neutralization blocking tests with the use of hyperimmune mouse ascitic fluids. RHA and SHA were unable to inhibit completely the RIP of each other by anti-RHA, and neutralization by anti-RHA was not blocked by SHA. This indicated that SHA is serologically related, but not identical, to RHA. SHA differed from RHA in that SHA lacked the “core” polypeptide but contained the two envelope polypeptides. In addition, SHA contained a polypeptide with a molecular weight of 16,500 daltons and a suggestion of several other proteins. These data, when considered with other evidence, suggest that SHA is a special form of “incomplete virus.” SCF was unable to inhibit the RIP of SHA or RHA or to block neutralizing antibodies. Further, anti-SCF did not neutralize RHA or precipitate significant levels of SHA or RHA. Polyacrylamide gel electrophoresis separated SCF from structural polypeptides by molecular size. This evidence suggests that SCF is a nonstructural antigen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLETT A. J., COOPER P. D. Some properties of the transmissible interfering component of vesicular stomatitis virus preparations. J Gen Microbiol. 1959 Dec;21:498–509. doi: 10.1099/00221287-21-3-498. [DOI] [PubMed] [Google Scholar]
- BREEDIS C., BERWICK L., ANDERSON T. F. Fractionation of Shope papilloma virus in cesium chloride density gradients. Virology. 1962 May;17:84–94. doi: 10.1016/0042-6822(62)90084-3. [DOI] [PubMed] [Google Scholar]
- BREITENFELD P. M., SCHAFER W. The formation of fowl plague virus antigens in infected cells, as studied with fluorescent antibodies. Virology. 1957 Oct;4(2):328–345. doi: 10.1016/0042-6822(57)90067-3. [DOI] [PubMed] [Google Scholar]
- Bauer H., Janda H. G. Group-specific antigen of avian leukosis viruses. Virus specificity and relation to an antigen contained in Rous mammalian tumor cells. Virology. 1967 Nov;33(3):483–490. doi: 10.1016/0042-6822(67)90124-9. [DOI] [PubMed] [Google Scholar]
- Brandt W. E., Buescher E. L., Hetrick F. M. Production and characterization of arbovirus antibody in mouse ascitic fluid. Am J Trop Med Hyg. 1967 May;16(3):339–347. doi: 10.4269/ajtmh.1967.16.339. [DOI] [PubMed] [Google Scholar]
- Brandt W. E., Cardiff R. D., Russell P. K. Dengue virions and antigens in brain and serum of infected mice. J Virol. 1970 Oct;6(4):500–506. doi: 10.1128/jvi.6.4.500-506.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., CRAWFORD E. M., WATSONDH The physical characteristics of polyoma virus. I. Two types of particle. Virology. 1962 Oct;18:170–176. doi: 10.1016/0042-6822(62)90002-8. [DOI] [PubMed] [Google Scholar]
- Crowell R. L. Specific cell-surface alteration by enteroviruses as reflected by viral-attachment interference. J Bacteriol. 1966 Jan;91(1):198–204. doi: 10.1128/jb.91.1.198-204.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner P., Dobos P. Investigations on the formation and interconversion of Sindbis virus hemagglutinins. Can J Microbiol. 1968 Jan;14(1):45–51. doi: 10.1139/m68-008. [DOI] [PubMed] [Google Scholar]
- HALPEREN S., EGGERS H. J., TAMM I. COMPLETE AND CORELESS HEMAGGLUTINATING PARTICLES PRODUCED IN ECHO 12 VIRUS-INFECTED CELLS. Virology. 1964 May;23:81–89. doi: 10.1016/s0042-6822(64)80010-6. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- KENT J. F., FIFE E. H., Jr Precise standardization of reagents for complement fixation. Am J Trop Med Hyg. 1963 Jan;12:103–116. doi: 10.4269/ajtmh.1963.12.103. [DOI] [PubMed] [Google Scholar]
- MUSSGAY M. STUDIES ON THE STRUCTURE OF A HEMAGGLUTINATING COMPONENT OF A GROUP A ARBO VIRUS (SINDBIS). Virology. 1964 Aug;23:573–581. doi: 10.1016/0042-6822(64)90241-7. [DOI] [PubMed] [Google Scholar]
- McCloud T. G., Brandt W. E., Russell P. K. Molecular size and charge relationships of the soluble complement-fixing antigens of dengue viruses. Virology. 1970 Jul;41(3):569–572. doi: 10.1016/0042-6822(70)90180-7. [DOI] [PubMed] [Google Scholar]
- Norrby E. Biological significance of structural adenovirus components. Curr Top Microbiol Immunol. 1968;43:1–43. doi: 10.1007/978-3-642-46118-7_1. [DOI] [PubMed] [Google Scholar]
- Ozer H. L., Takemoto K. K., Kirschstein R. L., Axelrod D. Immunochemical characterization of plaque mutants of simian virus 40. J Virol. 1969 Jan;3(1):17–24. doi: 10.1128/jvi.3.1.17-24.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHIM J. S., SMITH K. O., MELNICK J. L. Complete and coreless forms of reovirus (ECHO 10). Ratio of number of virus particles to infective units in the one-step growth cycle. Virology. 1961 Dec;15:428–435. doi: 10.1016/0042-6822(61)90110-6. [DOI] [PubMed] [Google Scholar]
- Russell P. K., Intavivat A., Kanchanapilant S. Anti-dengue immunoglobulins and serum beta 1 c-a globulin levels in dengue shock syndrome. J Immunol. 1969 Feb;102(2):412–420. [PubMed] [Google Scholar]
- Russell P. K., Nisalak A., Sukhavachana P., Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol. 1967 Aug;99(2):285–290. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Brandt W. E., Swanson J. L., McCown J. M., Buescher E. L. Physical and biological properties of dengue-2 virus and associated antigens. J Virol. 1970 Apr;5(4):524–532. doi: 10.1128/jvi.5.4.524-532.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A. Identification of the A protein as a structural component of bacteriophage R17. J Mol Biol. 1968 May 14;33(3):923–936. doi: 10.1016/0022-2836(68)90328-8. [DOI] [PubMed] [Google Scholar]
- Stevens T. M., Schlesinger R. W. Studies on the nature of dengue viruses. I. Correlation of particle density, infectivity, and RNA content of type 2 virus. Virology. 1965 Sep;27(1):103–112. doi: 10.1016/0042-6822(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Stollar V., Stevens T. M., Schlesinger R. W. Studies on the nature of dengue viruses. II. Characterization of viral RNA and effects of inhibitors of RNA synthesis. Virology. 1966 Oct;30(2):303–312. doi: 10.1016/0042-6822(66)90105-x. [DOI] [PubMed] [Google Scholar]
- Stollar V. Studies on the nature of dengue viruses. IV. The structural proteins of type 2 dengue virus. Virology. 1969 Nov;39(3):426–438. doi: 10.1016/0042-6822(69)90091-9. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Terman S. A. Relative effect of transcription-level and translation-level control of protein synthesis during early development of the sea urchin. Proc Natl Acad Sci U S A. 1970 Apr;65(4):985–992. doi: 10.1073/pnas.65.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON MAGNUS P. Incomplete forms of influenza virus. Adv Virus Res. 1954;2:59–79. doi: 10.1016/s0065-3527(08)60529-1. [DOI] [PubMed] [Google Scholar]
- Winter P. E., Yuill T. M., Udomsakdi S., Gould D., Nantapanich S., Russell P. K. An insular outbreak of dengue hemorrhagic fever. I. Epidemiologic observations. Am J Trop Med Hyg. 1968 Jul;17(4):590–599. doi: 10.4269/ajtmh.1968.17.590. [DOI] [PubMed] [Google Scholar]



