Summary
Background
Importin13 (IPO13) is a novel potential marker of corneal epithelial progenitor cells. We investigated the expression and localization of IPO13 in endometrial, endometriotic and endometrial carcinoma tissue.
Material/Methods
IPO13 expression in endometrial, endometriotic and endometrial carcinoma tissue was examined by immunohistochemistry, qPCR and Western blot.
Results
Immunohistochemistry studies showed that IPO13 protein was expressed mainly in cytoplasm of glandular epithelial cell and stromal cells. The rate of importin13-positive cells in proliferative phase endometrium was higher (by about 6-fold) than that in secretory endometrium (P<0.05) and the rate of importin13-positive cells in endometriosis and endometrial carcinoma was higher than that in normal secretory phase endometrial tissues (by about 4- and 9-fold, respectively). Immunofluorescence microscopy revealed co-localization of IPO13 with CD34, CD45, c-kit, telomerase, CD90 and CD146. QPCR revealed significantly increased IPO13 mRNA in endometriosis and endometrial carcinoma versus secretory phase endometrium (by about 2- and 10-fold, respectively). Western blot analysis showed that IPO13 protein is enhanced in endometriosis and endometrial carcinoma versus secretory phase endometrium (p<0.05).
Conclusions
These results demonstrate an increased expression of IPO13 in endometriosis and endometrial carcinoma, which could be involved in the pathogenesis of endometriosis and endometrial carcinoma; IPO13 can serve as an endometrial progenitor/stem cell marker.
Keywords: IPO13, endometrial stem cell, endometriosis, endometrial carcinoma
Background
Human endometrium is a cyclically regenerative tissue. Endometrial regeneration also follows parturition, almost complete resection and in post-menopausal women taking estrogen replacement therapy. The concept that endometrial stem/progenitor cells may be responsible for the highly regenerative capacity of human endometrium was proposed many years ago [1]. Candidate endometrial stem/progenitor cells were detected in mouse endometrium by the LRC technique [2]. At the same time, side population (sp) cells [3–5] of human endometrium were found. Clonogenic epithelial cells and stoma were identified in human endometrium, which suggests 2 types of adult stem/progenitor cells in human endometrium [6,7].
Endometrial stem/progenitor cells have critical roles in the physiological remodelling and regeneration of the human uterus and the pathogenesis of gynecological diseases, including endometriosis and endometrial cancer [8,9]. There are reports that clonogenic cells in a long-term culture were derived from eutopic endometrial cells [10]. Stem cells derived from bone marrow cells give rise to uterine epithelial cells and stroma in humans and mice, suggesting that bone marrow-derived stem cells may contribute to both normal tissue homeostasis and the pathogenesis of endometriosis [11,12]. Mesenchymal stem cells derived from bone marrow may be a source of endometrial stem/progenitor cells [13]. Recently, CD45-positive bone marrow cells were shown to contribute to mouse uterine epithelial cells [14]. Menstrual blood-derived mesenchymal cells exhibit stem cell properties such as the capability of self-renewal; they express multipotent markers such as Oct-4, and c-kit and can help repair damaged heart tissue in the nude rat model [15,16]. The evidence above may support the hypothesis that endometriosis is a disease of stem cell origin.
Cancer cells exhibit many features of stem cells, including high proliferative capacity, self-renewal and a high degree of plasticity [17]. It is possible that cancer stem cells (CSC) arise from human endometrial stem/progenitor cells, acquiring genetic mutations enabling their transformation into CSC, likely responsible for initiation, progression, metastasis and recurrence of endometrial carcinoma after treatment[18]. Some general adult stem cell markers – NAC1, c-kit (CD117) and oct4 – have been identified in human endometrium [19–21]; however, they are not restricted to stem cells. Therefore, there is a need to identify additional specific markers for endometrial stem/progenitor cells. Importin13 (IPO13), which is a new member of the importin beta super-family of nuclear transport proteins, has been verified as a unique bidirectional transport receptor protein related to cell development and physiologic function such as lung, brain, and heart embryonic development. It also plays an important role in maintaining the phenotype, high proliferative potential, and reduced differentiation of corneal epithelial progenitor cells [22]. IPO13 is crucial for normal and abnormal cellular differentiation [23].
To our knowledge there has been few studies describing the expression of IPO13 in endometrial tissue. In our previous study, we found IPO13 expression was decreased in endometrial polyps compared with normal endometrial tissue [24]. In this study we investigate the expression of IPO13 as a possible marker for endometrial stem/progenitor cells in normal human endometrium, endometriosis and endometrial cancer.
Material and Methods
Tissue Samples: This study protocol was approved by the Ethics Committee of the Chongqing Medical University, and informed consent was obtained from all patients according to The World Medical Association’s Declaration of Helsinki. Human endometrial samples were acquired from 80 patients aged 20–72 (mean 43) years undergoing surgery such as laparotomic hysterectomy and abdominal hysterectomy. A total of 160 samples of physiological endometrium (40 in proliferative phase and 40 in secretory phase,) and 80 samples of pathological endometrium (40 of endometriosis from endometriomas and 40 of endometrial carcinoma) were used for the study. All the pathologies were confirmed by pathological examination. Patients who had additional endometrial complications, including adenomyosis, dysfunctional uterine bleeding, polycystic ovary syndrome, and other hormone-dependent diseases, were excluded. All of the patients had regular menstrual cycles, did not receive hormone therapy during the 3 months before surgery, and were not pregnant or lactating during the study. There were no statistically significant differences between age groups (P>0.05). The specimens were washed with saline to remove blood, immediately frozen in liquid nitrogen, and stored at −80°.
Immunohistochemistry
The tissue samples were fixed in formalin, embedded in paraffin, sectioned (5 μm thick), deparaffinized in xylene, dehydrated in a graded series of ethanol, subjected to antigen retrieval in citrate buffer (pH 6.0; Sigma) for 30 min in a steamer and washed in phosphate-buffered saline (PBS). PBS was used for all subsequent washes and for antiserum dilution. Tissue sections were quenched sequentially in 3% hydrogen peroxide and blocked with PBS containing 10% donkey serum (Sigma) for 1 h at 37°C. Next, polyclonal goat anti-IPO13 antibody (1:100; NB100-1369; Novus Biologicals) was applied to the tissue sections at 4° overnight. Negative controls included omission of primary antibody and use of irrelevant primary antibodies. After several washes (3×3 min) to remove excess antibody, the slides were incubated with diluted (1:300) anti-goat biotinylated antibodies (Beijing Biosynthesis Biotechnology Co., Ltd, China) for 1 h. All the slides were washed in PBS and were incubated in avidin biotin peroxidase complex (ABC) (Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China) diluted 1: 300 in PBS for 30 min in humidified chambers at 37°. DAB (Beijing Biosynthesis Biotechnology Co., Ltd., China) was used as a chromogen and hematoxylin was used as a nuclear counter-stain.
Slides were evaluated independently by 3 pathologists for distribution and intensity of signal as described by De Falco et al. [25]. Intensity was scored from 0 to 3: 0 (absent immunopositivity); 1 (low immunopositivity); 2 (moderate immunopositivity); 3 (intense immunopositivity). The ipo13-positive cells were counted and an average of 22 fields was observed for each tissue.
Fluorescence microscope
All paraffin-embedded tissue sections were processed up to antigen retrieval, as described above. After blocking with 4% BSA in PBS, slides were incubated with the primary antibody overnight at 4°C, which included the following: monoclonal mouse anti-CD146 (1: 100; ZM-0299; Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China); polyclonal goat anti-IPO13 (1:100; NB100-1369; Novus Biologicals); polyclonal rabbit anti-telomerase (1:150; ZA-0239; Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China); polyclonal rabbit anti-c-kit (1:100; SC-168, Santa Cruz); polyclonal rabbit anti-CD90 (1:100; bs-0778R; Beijing Biosynthesis Biotechnology Co., Ltd., China); polyclonal rabbit anti-CD34 (1:100; bs-2038R; Beijing Biosynthesis Biotechnology Co., Ltd., China); polyclonal rabbit anti-CD45(1:150; bs-0522R; Beijing Biosynthesis Biotechnology Co., Ltd., China). Negative controls included omission of primary antibody and use of irrelevant primary antibodies. The tissues were then incubated with the secondary antibodies, including FITC-conjugated donkey anti-rabbit (1: 500; bsF-0295D; Beijing Biosynthesis Biotechnology Co., Ltd, China), FITC-conjugated Rabbit Anti-mouse IgG (1:500; bsF-0296R; Beijing Biosynthesis Biotechnology Co., Ltd., China), Cy3-conjugated donkey anti-goat IgG (H+L)(1;400;A0502; Beyotime Institute of Biotechnology, Jiangsu, China) in the dark for 1 h at room temperature. Following incubation, the slides were washed 3 times with PBS and cell nuclei were stained with DAPI (Sigma). Fluorescence was detected using fluorescence microscopy and images from the different fluorescence channels were merged using Adobe Photoshop CS 8.01.
Quantitative real-time polymerase chain reaction detection of the mRNA of IPO13
Total RNA was extracted from normal human endometrium, endometriosis and endometrial carcinoma using the RNA prep pure Plant Kit (Tiangen Biotech Co., Ltd). First-strand cDNA was synthesized using a PrimeScriptTM RT-PCR Kit (TaKaRa Biotechnology Co., Ltd.). IPO13 mRNA expression levels were examined by quantitative real-time PCR with the iQ5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) and All-in-One qPCR Mix (GeneCopoeia, Inc.). β-actin values were used for normalization. Primers used for SYBR Green assay were purchased from GeneCopoeia, Inc. (HQP023057, HQP016381). PCR amplifications were started with a 10 min denaturation step at 95°C, followed by 40 amplification cycles (10 sec at 95°C, 20 sec at 60°C, 10 sec at 72°C). Relative quantification of mRNA was performed using the comparative threshold cycles (CT) method. The value was used to plot the gene expression employing the formula 2−ΔΔCT.
Western blot analysis
Expression of IPO13 protein was analyzed by Western blotting as previously described [26]. The primary antibodies used included polyclonal goat anti-IPO13 (1:500; NB100-1369; Novus Biologicals) and polyclonal rabbit anti-β-actin (1:500; ZA-0239; Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China). The bands were detected via enhanced chemiluminescence (ECL) reagent (Beyotime Institute of Biotechnology, Jiangsu, China) and quantified by ImageQuant 3.3 software (Molecular Dynamics).
Statistical evaluation
All values were expressed as mean ± SEM. Comparison of 2 means was made with Student’s t-test. A p value of <0.05 was considered statistically significant.
Results
Localization of IPO13 in endometrium, endometriosis and endometrial carcinoma
In the samples examined we observed that in proliferative phase, IPO13 was intensively expressed in glandular epithelium and stromal cells (Figure 1A). In secretory phase, IPO13 was expressed at a low level in glandular epithelium, whereas no immunopositivity was present in the stromal cells (Figure 1B). By contrast, IPO13 immunopositivity in the endometriosis was increased to an almost moderate level of expression in the glandular epithelium and stroma (Figure 1C). In the endometrial cancer, IPO13 was intensively expressed in the glands and moderately expressed in the stroma (Figure 1D).
Figure 1.
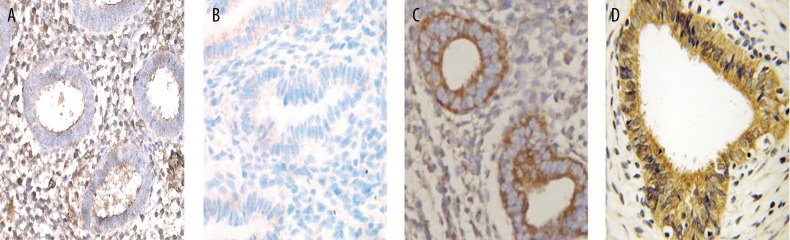
Representative immunohistochemical staining patterns of IPO13 in human proliferative phase endometrium, secretory phase endometrium, endometriosis and endometrial carcinoma. (A) IPO13-expressing cells in proliferative phase endometrium; ×400 magnification. (B) IPO13-expressing cells in secretory phase endometrium; ×400 magnification. (C) IPO13-expressing cells in endometriosis; ×200 magnification. (D) IPO13-expressing cells in endometrial carcinoma; ×400 magnification.
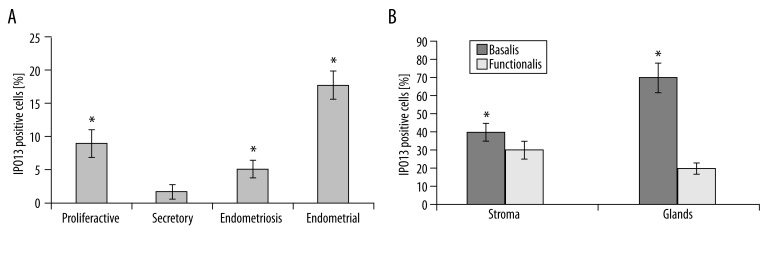
The number of IPO13-expressing cells was significantly increased by about 6-fold during the proliferative phase compared to the secretory phase (P<0.05). IPO13-positive cell numbers were significantly increased by about 4-fold in endometriotic tissue compared to secretory endometrium (P<0.05). IPO13-positive cell numbers were significantly increased by about 9-fold in endometrial carcinoma compared to secretory endometrium (P<0.05) (Figure 2A). We also observed that the percentage of IPO13-positive cells in the basalis of proliferative endometrium was more than that in the functionalis of the endometrial tissues (Figure 2B).
Figure 2.
Distribution of IPO13-positive cells in proliferative phase endometrium, secretory phase endometrium, endometriosis and endometrial carcinoma. (A) Significantly increased number of IPO13-positive cells in endometriosis and endometrial carcinoma vs. secretory phase endometrium. (B) Significantly increased percentage of IPO13-positive cells in the basalis compared to the functionalis layer of proliferative phase endometrium. * p<0.05; error bars, SEM.
The expression of IPO13 in putative endometrial stem/progenitor cells
IPO13 were labeled red; CD90, CD34, CD45, telomerase, CD146 and c-kit were labeled green; nuclei were stained blue; and yellow staining in the merged pictures represents co-localization of the 2 progenitor cell marker proteins. IPO13-positive cells were present in the vicinity of endometrial glands (Figure 4C), in the endometrial stroma (Figure 3A–C) and in the endometrial tissues (Figure 4A, B).
Figure 4.
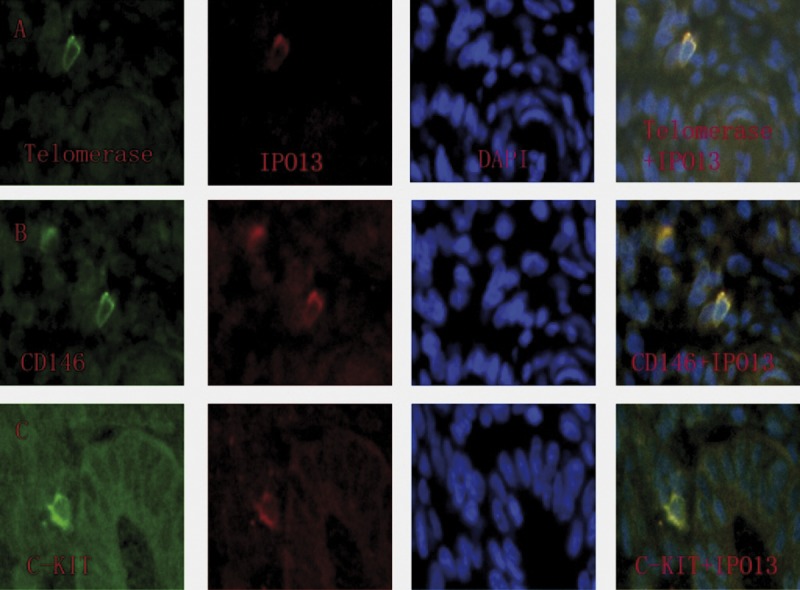
Co-localization of IPO13, telomerase, CD146 and c-kit in human endometrium. The sections were immunostained with a goat polyclonal IPO13 antibody (red fluorescent secondary antibody), a mouse monoclonal CD146 antibody (green fluorescent secondary antibody) and polyclonal rabbit antibodies (green fluorescent secondary antibody) including anti-telomerase, anti-telomerase and anti-c-kit. Yellow staining in the merged pictures denotes co-localization of the 2 progenitor cell marker proteins. Blue, DAPI nuclear staining. (A) Putative endometrial progenitor cell displaying co-localization of IPO13 and telomerase. (B) Putative endometrial progenitor cell displaying co-localization of IPO13 and CD146. (C) Putative endometrial progenitor cell displaying co-localization of IPO13 and c-kit.
Figure 3.
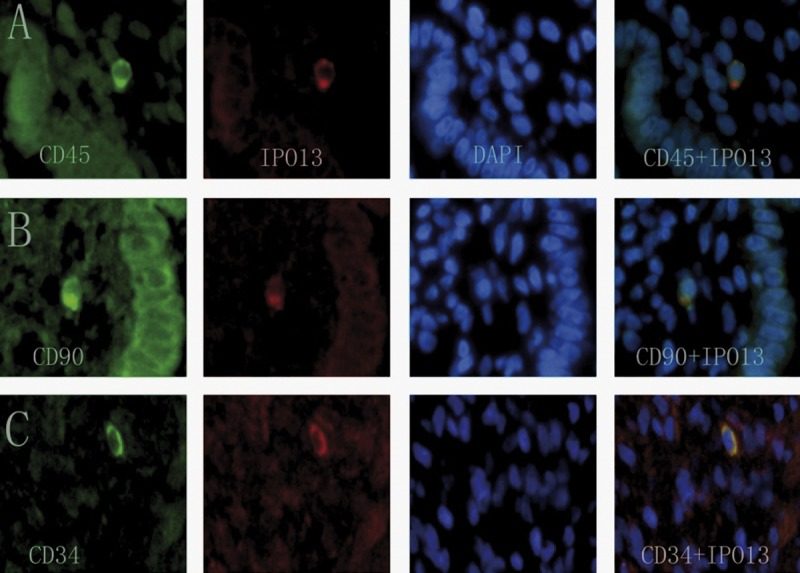
Co-localization of IPO13 and CD90, CD34, CD45 in human endometrium. The sections were immunostained with a goat polyclonal IPO13 antibody (red fluorescent secondary antibody) and polyclonal rabbit antibodies (green fluorescent secondary antibody) including anti-CD90, anti-CD34 and anti-CD45. Yellow staining in the merged pictures denotes co-localization of the 2 progenitor cell marker proteins. Blue, DAPI nuclear staining. (A) Putative endometrial progenitor cell displaying co-localization of IPO13 and CD45. (B) Putative endometrial progenitor cell displaying co-localization of IPO13 and CD90. (C) Putative endometrial progenitor cell displaying co-localization of IPO13 and CD34.
Expression of IPO13 mRNA in endometrium, endometriosis and endometrial carcinoma
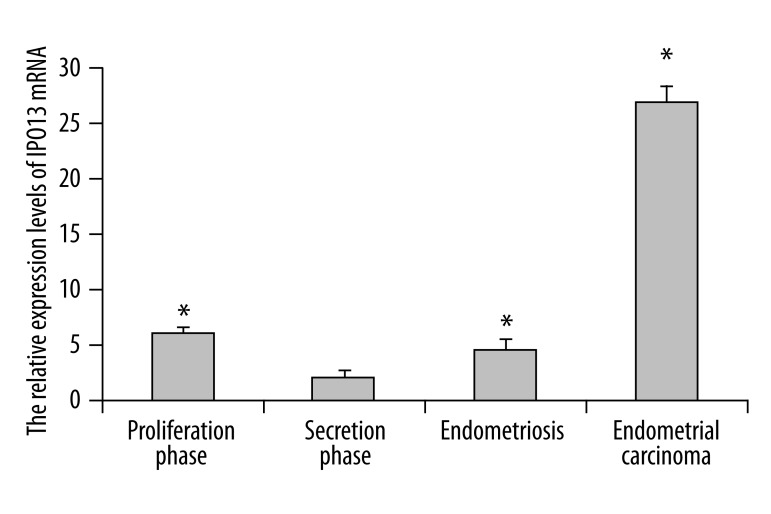
IPO13 mRNA was detected in all tissues studied, with the highest expression in the endometrial carcinoma. The expression of IPO13 mRNA in secretory phase endometrium was decreased by about 10-fold over that in endometrial carcinoma (p<0.05) (Figure 5). IPO13 mRNA expressed in endometriosis was increased by about 2-fold over that in secretory phase endometrium (p<0.05) (Figure 5).
Figure 5.
Quantitative real-time PCR analysis of IPO13 mRNA expression in human endometrium, endometriosis and endometrial carcinoma. Relative increase of IPO13 expression in endometriosis and endometrial carcinoma vs. secretory myometrium, as calculated by the 2−ΔΔCt method. Error bars, SEM; * p<0.05.
Western blot of IPO13 in endometrium, endometriosis and endometrial carcinoma
The expression level of IPO13 protein in endometriosis (0.81±0.17) and endometrial carcinoma (0.94±0.10) was significantly higher than that in the secretory endometrium (0.45±0.10*) (P<0.05). There was no significant difference between proliferative endometrium and endometriosis and endometrial carcinoma (P>0.05) (Figure 6 and Table 1).
Figure 6.
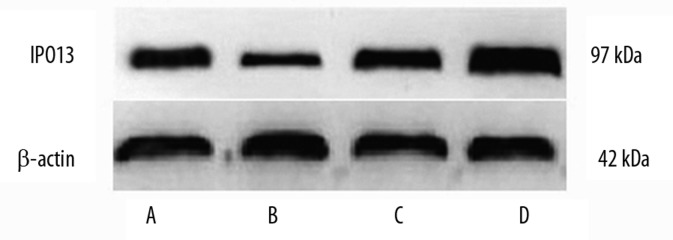
Western blot analysis of IPO13 in human endometrium, endometriosis and endometrial carcinoma. The upper and lower panels show the intensity of IPO13 and β-actin bands, respectively. (A) proliferative endometrium (B) secretory endometrium (C) endometriosis (D) endometrial carcinoma
Table 1.
The relative expression levels of IPO13 proteins in normal endometrial tissues, endometriosis and endometrial carcinoma.
| Cases | Proliferative endometrium | Secretory endometrium | Endometriosis | Endometrial carcinoma |
|---|---|---|---|---|
| 40 | 0.62±0.11* | 0.45±0.10 | 0.81±0.17* | 0.94±0.10* |
Data given as mean ±SEM.
p<0.05, vs. control endometrium at the secretory endometrium, Error bars, SEM.
Discussion
In the present study we have characterized the expression of IOP13 in human endometrium, endometriosis and endometrial carcinoma, showing IOP13 expression in the different endometrial phases. We observed increased expression of IPO13 in proliferative phase of the endometrium and a decreased expression of IPO13 in secretory phase endometrial tissue. We found that the percentage of IPO13-positive cells in the basalis of proliferative endometrium was more than that in the functionalis of the endometrial tissues (Figure 2B). Our findings support the hypotheses that the putative endometrial stem/progenitor cells may localize at the lower region of the stroma. In corneal epithelial progenitor cells, IPO13 is important in the maintenance of “stemness” [22]. Telomerase is a reverse transcriptase whose activity is associated with the immortality of IPS cells (induced pluripotent stem cells), embryonic stem cells, germ cells and cancer cells [27–31]. Endometriosis and endometrial carcinoma are associated with aberrant endometrial expression of telomerase and increased telomere activity [32,33]. C-kit is a stem cell factor receptor that has been described as an undifferentiation marker in the human endometrium [21]. CD90 and CD146 are 2 well-known MSC markers, and endometrial mesenchymal stem-like cells are prospectively isolated as CD146 (+) PDGF-Rβ (+) cells [34,35]. The co-localization of IPO13-positive cells in human endometrium with telomerase, c-kit, CD90, and CD146, together with increased IPO13 expression in proliferative endometrium, endometriosis and endometrial carcinoma, emphasize the stem cell character of IPO13-expressing cells.
The aberrant expression of telomerase and c-kit in endometrium mediates alterations in cell proliferation, contributing to the pathogenesis of endometriosis [36–38]. Ipo13 is critical for normal and abnormal cellular differentiation and is expressed by human limbal basal epithelial cells; it plays an important role in maintaining the phenotype, high proliferative potential, and less differentiation of corneal epithelial progenitor cells [22,23]. In the presence of IPO13 inhibitor, IPO13 expression and the proliferative capacity decreased in human limbal epithelial clones, accompanied by cell differentiation [22]. We speculate that IPO13 inhibitor may decrease the proliferative capacity and the number of clones in endometriotic lesions; IPO13 inhibitor might be a useful approach for the treatment of endometriosis.
The hematopoietic stem cell can differentiate into liver cells, muscle cells and nerve cells under certain conditions [39,40]. Bone-marrow transplantation experiments in mice and humans have demonstrated that bone marrow-derived stem cells have the potential to differentiate into endometrial tissue [11,12]. CD45-positive blood cells give rise to uterine epithelial cells in mice [14]. The endometrial side population (SP) cells express CD34, CD90 and CD146. In our study we found the IPO13-positive cells co-localized with CD34, CD45, telomerase, c-kit, CD90 and CD146, which suggests that IPO13 may be a putative endometrial stem/progenitor cell marker.
Emerging evidence indicates that cancer stem cells are responsible for endometrial carcinoma, with abnormal expression of c-kit and telomerase [18,33,41]. Similar to c-kit and telomerase expression in endometrial carcinoma [33,41], the expression of IPO13 was significantly higher in endometrial carcinoma compared to secretory phase endometrium. IPO13 is a transport receptor protein; the substrates transported by IPO13 include pax-6, pax-3, ubc9 and ElF1A. Pax-6 is important for cell growth, survival and differentiation [42]. Pax-3 is a transcription factor that is expressed in the neural tube and neural crest and regulates neural tube closure by inhibiting p53-dependent apoptosis [43]. Ubc9 is an E2-conjugating enzyme that promotes breast cell invasion and metastasis [44]. In our study the expression of IPO13 in endometrium was increased; therefore, we suspected IPO13 may be associated with invasion and metastasis in endometrial cancer.
Conclusions
The present study has, for the first time, demonstrated the expression of IPO13 in endometrial carcinoma. An increased expression of IPO13 in proliferative phase of the endometrium, and the IPO13-positive cells, co-localized with CD34, CD45, telomerase, c-kit, CD90 and CD146. Increased expression of IPO13 in endometriosis and endometrial carcinoma compared to secretory endometrium might support the hypotheses that these diseases originate from stem cells.
Footnotes
Conflict of interest statement
We declare that we have no conflict of interest.
Source of support: This work was supported by a project of the Natural Science Foundation of Chongqing (CSTC: 2008BB5239)
References
- 1.Lucot JP, Coulon C, Collinet P, et al. Surgical therapeutic management for menorrhagia. J Gynecol Obstet Biol Reprod (Paris) 2008;37(Suppl 8):S398–404. doi: 10.1016/S0368-2315(08)74780-X. [DOI] [PubMed] [Google Scholar]
- 2.Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24(6):1529–38. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- 3.Kato K, Yoshimoto M, Kato K, et al. Characterization of side-population cells in human normal endometrium. Hum Reprod. 2007;22(5):1214–23. doi: 10.1093/humrep/del514. [DOI] [PubMed] [Google Scholar]
- 4.Masuda H, Matsuzaki Y, Hiratsu E, et al. Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS One. 2010;5(4):e10387. doi: 10.1371/journal.pone.0010387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuji S, Yoshimoto M, Takahashi K, et al. Side population cells contribute to the genesis of human endometrium. Fertil Steril. 2008;90(Suppl 4):1528–37. doi: 10.1016/j.fertnstert.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70(6):1738–50. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 7.Gargett CE, Schwab KE, Zillwood RM, et al. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80(6):1136–45. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maruyama T, Masuda H, Ono M, et al. Human uterine stem/progenitor cells: their possible role in uterine physiology and pathology. Reproduction. 2010;140(1):11–22. doi: 10.1530/REP-09-0438. [DOI] [PubMed] [Google Scholar]
- 9.Gargett CE, Chan RW, Schwab KE. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol Cell Endocrinol. 2008;288(1–2):22–29. doi: 10.1016/j.mce.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka T, Nakajima S, Umesaki N. Cellular heterogeneity in long-term surviving cells isolated from eutopic endometrial, ovarian endometrioma and adenomyosis tissues. Oncol Rep. 2003;10(5):1155–60. [PubMed] [Google Scholar]
- 11.Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–85. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 12.Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–86. doi: 10.1634/stemcells.2006-0828. [DOI] [PubMed] [Google Scholar]
- 13.Aghajanova L, Horcajadas JA, Esteban FJ, Giudice LC. The bone marrow-derived human mesenchymal stem cell: potential progenitor of the endometrial stromal fibroblast. Biol Reprod. 2010;82(6):1076–87. doi: 10.1095/biolreprod.109.082867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bratincsak A, Brownstein MJ, Cassiani-Ingoni R, et al. CD45-positive blood cells give rise to uterine epithelial cells in mice. Stem Cells. 2007;25(11):2820–26. doi: 10.1634/stemcells.2007-0301. [DOI] [PubMed] [Google Scholar]
- 15.Patel AN, Park E, Kuzman M, et al. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant. 2008;17(3):303–11. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- 16.Hida N, Nishiyama N, Miyoshi S, et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26(7):1695–704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- 17.Yao T, Chen Q, Zhang B, et al. The expression of ALDH1 in cervical carcinoma. Med Sci Monit. 2011;17(8):HY21–26. doi: 10.12659/MSM.881886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubbard SA, Friel AM, Kumar B, et al. Evidence for cancer stem cells in human endometrial carcinoma. Cancer Res. 2009;69(21):8241–48. doi: 10.1158/0008-5472.CAN-08-4808. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa M, Nakayama K, Yeasmin S, et al. NAC1, a potential stem cell pluripotency factor expression in normal endometrium, endometrial hyperplasia and endometrial carcinoma. Int J Oncol. 2010;36(5):1097–103. doi: 10.3892/ijo_00000591. [DOI] [PubMed] [Google Scholar]
- 20.Matthai C, Horvat R, Noe M, et al. Oct-4 expression in human endometrium. Mol Hum Reprod. 2006;12(1):7–10. doi: 10.1093/molehr/gah254. [DOI] [PubMed] [Google Scholar]
- 21.Cho NH, Park YK, Kim YT, et al. Lifetime expression of stem cell markers in the uterine endometrium. Fertil Steril. 2004;81(2):403–7. doi: 10.1016/j.fertnstert.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Tao T, Tang J, et al. Importin 13 serves as a potential marker for corneal epithelial progenitor cells. Stem Cells. 2009;27(10):2516–26. doi: 10.1002/stem.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang J, Ke G, You W, et al. Interaction between importin 13 and myopodin suggests a nuclear import pathway for myopodin. Mol Cell Biochem. 2008;307(1–2):93–100. doi: 10.1007/s11010-007-9588-1. [DOI] [PubMed] [Google Scholar]
- 24.Hu J, Yuan R. The expression levels of stem cell markers importin13, c-kit, CD146, and telomerase are decreased in endometrial polyps. Med Sci Monit. 2011;17(8):BR221–27. doi: 10.12659/MSM.881901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Falco M, Fedele V, Cobellis L, et al. Pattern of expression of cyclin D1/CDK4 complex in human placenta during gestation. Cell Tissue Res. 2004;317(2):187–94. doi: 10.1007/s00441-004-0880-z. [DOI] [PubMed] [Google Scholar]
- 26.He HJ, Zhu TN, Xie Y, et al. Pyrrolidine dithiocarbamate inhibits interleukin-6 signaling through impaired STAT3 activation and association with transcriptional coactivators in hepatocytes. J Biol Chem. 2006;281(42):31369–79. doi: 10.1074/jbc.M603762200. [DOI] [PubMed] [Google Scholar]
- 27.Shay JW, Wright WE. Telomeres and telomerase in normal and cancer stem cells. FEBS Lett. 2010;584(17):3819–25. doi: 10.1016/j.febslet.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marion RM, Strati K, Li H, et al. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell. 2009;4(2):141–54. doi: 10.1016/j.stem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Schieker M, Gulkan H, Austrup B, et al. Telomerase activity and telomere length of human mesenchymal stem cells. Changes during osteogenic differentiation. Orthopade. 2004;33(12):1373–77. doi: 10.1007/s00132-004-0739-8. [DOI] [PubMed] [Google Scholar]
- 30.Sekulovic S, Gylfadottir V, Vulto I, et al. Prolonged self-renewal activity unmasks telomerase control of telomere homeostasis and function of mouse hematopoietic stem cells. Blood. 2011;118(7):1766–73. doi: 10.1182/blood-2010-11-319632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serakinci N, Graakjaer J, Kolvraa S. Telomere stability and telomerase in mesenchymal stem cells. Biochimie. 2008;90(1):33–40. doi: 10.1016/j.biochi.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Hapangama DK, Turner MA, Drury JA, et al. Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Hum Reprod. 2008;23(7):1511–19. doi: 10.1093/humrep/den172. [DOI] [PubMed] [Google Scholar]
- 33.Boggess JF, Zhou C, Bae-Jump VL, et al. Estrogen-receptor-dependent regulation of telomerase activity in human endometrial cancer cell lines. Gynecol Oncol. 2006;103(2):417–24. doi: 10.1016/j.ygyno.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 34.Gronthos S, Zannettino AC, Hay SJ, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116(Pt 9):1827–35. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 35.Schwab KE, Hutchinson P, Gargett CE. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23(4):934–43. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S, Verfaillie C, Chmielewski D, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17(11):3028–40. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 37.Forte A, Schettino MT, Finicelli M, et al. Expression pattern of stemness-related genes in human endometrial and endometriotic tissues. Mol Med. 2009;15(11–12):392–401. doi: 10.2119/molmed.2009.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hapangama DK, Turner MA, Drury JA, et al. Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Hum Reprod. 2008;23(7):1511–19. doi: 10.1093/humrep/den172. [DOI] [PubMed] [Google Scholar]
- 39.Dalakas E, Newsome PN, Boyle S, et al. Bone marrow stem cells contribute to alcohol liver fibrosis in humans. Stem Cells Dev. 2010;19(9):1417–25. doi: 10.1089/scd.2009.0387. [DOI] [PubMed] [Google Scholar]
- 40.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290(5497):1775–79. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 41.Slomovitz BM, Broaddus RR, Schmandt R, et al. Expression of imatinib mesylate-targeted kinases in endometrial carcinoma. Gynecol Oncol. 2004;95(1):32–36. doi: 10.1016/j.ygyno.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 42.Mascarenhas JB, Young KP, Littlejohn EL, et al. PAX6 is expressed in pancreatic cancer and actively participates in cancer progression through activation of the MET tyrosine kinase receptor gene. J Biol Chem. 2009;284(40):27524–32. doi: 10.1074/jbc.M109.047209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pani L, Horal M, Loeken MR. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: implications for Pax-3- dependent development and tumorigenesis. Genes Dev. 2002;16(6):676–80. doi: 10.1101/gad.969302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu S, Sachdeva M, Wu F, et al. Ubc9 promotes breast cell invasion and metastasis in a sumoylation-independent manner. Oncogene. 2010;29(12):1763–72. doi: 10.1038/onc.2009.459. [DOI] [PMC free article] [PubMed] [Google Scholar]