Summary
Bacteria have been found to grow predominantly in biofilms. The initial stage includes the attachment of bacteria to the substratum. Bacterial growth and division then leads to the colonization of the surrounding area and the formation of the biofilm. The environment in a biofilm is not homogeneous; the bacteria in a multispecies biofilm are not randomly distributed, but rather are organized to best meet their needs.
Although there is an initial understanding on the mechanisms of biofilm-associated antimicrobial resistance, this topic is still under investigation. A variety of approaches are being explored to overcome biofilm-associated antimicrobial resistance. A greater understanding of biofilm processes should lead to novel, effective control strategies for biofilm control and a resulting improvement in patient management.
Keywords: biofilm, colonization, community, antimicrobial resistance
Background
Microorganisms have primarily been characterized as planktonic, freely suspended cells and described on the basis of their growth characteristics in nutritionally rich culture media [1]. Van Leeuwenhoek first described that microorganisms attach to and grow universally on exposed surfaces, which led to studies that revealed surface-associated microorganisms (biofilms) exhibited a distinct phenotype with respect to gene transcription and growth rate [2].
In biofilms, bacteria adopt a different phenotype [3], and the component cells of biofilms have been shown to communicate by intercellular signals [4]. The pathogenesis of many orthopaedic infections is related to the presence of microorganisms in biofilms [5–7].
Biofilm development on surfaces is a dynamic stepwise process involving adhesion, growth, motility and extracellular polysaccharide production. On every biomaterial surface there is as a “race for the surface”, involving extracellular matrix (ECM) proteins, host cells (fibroblasts, osteoblasts, endothelial cells), and bacteria [6,8]. The ECM is a biologically active layer composed of a complex mixture of macromolecules such as fibronectin, fibrinogen, albumin, vitronectin, and collagen [6]. Host cell adhesion, migration, proliferation, and differentiation are all influenced by the composition and structural organization of the surrounding ECM [9]. However, the ECM not only serves as a substrate for host cells, but also for colonizing bacteria. If host cells such as fibroblasts arrive at the biomaterial surface and establish secure bonds, bacteria are confronted with a living, integrated cellular surface. Such integrated viable cell layers with functional host defense mechanisms can resist bacterial attachment and colonization [8]. However, it has been found that bacteria (eg, Staphylococcus aureus) express many surface adhesion molecules that promote attachment to plasma and ECM proteins of host cells, or those ECM proteins anchored onto metal or polymer surfaces [10,11].
Although biofilms that colonize orthopaedic devices have been studied extensively, there are many areas that need further clarification regarding biofilm structure, cell community composition and pathophysiologic activity.
Historical Overview
Van Leeuwenhoek first observed microorganisms on tooth surfaces and can be credited with the discovery of microbial biofilms [2].
Jones et al. [12] used scanning and transmission electron microscopy to examine biofilms on trickling filters in a wastewater treatment plant, showing them to be composed of a variety of organisms (based on cell morphology). By using a specific polysaccharide-stain called Ruthenium red and coupling this with osmium tetroxide fixative, these researchers were also able to show that the matrix material surrounding and enclosing cells in these biofilms is polysaccharide.
Prior to 1978, biofilms had been described in aquatic systems [13–15] without determination of the proportion of bacteria in a given ecosystem. In 1978 Geesey et al. [16] adapted recovery methods for quantitative determination of biofilm bacteria in a pristine mountain stream. These methods allowed direct comparison, in number and activity, between planktonic (free-living) and biofilm bacteria of the same aquatic system. This study demonstrated that bacteria in the biofilm clearly predominate in numbers and in metabolic activity, leading to widespread application of the same methods in natural [17], industrial [18], and medical [19,20] ecosystems. These biofilm populations have a very significant metabolic activity and predominate in virtually all nutrient-sufficient aquatic systems irrespective of system geometry and type of ecosystem involved [21–23].
The above methods of quantitative analysis allowed Lappin-Scott and Costerton [23] to predict the extent of biofilm formation in a particular aquatic system, based on the following principles: 1. Metabolically active (vegetative) bacteria show a remarkable avidity for adhesion to surfaces, and this tendency is especially pronounced in wild-type cells in natural environments. 2. The extent of biofilm accretion on surfaces in any aquatic system is controlled by the amount of nutrients available for cell replication and for exopolysaccharide production. 3. In extremely oligotrophic environments, organic nutrients tend to associate with available surfaces and to trigger local biofilm development; however, bacteria in general do not adhere on surfaces in nutrient-deficient environments.
Biofilm – Definition and Formation Procedure
A biofilm can be defined as a layer-like aggregation of cells and cellular products attached to a solid surface or substratum [2,24,25]. An established biofilm structure comprises microbial cells and extracellular polymeric substances and provides an environment for the exchange of genetic material between cells [25] (Figure 1).
Figure 1.
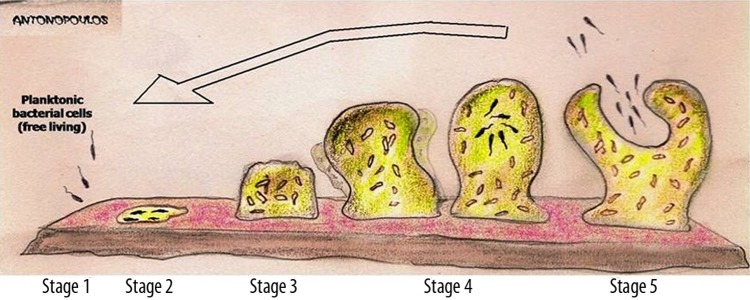
Diagram showing the development of a biofilm as a five-stage process. Stage 1: initial attachment of cells to the surface. Stage 2: production of extracellular polymeric substance. Stage 3: early development of biofilm architecture (colonization). Stage 4: maturation of biofilm architecture. Stage 5: dispersion of single cells from the biofilm. In the final stage, when environmental conditions become unfavorable, some of the bacteria may detach and swim away to find a surface in a more favorable environment.
The biofilm architecture is spatially heterogeneous, constantly changing through external and internal processes. Although macroscopically an idealized biofilm is a thin homogeneous layer, microscopically it is a nonuniform structure characterized by variable thickness and polymer densities. This heterogeneity may play an important role in hydrodynamic fouling, microbially influenced corrosion, substrate conversion and biocide efficacy. Furthermore, owing to their irregular surface, biofilms increase a fluid’s functional resistance and shear stress. These effects, in turn, influence the effective diffusion coefficient in aerobic biofilms, where the oxygen distribution strongly depends on flow conditions and on the biofilm’s structure.
A large portion of biofilm matrix, depending on the specific system under investigation, is actually water (up to 97%) [26,27]. The water can be bound within the capsules of microbial cells or can exist as a solvent with physical properties such as viscosity determined by the solutes dissolved in it. Viscosity within the biofilm matrix is integral to the diffusion processes that occur [28]. Apart from water and microbial cells, the biofilm matrix includes secreted polymers, absorbed nutrients and metabolites, products from cell lysis, and particulate material and detritus from the immediate surrounding environment. All major classes of macromolecules – proteins, polysaccharides, DNA and RNA – can be present, in addition to peptidoglycans, lipids, and phospholipids.
Within biofilms, microorganisms organize communities with structural and functional heterogeneity similar to that of a multicellular organism; interstitial voids between microcolonies can be considered to serve as a rudimentary circulatory system [7]. Cell-to-cell signaling (eg, quorum-sensing) induces biofilm microorganisms to change patterns of gene expression. Quorum sensing is the ability of a bacterial colony to sense its size and, in response, to regulate its activity. At certain population densities, intercellular signals activate genes involved in biofilm differentiation.
Living within a biofilm represents a basic survival mechanism against environmental influences, including host immune responses (eg, opsonization, phagocytosis, and complement-mediated lysis) and antimicrobial agents. Polymorphonuclear neutrophils can attach to, penetrate, and produce cytokines in, maturing and fully matured Staphylococcus aureus biofilm [29]; nevertheless, these efforts are usually insufficient to clear the bacteria [30]. Furthermore, ineffective attempts to engage in phagocytosis may result in release of cytotoxic and proteolytic substances contributing to tissue injury and ultimately to periprosthetic osteolysis in cases of orthopaedic implants [30].
The genetic basis of biofilm formation has been investigated for a number of bacterial species, including Escherichia coli[31], Pseudomonas aeruginosa[32] and Vibrio cholera[33]. These studies used randomly generated mutant species grown on plates [34–36]. After removal of planktonic forms and staining with crystal violet, cells with no staining correspond to mutants that are defective for mature biofilm formation. These genetic screens for biofilm-defective mutants have shown that the initial interaction with the surface is accelerated by force-generating organelles such as type IV pili and flagella. Once temporary contact with the surface is made, bacteria use either flagella or type IV pili to move along the surface until other bacteria are encountered and microcolonies are formed or enlarged [31–33]. Finally, exopolysaccharide production is necessary to stabilize the pillars of the biofilm [33].
Why do Bacteria form Biofilms?
Jefferson suggested 4 promoting aspects for biofilm formation during infection: (1) protection from harmful conditions in the host (defense), (2) sequestration in a nutrient-rich area (colonization), (3) utilization of cooperative benefits (community), and (4) bacteria normally grow as biofilms and planktonic cultures are an in vitro artifact (biofilms are the default mode of growth) [37].
According to others, there are 5 stages in the growth cycle of a biofilm, with common characteristics independent of the phenotype of the organisms [38]. Stage I is the attachment phase, which can take only seconds to activate and is likely induced by environmental signals. These signals vary by organisms, but they include changes in nutrients and nutrient concentrations, pH, temperature, oxygen concentration, osmolality and iron.
Rough surfaces are more susceptible to biofilm formation, likely due to reduction of shear forces and increased surface area. Studies indicate that biofilms also tend to form more readily on hydrophobic materials like Teflon and other plastics than on glass and metal. The initial binding in stage I is reversible, as some cells detach. During this stage, bacterial cells exhibit a logarithmic growth rate. Stage II is characterized as irreversible binding and begins minutes after stage I. After adhering to the epithelial surface, the bacteria begin to multiply while emitting chemical signals that “inter-communicate” between bacterial cells. Once the signal intensity exceeds a certain threshold level, the genetic mechanisms underlying exopolysaccharide (EPS) production are activated, which is able to trap nutrients and planktonic bacteria [4]. During stage II, cell aggregates are formed and motility is decreased when cell aggregates become progressively layered to a thickness greater than 10 μm; the biofilm stage III also known as maturation I. When biofilms reach their ultimate thickness, generally greater than 100 mm, this is called stage IV or maturation II. During stage V, cell dispersion is noted. Some of the bacteria develop the planktonic phenotype and leave the biofilm. This begins several days after stage IV [39].
Biofilms are also resistant to phagocytosis, and the phagocytes that attempt an assault on the biofilm may actually do more harm to surrounding tissues than to the biofilm itself. The chronic nature of certain infections is usually due to the development of a resilient biofilm. The invulnerability of biofilms is not completely understood, but is likely dependent upon a number of biofilm-specific characteristics, including slow growth and physiologic heterogeneity of the inhabitants. Another important aspect that fortifies biofilm resistance is the sticky matrix, which may contain DNA and other polymers, but in general is predominantly composed of exopolysaccharides [40,41].
Bacteria have a number of strategies to ensure their viability in the human host. Overall, bacteria produce an impressive array of autolysin – adhesins that appear to have evolved as a means to inhabit the human host. The finding that carbon catabolite-induced gene regulation plays a critical role in biofilm formation [42] also supports the hypothesis that biofilm formation is a mechanism for organisms to remain viable in the favorable environment of the human host.
Bacterial cells do not differentiate, but rather respond to the environment by adapting their gene expression to meet occasional needs. Thus, it is more accurate to refer to biofilms as interactive communities rather than comparing them to multicellular organisms. Nonetheless, living in a community provides its members a number of benefits such as resistance to environmental changes, distribution of the metabolic burden, gene transfer, and selfless behavior [30].
Pathogenesis of Implant-Associated Infections
Biofilms may form on a wide variety of surfaces, including living tissues, indwelling medical devices, industrial or potable water system piping, or natural aquatic systems.
The water system biofilm is highly complex, containing corrosion products, clay material, freshwater diatoms, and filamentous bacteria. The biofilm on a medical device, on the other hand, appears to be composed of a single coccoid organism and the associated extracellular polymeric substance matrix.
Implant-associated infections microorganisms live clustered together in a highly hydrated extracellular matrix attached to a surface [43]. Depletion of nutrients and/or waste product accumulation in biofilms causes microorganisms to enter a slow-growing or non-growing (stationary) state, rendering them up to 1000 times more resistant to most antimicrobial agents than their planktonic (free-living) counterparts [1,44].
Adherence of microorganisms to the surface of the implant involves rapid attachment by specific factors (eg, adhesins) or nonspecific factors (eg, surface tension, hydrophobicity, and electrostatic forces) [45]. This initial phase is followed by an accumulative phase during which bacterial cells adhere to each other and form a biofilm. The presence of a foreign body has been shown to significantly increase susceptibility to infection. This increased susceptibility to infection is at least partially due to a locally acquired granulocyte defect [46].
Infections associated with fracture fixation can occur exogenously in cases of open trauma (pre-operatively), during insertion of the fixation device (intra-operatively), or during disturbed wound healing (post-operatively) [47–49]. Hematogenous infection is less frequent and is commonly associated with bacteraemia originating from skin, respiratory, dental, or urinary tract infections [50]. Stainless steel, titanium and titanium alloys are the most commonly used materials for orthopaedic implants, but biodegradable polymers such as poly -L-lactide are also utilized in orthopaedic and maxillofacial surgery.
The differences between stainless steel and titanium are well documented [51–54], with stainless steel implants being associated with significantly greater infection rates than titanium implants [52,53]. A possible reason for this could be that soft tissue adheres firmly on titanium-implant surfaces [10,54] but a known reaction to steel implants is the formation of a fibrous capsule, enclosing a liquid-filled void [8,52]. Bacteria can spread and multiply freely in this unvascularized space, which is also less accessible to the host defense mechanisms. Adhesion and proliferation of fibroblasts is inhibited on titanium alloy surfaces [56]. In vitro studies of the reaction of bacteria to titanium alloys have yielded contradictory results. Delmi et al. [57] and Ha et al. [58] reported extensive S. aureus and S. epidermidis adhesion and biofilm formation on titanium alloys as opposed to stainless steel, whereas Gracia et al. [59] found no significant differences for S. aureus between titanium alloy and stainless steel surfaces. From a clinical point of view, the prevention of initial bacterial adhesion is of utmost importance, since mature biofilms are very difficult to treat. Possible solutions include implant surface modifications by altering the topography and/or surface chemistry of the biomaterial, or by using an antimicrobial or protein-resistant coating [7].
Mechanisms of the Antimicrobial Resistance
The production of an exopolysaccharide matrix, or glycocalyx, is one of the distinguishing characteristics of biofilms. It has been suggested that this matrix, among other functions, prevents the entry of antibiotics into the community (Figure 2) [60]. This extracellular matrix may physically restrict the diffusion of antimicrobial agents. Nutrient and/or oxygen depletion and waste product accumulation may cause bacteria to enter a non-growing (stationary) state, providing protection from growth-dependent antimicrobial action. A subpopulation of bacteria may differentiate into a phenotypically resistant state. Furthermore, organisms may express biofilm-specific antimicrobial resistance genes that are not required for biofilm formation [7].
Figure 2.
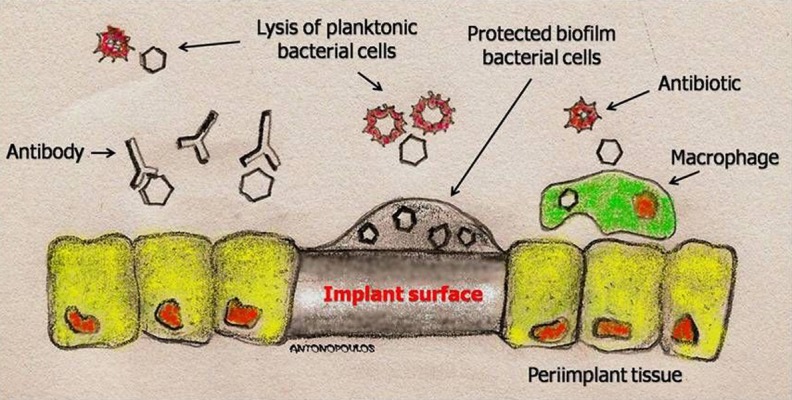
Schematic representation of planktonic bacterial cells, killed by antibiotics and the immune system, and biofilm microorganisms, attached to a surface and protected in an extracellular matrix.
Anderl et al. [61] cultured Clebsiella pneumoniae colony biofilms on agar plates with and without antibiotics. By placing a filter on top of the colony, they were able to directly search for antibiotic diffusion through the colony by performing a standard zone of inhibition assay with the filter. This breakthrough study showed that ampicillin was unable to penetrate the biofilm, irrespective of the presence of ampicillin-degrading enzyme β-lactamase. Ampicillin was unable to diffuse in mutant colonies that lack the ability to produce β-lactamase, suggesting that other mechanisms contribute to the resistance of these colonies.
Pseudomonas aeruginosa biofilms formed by an alginate-overproducing strain show a highly structured architecture and are more resistant to tobramycin than are biofilms formed by an isogenic non-mucoid strain [62]. Mah et al. [63] recently identified a gene (ndvB), the absence of which results in the formation of P. aeruginosa colonies without biofilm-specific resistance to antimicrobial agents. The ndvB locus is required for the synthesis of periplasmic glucose polymers that interact with tobramycin, apparently preventing the drug from reaching its site of action. Whether such a process occurs in staphylococci as well is not known, but could explain the poor activity of glycopeptides against S. epidermidis biofilms [64].
Fux et al. [65] studied oxacillin resistance of detached S. aureus biofilm particles that formed emboli. These emboli can explain the high rate of symptomatic metastatic infections of S. aureus. Cells within emboli are at a stationary state of growth and their formation is possibly promoted in nutrient deficient environments.
Diagnosis of Orthopaedic Biofilm Infections
Diagnosis of biofilm infections is always complicated by the fact that matrix-enclosed sessile bacteria are less immunogenic and elicit a reduced inflammatory response, as opposed to the response elicited by an analogous amount of planktonic free-living bacterial cells [4,66].
Because of the lack of sensitivity of conventional microbiologic methods, molecular methods (eg, polymerase chain reaction [PCR] and fluorescence in situ hybridization [FISH]) are more suitable for detection of biofilm infections. The humoral and cellular responses of patients are very useful for detection of developing biofilms in cases of implanted orthopaedic materials [67]. The humoral system reacts to immunogenic epitopes on the surface of bacteria by producing specific antibodies. These antibodies are not useful against biofilms because bacteria in biofilms produce surface proteins that are very distinct from those on the surface of planktonic cells of the same species [5].
Several tests based on molecular and immunologic methods are currently available for the diagnosis of biofilm infections of bones and joints. These new methods can be combined with imaging modalities, allowing bacterial communities to be located with some degree of accuracy. Anti-biofilm antibodies can be tagged with specific “opacity markers” for various types of scans. Positive enzyme-linked immunosorbent assay (ELISA) tests could be informative as a diagnostic tool, whereas antibody-based imaging could help localization and clinical treatment [66].
The difficulty of identifying biofilm infections in vivo has led to the outline of specific criteria for diagnosing biofilm infections from clinical specimens by Parsek et al. [67]: a) pathogenic bacteria are associated with a surface; b) direct examination of infected tissue demonstrates aggregated cells in cell clusters encased in a matrix, which may be of bacterial and host origin; c) infection is confined to a particular site in the host; d) recalcitrance to antibiotic treatment despite demonstrated susceptibility of planktonic bacteria; e) culture-negative result in spite of clinically documented high suspicion of infection (since localized bacteria in a biofilm may be missed in a conventional blood sample or aspirate); and f) ineffective host clearance evidenced by the location of bacterial cell clusters (macrocolonies) in discrete areas in the host tissue associated with host inflammatory cells.
Can We Prevent Colonization and Formation of Biofilm?
Any plastic or metal biomaterial that is placed into the body should, ideally, be perfectly innocuous [66]. Research in the water industry has shown that surfaces are very similar in their tendency to attract planktonic cells, and that the contamination of surfaces by organic materials (especially residual biofilm matrices) accelerates this process by at least 10-fold [1].
In the process of manufacturing orthopaedic implants, machining techniques (especially those that use a wet interface between the tool assembly and the implant) can lead to biofilm development. Sterilization (eg, with ethylene oxide) kills the bacteria in these biofilms, but fails to remove the residue of their matrices. These deposits must be removed before the devices can be implanted. Techniques with enzyme treatments are available for the removal of biofilm residues.
One of the most practical strategies for the prevention of colonization and consequent biofilm formation is the use of materials and coatings that release antibiotics into the surrounding tissues and fluids. Ideally, these materials will release antibiotics in concentrations lethal to any planktonic cell in the area to prevent biofilm formation [8,66].
Topography and chemical properties of biomaterials surface could be modified to alter the propensity for bacteria adhesion and subsequent biofilm formation [69–71]. Using electro-polished titanium and titanium alloy (Ti-6Al-7Nb) could be a solution for avoiding infections associated with intramedullary nailing systems, as there are indications that staphylococci adhere more to standard titanium alloy nails in vitro[57] and in vivo[58]. Another possibility is to coat titanium or stainless steel with nitrogen ions, which affects the resistivity and chemical topography of the surface [72]. Titanium nitride coatings are known to induce fibroblast attachment and growth [73,74], minimizing the adhesion of S. aureus[75], S. epidermis[72,76], Streptococcus mutans and Pseudomonas aeruginosa[72].
Approaches to reduce protein absorption, bacterial attachment and biofilm formation on biomaterial surfaces include protein coatings such as heparin [70] or albumin [77,78], surface modification by hydrophilic chains [79], phosphorylcholine-modified polymer coatings [80] and poly(ethylene glycol)-based coatings [81,82].
Use of local antibiotics to supplement systemic therapy has been proven effective in controlling orthopaedic infections [83,84]; thus there has been an interest in coating implants (stainless steel, titanium, or titanium alloy) with a thin layer of antibiotic-loaded biocompatible, biodegradable polymer such as polylactic-co-glycolic acid (PLGA) [85,86] and poly(D,L-lactide) (PDLLA) [83,87]. Various antibiotics have been studied, including gentamicin [83,86], ciprofloxacin [85] and vancomycin [83,87]. However, the main concern is the development of resistant bacteria [87], which is more likely if a combination of antibiotics is used [88]. To prevent this, the concentration of the antibiotic eluted from the implant must remain above the minimal inhibitory concentration (MIC) value for a sufficient amount of time.
A novel idea to prevent bacterial colonization on external fixation pins and wires was described by Forster et al. [89], who fitted gentamicin-coated polyurethane sleeves over the pins and wires of the external fixation device. The sleeves substantially reduced the incidence of pin tract infections caused by S. epidermidis, and elution tests revealed that the concentration of gentamicin in the pin tract remained above the 4 μg/ml MIC value recommended for gentamicin for up to 26 weeks.
A new approach to the prevention of the colonization of prostheses is under investigation, as intercellular communication in the biofilm can be altered or interrupted. Intercellular communication is vital for biofilm formation and maturation. Intercellular signals are simple acyl homoserine lactones (AHLs), in the case of gram-negative bacteria [90], and gram-positive bacteria use equally simple cyclic octapeptides for the same purpose [91].
Conclusions
Bacterial cells grow in the biofilm phenotype as a part of their successful strategy to colonize most of this planet and most of its life forms. We have only recognized this distinct phenotype as the predominant mode of bacterial growth for the last 2 decades. Understanding how microbes gather into biofilm communities and maintain diversity remains one of the central questions of microbiology, requiring an understanding of microbes as communal rather then individual organisms. Biofilm formation is a crucial step in the pathogenesis of many subacute and chronic bacterial infections, including foreign body-related infections. Biofilms are difficult to eradicate with conventional antimicrobial agents. Bacterial biofilms have several potential antimicrobial resistance mechanisms. Antimicrobial resistance mechanisms may act concurrently, and in some cases, synergistically. Persisting cells play a major role in the tolerance of biofilm bacteria to antimicrobial agents. Understanding the mechanisms involved in biofilm-associated antimicrobial resistance is key to development of new therapeutic strategies.
Acknowledgments
The authors would like to give our warmest thanks to our dear colleague Antonopoulos Dimitrios, MD, who performed the skillful penmanship of the pictures.
Footnotes
Conflict of interest statement
The authors confirm that there are no conflicts of interest for the above manuscript.
Source of support: Self financing
References
- 1.Donlan RM. Biofilms: Microbial Life on Surfaces. Emerging Infectious Diseases. 2002;8(9):881–90. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costerton JW, Geesey GG, Cheng KJ. How bacteria stick. Sci Am. 1978;238:86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- 3.Costerton JW, Lewandowski Z, Caldwell DE, et al. Microbial biofilms. Ann Rev Micro. 1995;49:711–45. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 4.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 5.Sauer K, Camper AK, Ehrlich GD, et al. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol. 2002;184:1140–54. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies DG, Parsek MR, Pearson JP, et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–98. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 7.Patel R. Biofilms and Antimicrobial Resistance. Clin Orthop. 2005;437:437, 41–47. doi: 10.1097/01.blo.0000175714.68624.74. [DOI] [PubMed] [Google Scholar]
- 8.Harris LG, Richards RG. Staphylococci and implant surfaces: a review. Injury. 2006;37:S3–S14. doi: 10.1016/j.injury.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Petty W, Spanier S, Shuster JJ, Silverthorne C. The influence of skeletal implants on incidence of infection. Experiments in a canine model. J Bone Joint Surg Am. 1985;67(8):1236–44. [PubMed] [Google Scholar]
- 10.Gristina A. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–95. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 11.Ruoslahti E. Integrins as receptors for extracellular matrix. In: Hay ED, editor. Cell biology of extracellular matrix. 2nd ed. New York: Plenum Press; 1991. pp. 343–63. [Google Scholar]
- 12.Francois P, Vaudaux P, Foster TJ, Lew DP. Host-bacteria interactions in foreign body infections. Infect Control Hosp Epidemiol. 1996;17(8):514–20. doi: 10.1086/647358. [DOI] [PubMed] [Google Scholar]
- 13.Patti JM, Hook M. Microbial adhesins recognizing extracellular matrix macromolecules. Curr Opin Cell Biol. 1994;6(5):752–58. doi: 10.1016/0955-0674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 14.Marshall KC. Biofilms: an overview of bacterial adhesion, activity, and control at surfaces. Am Soc Microbiol News. 1992;58:202–7. [Google Scholar]
- 15.Jones HC, Roth IL, Saunders WM., III Electron microscopic study of a slime layer. J Bacteriol. 1969;99:316–25. doi: 10.1128/jb.99.1.316-325.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall KC, Stout R, Mitchell R. Mechanisms of the initial events in the sorption of marine bacteria to surfaces. J Gen Microbiol. 1971;68:337–48. [Google Scholar]
- 17.Zobell CE. The effect of solid surfaces upon bacterial activity. J Bacteriol. 1943;46:39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geesey GG, Mutch R, Costerton JW, Green RB. Sessile bacteria: an important component of the microbial population in small mountain streams. Limnol Oceanogr. 1978;23:1214–23. [Google Scholar]
- 19.Cunningham AB. Hydrodynamics and solute transport at the fluid-biofilm interface. 1989;27a:19–31. See Ref. [Google Scholar]
- 20.Boivin J, Costerton JW. Biofilms and biodeterioration. In: Rossmore HW, editor. Biodeterioration and Biodegradation. 8th ed. London: Elsevier Appl Sci; 1991. pp. 53–62. [Google Scholar]
- 21.Costerton JW, Anwar H. Pseudomonas aeruginosa: the microbe and pathogen. In: Baltch A, Smith P, editors. Pseudomonas aeruginosa Infections and Treatment. New York: Dekker; 1994. pp. 1–18. [Google Scholar]
- 22.Khoury AE, Lam K, Ellis BD, Costerton JW. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 1992;38:174–78. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Lappin-Scott HM, Costerton JW, editors. Microbial Biofilms. Cambridge: Cambridge Univ. Press; 1995. [Google Scholar]
- 24.Lappin-Scott HM, Costerton JW. Bacterial biofilms and surface fouling. Biofouling. 1989;1:323–42. [Google Scholar]
- 25.Rodriguez RF, Zamora JM, Salinas-Rodriguez E, Izquierdo E. Stochastic modeling of some aspects of biofilm behavior. Rev Mex Fis. 2003;49(2):132–43. [Google Scholar]
- 26.Zhang XQ, Bishop PL, Kupferle MJ. Measurement of polysaccharides and proteins in biofilm extracellular polymers. Water Sci Technol. 1998;37:345–48. [Google Scholar]
- 27.Sutherland IW. The biofilm matrix – an immobilized but dynamic microbial environment. Trends in Microbiology. 2001;9(5):222–27. doi: 10.1016/s0966-842x(01)02012-1. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt J, Flemming H-C. Water binding in biofilms. Water Sci Technol. 1999;39:77–82. [Google Scholar]
- 29.Leid JG, Shirtliff ME, Costerton JW, Stoodley AP. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70:6339–45. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner C, Kondella K, Bernschneider T, et al. Post-traumatic osteomyelitis: Analysis of inflammatory cells recruited into the site of infection. Shock. 2003;20:503–10. doi: 10.1097/01.shk.0000093542.78705.e3. [DOI] [PubMed] [Google Scholar]
- 31.Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–93. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 32.O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 33.Watnick PI, Kolter R. Steps in the development of a Vibrio cholerae biofilm. Mol Microbiol. 1999;34:586–95. doi: 10.1046/j.1365-2958.1999.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowan MM, Fletcher M. Rapid screening methods for detection of bacterial mutants with altered adhesion abilities. J Microbiol Methods. 1987;7:241–49. [Google Scholar]
- 35.Genevaux P, Muller S, Bauda P. A rapid screening procedure to identify mini-Tn10 insertion mutants of Escherichia coli K-12 with altered adhesion properties. FEMS Microbiol Lett. 1996;142:27–30. doi: 10.1111/j.1574-6968.1996.tb08402.x. [DOI] [PubMed] [Google Scholar]
- 36.O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–61. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 37.Jefferson KK. What drives bacteria to produce a biofilm? FEMS Microbiol Lett. 2004;236(2):163–73. doi: 10.1016/j.femsle.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Aparna MS, Yadav S. Biofilms: microbes and disease. Braz J Infect Dis. 2008;12(6):526–30. doi: 10.1590/s1413-86702008000600016. [DOI] [PubMed] [Google Scholar]
- 39.John GT, Donale CL. Biofilms: architects of disease. In: Connie RM, Donald CL, George M, editors. Textbook of diagnostic microbiology. 3rd ed. Saunders; 2007. pp. 884–95. [Google Scholar]
- 40.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirtliff ME, Mader JT, Camper AK. Molecular interactions in biofilms. Chem Biol. 2002;9:859–71. doi: 10.1016/s1074-5521(02)00198-9. [DOI] [PubMed] [Google Scholar]
- 42.O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 43.Trampuz A, Zimmerli W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury. 2006;37:S59–66. doi: 10.1016/j.injury.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358(9276):135–38. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 45.Darouiche RO. Device-associated infections: a macroproblem that starts with microadherence. Clin Infect Dis. 2001;33(9):1567–72. doi: 10.1086/323130. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerli W, Lew PD, Waldvogel FA. Pathogenesis of foreign body infection: Evidence for a local granulocyte defect. J Clin Invest. 1984;73(4):1191–200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arens S, Hansis M, Schlegel U, et al. Infection after open reduction and internal fixation with dynamic compression plates-clinical and experimental data. Injury. 1996;27(Suppl 3):27–33. doi: 10.1016/0020-1383(96)89029-2. [DOI] [PubMed] [Google Scholar]
- 48.Arens S, Kraft C, Schlegel U, et al. Susceptibility to local infection in biological internal fixation. Experimental study of open vs. minimally invasive plate osteosynthesis in rabbits. Arch Orthop Trauma Surg. 1999;119(1–2):82–85. doi: 10.1007/s004020050361. [DOI] [PubMed] [Google Scholar]
- 49.Benson DR, Riggins RS, Lawrence RM, et al. Treatment of open fractures: a prospective study. J Trauma. 1983;23(1):25–30. doi: 10.1097/00005373-198301000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Law MD, Jr, Stein RE. Late infection in healed fractures after open reduction and internal fixation. Orthop Rev. 1993;22(5):545–52. [PubMed] [Google Scholar]
- 51.Melcher GA, Claudi B, Schlegel U, et al. Influence of type of medullary nail on the development of local infection. An experimental study of solid and slotted nails in rabbits. J Bone Joint Surg Br. 1994;76(6):955–59. [PubMed] [Google Scholar]
- 52.Arens S, Schlegel U, Printzen G, et al. Influence of materials for fixation implants on local infection. An experimental study of steel versus titanium DCP in rabbits. J Bone Joint Surg Br. 1996;78(4):647–51. [PubMed] [Google Scholar]
- 53.Chang CC, Merritt K. Infection at the site of implanted materials with and without preadhered bacteria. J Orthop Res. 1994;12(4):526–31. doi: 10.1002/jor.1100120409. [DOI] [PubMed] [Google Scholar]
- 54.Perren SM. The concept of biological plating using the limited contact-dynamic compression plate (LC-DCP). Scientific background, design and application. Injury. 1991;22(Suppl 1):1–41. [PubMed] [Google Scholar]
- 55.Woodward SC, Salthouse TN. The tissue response to implants and its evaluation by light microscopy. In: von Recum AF, editor. Handbook of Biomaterial Evaluation. New York: Macmillan; 1986. pp. 364–78. [Google Scholar]
- 56.Meredith DO, Eschbach L, Wood MA, et al. Human fibroblast reactions to standard and electropolished titanium and Ti–6al–7nb, and electropolished stainless steel. J Biomed Mater Res A. 2005;75(3):541–55. doi: 10.1002/jbm.a.30457. [DOI] [PubMed] [Google Scholar]
- 57.Delmi M, Vaudaux P, Lew DP, Vasey H. Role of fibronectin in staphylococcal adhesion to metallic surfaces used as models of orthopaedic devices. J Orthop Res. 1994;12(3):432–38. doi: 10.1002/jor.1100120316. [DOI] [PubMed] [Google Scholar]
- 58.Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine. 2005;30(1):38–43. doi: 10.1097/01.brs.0000147801.63304.8a. [DOI] [PubMed] [Google Scholar]
- 59.Gracia E, Fernandez A, Conchello P, et al. Adherence of Staphylococcus aureus slime-producing strain variants to biomaterials used in orthopaedic surgery. Int Orthop. 1997;21(1):46–51. doi: 10.1007/s002640050116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends in Microbiology. 2001;(9):34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 61.Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella pneumonia biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2000;44:1818–24. doi: 10.1128/aac.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hentzer M, Teitzel GM, Balzer GJ, et al. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol. 2001;183:5395–401. doi: 10.1128/JB.183.18.5395-5401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mah TF, Pitts B, Pellock B, et al. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 2003;426:306–10. doi: 10.1038/nature02122. [DOI] [PubMed] [Google Scholar]
- 64.Konig C, Schwank S, Blaser J. Factors compromising antibiotic activity against biofilms of Staphylococcus epidermidis. Eur J Clin Microbiol Infect Dis. 2001;20:20–26. doi: 10.1007/pl00011232. [DOI] [PubMed] [Google Scholar]
- 65.Fux CA, Wilson S, Stoodley P. Detachment characteristics and oxacillin resistance of Staphyloccocus aureus biofilm emboli in an in vitro catheter infection model. J Bacteriol. 2004;186:4486–91. doi: 10.1128/JB.186.14.4486-4491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costerton JW. Biofilm Theory Can Guide the Treatment of Device-Related Orthopaedic Infections. Session I: biofilms in Orthopaedic infections. Clin Orthop. 2005;437:7–11. doi: 10.1097/00003086-200508000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 68.Selan L, Passariello L, Rizzo L, et al. Diagnosis of vascular graft infections with antibodies against staphylococcal slime antigens. Lancet. 2002;359:2166–68. doi: 10.1016/S0140-6736(02)09086-4. [DOI] [PubMed] [Google Scholar]
- 69.Lange R, Lüthen F, Beck U, et al. Cell-extracellular matrix interaction and physico-chemical characteristics of titanium surfaces depend on the roughness of the material. Biomol Eng. 2002;9(2–6):255–61. doi: 10.1016/s1389-0344(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 70.Nagaoka S, Kawakami H. Inhibition of bacterial adhesion and biofilm formation by a heparinized hydrophilic polymer. ASAIO J. 1995;41(3):M365. doi: 10.1097/00002480-199507000-00032. [DOI] [PubMed] [Google Scholar]
- 71.Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20(23–24):2311–21. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 72.Koerner RJ, Butterworth LA, Mayer IV, et al. Bacterial adhesion to titanium-oxy-nitride (TiNOX) coatings with different resistivities: a novel approach for the development of biomaterials. Biomaterials. 2002;23(14):2835–40. doi: 10.1016/s0142-9612(01)00404-5. [DOI] [PubMed] [Google Scholar]
- 73.Cyster LA, Parker KG, Parker TL, Grant DM. The effect of surface chemistry and nanotopography of titanium nitride (TiN) films on 3T3-L1 fibroblasts. J Biomed Mater Res A. 2003;67(1):138–47. doi: 10.1002/jbm.a.10087. [DOI] [PubMed] [Google Scholar]
- 74.Groessner-Schreiber B, Neubert A, Müller WD, et al. Fibroblast growth on surface-modified dental implants: an in vitro study. J Biomed Mater Res. 2003;64(4):591–99. doi: 10.1002/jbm.a.10417. [DOI] [PubMed] [Google Scholar]
- 75.Harris LG, Richards RG. Staphylococcus aureus adhesion to different treated titanium surfaces. J Mater Sci Mater Med. 2004;15(4):311–14. doi: 10.1023/b:jmsm.0000021093.84680.bb. [DOI] [PubMed] [Google Scholar]
- 76.Poortinga AT, Bos R, Busscher HJ. Charge transfer during staphylococcal adhesion to TiNOX coatings with different specific resistivity. Biophys Chem. 2001;91(3):273–79. doi: 10.1016/s0301-4622(01)00177-6. [DOI] [PubMed] [Google Scholar]
- 77.Galliani S, Viot M, Cremieux A, et al. Early adhesion of bacteremic strains of Staphylococcus epidermidis to polystyrene: influence of hydrophobicity, slime production, plasma, albumin, fibrinogen, and fibronectin. J Lab Clin Med. 1994;123(5):685–92. [PubMed] [Google Scholar]
- 78.Kinnari TJ, Peltonen LI, Kuusela P, et al. Bacterial adherence to titanium surface coated with human serum albumin. Otol Neurotol. 2005;26(3):380–84. doi: 10.1097/01.mao.0000169767.85549.87. [DOI] [PubMed] [Google Scholar]
- 79.Mori Y, Nagaoka S, Takiuchi H, et al. A new antithrombogenic material with long polyethyleneoxide chains. Trans Am Soc Artif Intern Organs. 1982;28:459–63. [PubMed] [Google Scholar]
- 80.Ruiz L, Fine E, Vörös J, et al. Phosphorylcholine-containing polyurethanes for the control of protein adsorption and cell. J Biomater Sci Polym Ed. 1999;10(9):931–55. doi: 10.1163/156856299x00540. [DOI] [PubMed] [Google Scholar]
- 81.Harris LG, Tosatti S, Wieland M, et al. Staphylococcus aureus adhesion to titanium oxide surfaces coated with nonfunctionalized and peptide-functionalized poly (L-lysine) – grafted-poly (ethylene glycol) copolymers. Biomaterials. 2004;25(18):4135–48. doi: 10.1016/j.biomaterials.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 82.Desai NP, Hossainy SF, Hubbell JA. Surface-immobilized polyethylene oxide for bacterial repellence. Biomaterials. 1992;13(7):417–20. doi: 10.1016/0142-9612(92)90160-p. [DOI] [PubMed] [Google Scholar]
- 83.Calhoun JH, Mader JT. Treatment of osteomyelitis with a biodegradable antibiotic implant. Clin Orthop Relat Res. 1997;341:206–14. [PubMed] [Google Scholar]
- 84.Garvin KL, Miyano JA, Robinson D, et al. Polylactide/polyglycolide antibiotic implants in the treatment of osteomyelitis. A canine model. J Bone Joint Surg Am. 1994;76(10):1500–6. doi: 10.2106/00004623-199410000-00009. [DOI] [PubMed] [Google Scholar]
- 85.Makinen TJ, Veiranto M, Knuuti J, et al. Efficacy of bioabsorbable antibiotic containing bone screw in the prevention of biomaterial-related infection due to Staphylococcus aureus. Bone. 2005;36(2):292–99. doi: 10.1016/j.bone.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 86.Price JS, Tencer AF, Arm DM, Bohach GA. Controlled release of antibiotics from coated orthopedic implants. J Biomed Mater Res. 1996;30(3):281–86. doi: 10.1002/(SICI)1097-4636(199603)30:3<281::AID-JBM2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 87.Vaudaux P, Francois P, Berger-Bachi B, Lew DP. In vivo emergence of subpopulations expressing teicoplanin or vancomycin resistance phenotypes in a glycopeptidesusceptible, methicillin-resistant strain of Staphylococcus aureus. J Antimicrob Chemother. 2001;47(2):163–70. doi: 10.1093/jac/47.2.163. [DOI] [PubMed] [Google Scholar]
- 88.Tambe SM, Sampath L, Modak SM. In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. J Antimicrob Chemother. 2001;47(5):589–98. doi: 10.1093/jac/47.5.589. [DOI] [PubMed] [Google Scholar]
- 89.Forster H, Marotta JS, Heseltine K, et al. Bactericidal activity of antimicrobial coated polyurethane sleeves for external fixation pins. J Orthop Res. 2004;22(3):671–77. doi: 10.1016/j.orthres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Fuqua WC, Winans EP, Greenberg EP. Quorum sensing in bacteria: The Lux R - Lux I family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–75. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balaban N, Goldkorn T, Nhan RT, et al. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcus aureus. Science. 1998;280:438–40. doi: 10.1126/science.280.5362.438. [DOI] [PubMed] [Google Scholar]


