Summary
Background
Celecoxib has a positive effect on human osteoarthritic cartilage, but the mechanisms remain unclear. The aim of this study was to test whether celecoxib could inhibit the apoptosis of chondrocyte and ameliorate type II collagen synthesis to relieve symptoms of OA (osteoarthritis).
Material/Methods
130 Wistar rats were randomly divided into 4 groups as celecoxib (CE), ibuprofen (IBP), indomethacin (IN) and normal saline group (NS). The osteoarthritis was induced by the excision of the left Achilles tendon. At the 3th, 6th, 9th month of treatment, the histological structure of articular cartilage was observed using HE staining. Type II collagen was examined using immunohistochemistry. Chondrocyte apoptosis was detected by TUNEL staining, and the change of ultra-microstructure of chondrocyte was examined through a transmission electron microscope.
Results
CE reduced the OA-like histological changes and suppressed chondrocyte apoptosis. However, IN or IBP had deleterious effects on articular cartilage and enhanced the chondrocyte apoptosis. IBP promoted the expression of type II collagen, and IN inhibited its expression, but had no effect in the CE group.
Conclusions
CE had favorable action on OA progression, and may be the ideal choice in the treatment of chronic destructive joint disease where anti-inflammatory drugs need to be used for a prolonged period.
Keywords: nonsteroidal anti-inflammatory drugs, chondrocyte, osteoarthritis, apoptosis
Background
Osteoarthritis (OA) is the most common form of arthritis and the leading cause of disability, especially among older adults. The disease process leads to limitation of joint movement, joint deformity, tenderness, inflammation, and severe pain. OA is characterized by progressive loss of cartilage from the articulating surfaces of diarthrodial joints [1,2]. Although the pathophysiologic mechanisms of osteoarthritis remain unresolved, chondrocytes in OA cartilage demonstrated morphologic changes that are characteristic features of apoptosis, which suggests apoptosis plays an important role in the development of OA [3–6].
Conventional approaches for the treatment of OA range from conservative measures, to surgical intervention, and eventually joint replacement [7–9]. Currently, there is no curative treatment for the disease. Even though total joint replacement surgery is relatively successful for the treatment for OA, the lifespan of artificial joints is limited, and there are still significant problems such as implant loosening and failure, and the expensive cost. Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most widely prescribed drugs in the treatment of osteoarthritis (OA) to alleviate pain and thereby maintain the ability to perform normal daily physical activities, even in treatment of knee replacement surgery. The clinical efficacy of NSAIDs is primarily related to the inhibition of cyclooxygenase 2 (COX-2) but not cyclooxygenase 1. Celecoxib, as one of the first selective COX-2 inhibitors, has been shown to be an effective anti-inflammatory and analgesic drug in patients with rheumatoid arthritis and OA, comparable to that of traditional NSAIDs, such as naproxen, diclofenac and ibuprofen [9–12]. A significant reduction of gastrointestinal adverse events with selective COX-2 inhibitors compared to non-selective NSAIDs has been frequently demonstrated. Currently, several in vitro studies have shown celecoxib has a positive effect on human osteoarthritic cartilage, but it remains controversial as to what effects these agents have on the progression of OA.
Apoptotic death of articular chondrocytes has been implicated in the pathogenesis of OA [13]. Herein, based on surgically-induced osteoarthritis model, we performed a study to determine whether celecoxib could inhibit the apoptosis of chondrocytes and ameliorate type II collagen synthesis to relieve symptoms of OA.
Material and Methods
Animals
One hundred and thirty Wistar rats (3~4 months old) were purchased from the laboratory animal center, Chongqing Medical University. An OA model in Wistar rats was induced using the surgical resection of the left Achilles tendon, resulting in a decrease in joint stress, performed as previously described [14]. The left knee was used as the experimental side and the right knee as the control side. The experimental protocol was approved by the animal study committee at our institution.
The experiments were done with reference to the long-term toxicity test methods in the Methodology of Pharmacological Experiments [15]. Animals were randomly divided into 4 groups: celecoxib group (CE), Ibprofen group (IBP), indomethacin group (IN) and normal saline group (NS). The daily drug dosages were: CE 24 mg/kg (American Silver Pharmaceutical Company), IBP 72 mg/kg (Chongqing Southwest Pharmaceutical CO.LTD.), IN 9 mg/kg (Chongqing Kerui Pharmacy CO.LTD.), and NS (Sichuan Kelun Pharmaceutical CO. LTD.). The drug was administered to rats by daily oral gavage. Periods of administration were 3, 6, and 9 months. If there were more than 50 g in the weight difference between rats, the drug would be administered individually.
HE staining observation
At the end of the 3rd, 6th, and 9th months of treatment after the surgically-induced model, the rats were killed. The knees were dissected from each animal, then fixed in 4% paraformaldehyde and 70% ethanol, and decalcified with 10% EDTA. Decalcified samples were paraffin embedded and sectioned.
After HE staining, chondrocytes, cartilage surface, cartilage matrix and tide line were observed with the microscope.
Immunohistochemical study of type II collage in chondrocyte
Type II collagen antibody, the SABC kit and DAB are purchased from Boston Corp., Wuhan. The IHC stainings of cartilage matrix and chondrocyte were observed and photographed using an Olympus microscope. Beijing aviation medical image analysis system was adopted to calculate the average density of positive staining in every field. Five fields are obtained from every sample, which are represented as mean ±S.E.M.
Chondrocyte apoptosis detection
Chondrocyte apoptosis was detected by TUNEL staining. The apoptosis detection kit was purchased from Mannheim Company (Germany), and the procedure of TUNEL detection was performed according to the manual. The stainings were observed and photographed using an Olympus microscope. The test included a positive and negative control. The positive cells presented yellow particles distributed throughout the nuclear material, and the negative cells showed blue staining (hematoxylin). The positive and negative cells were counted in high-power microscope fields. Apoptosis proportion was calculated by the formula: the number of positive cells/the total number of cells. Five hundred chondrocytes were counted in every slide and per 1000 chondrocytes in different periods of each group. The means represents the apoptosis proportion for every sample.
Transmission electron microscope observation
The rats were killed by vertebrae dislocation, the left knees were exposed and the weight loading area of femoral condyle (2×2 mm) was obtained. The specimens were fixed with 1% osmium tetroxide, decalcified with 10% EDTA, dehydrated in a graded series of ethanol and acetone, and finally embedded in epoxy resin and semithin section. Ultrathin sections were cut using an ultramicrotome, double stained, and examined and photographed with a transmission electron microscope.
Statistical analysis
The quantitative and semi-quantitative data analyses, including the analysis of variance, and Q-test, were performed with SAS 6.12 software, and the significance level was set at α=0.05.
Results
Observation an animal model of OA
The change of early OA appeared 2 months after the surgery. The surface of articular cartilage became slightly rough, where is the focus of the stress. The HE stain displayed light and the chondrocyte showed mild hyperplasia. The toluidine blue staining was mildly uneven. Because of death of the rats in the IN group, the experiment in the 9th month could not be completed.
Histological observation of articular cartilage
At the 3rd, 6th, 9th months of treatment after surgically induced animal model of OA, the NS group and CE group showed similar in different periods, which was the progressive development of OA. In the 3rd month, the surface of the stress concentration area was rough, and double-columnar, nested chondrocyte hyperplasia was observed. In the 6th month, the superficial layer of articular cartilage shed, and there was a large amount of chondrocyte hyperplasia. In the 9th month, the superficial layer of the articular cartilage became rougher. The chondrocytes in the NS group decreased, while the chondrocytes in the CE group slightly decreased. To the IN group, in the 3rd month the layer of the cartilage became thinner and appeared villous. Chondrocytes were arranged in irregular clusters. At the 6th month, the changes as mentioned above further progressed, and chondrocyte necrosis occurred. In the IBP group, in the 9th month, the thickness of the articular cartilage became thin and deteriorated. Chondrocytes were arranged irregularly, and necrosis of chondrocytes was reduced.
The expression of type II collagen in chondrocytes
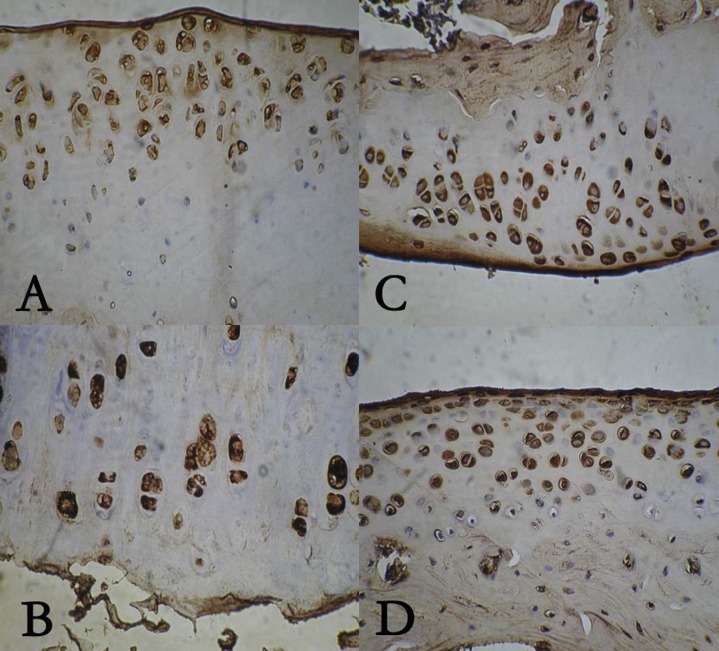
The expression of type II collagen was detected using immunohistochemistry (Figure 1). As shown in Table 1, at the 3rd, 6th and 9th months, IBP promoted the expression of type II collagen, but IN inhibited its expression. CE had no effect on the expression of collagen type II.
Figure 1.
The expression of type II collagen in celecoxib group (A), ibuprofen group (B), indomethacin group (C), normal saline group (D).
Table 1.
The expression of type II collagen in four groups.
| CE | IBP | IN | NS | |
|---|---|---|---|---|
| 3rd month | 0.53±0.11** | 0.54±0.07** | 0.35±0.06 | 0.43±0.10 |
| 6rd month | 0.53±0.04** | 0.66±0.03*,** | 0.39±0.05 | 0.46±0.09 |
| 9rd month | 0.44±0.02 | 0.64±0.15*,*** | 0.39±0.16 |
CE – celecoxib group, n(3M)=9, n(6M)=8, n(9M)=8; IBP – ibuprofen group, n(3M)=8, n(6M)=7, n(9M)=6;p IN – indomethacin group, n(3M)=7, n(6M)=5, n(9M)=0; NS – normal saline group, n(3M)=9, n(6M)=9, n(9M)=8;
p<0.01, compared with NS group;
p<0.01, compared with IN group;
p<0.01, compared with IBP group.
Detection of chondrocyte apoptosis
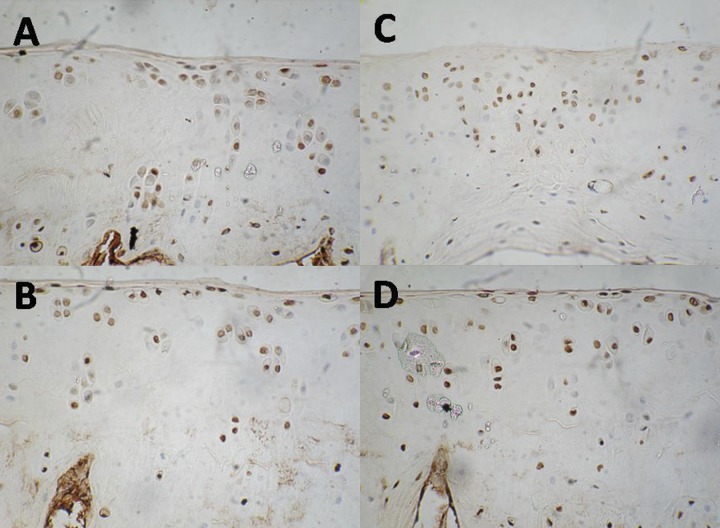
Chondrocyte apoptosis was detected by TUNEL staining (Figure 2). As shown in Table 2, at the 3rd month of treatment, CE suppressed chondrocyte apoptosis, while IBP and IN promoted chondrocyte apoptosis, especially IN. At the 6th month of treatment, CE still inhibited chondrocyte apoptosis. IBP significantly increased chondrocyte apoptosis, but chondrocyte apoptosis maximized at the 3th month of treatment, and then began to reduce. At the 9th month of treatment, CE still inhibited chondrocyte apoptosis. Taken together, CE inhibited chondrocyte apoptosis and retained the degeneration of cartilage, while IN and IBP promoted chondrocyte apoptosis and aggravated the degeneration of cartilage.
Figure 2.
Chondrocyte apoptosis in celecoxib group (A), ibuprofen group (B), indomethacin group (C), normal saline group (D).
Table 2.
Chondrocyte apoptosis in four groups unit: 10-2.
| CE | IBP | IN | NS | |
|---|---|---|---|---|
| 3rd month | 14.1±2.67*,**,*** | 27.9±1.57*,** | 47.4±2.24* | 17.2±1.96 |
| 6rd month | 22.6±2.62*,*** | 50.5±1.92*,** | 20.67±3.73* | 33.1±2.31 |
| 9rd month | 29.7±3.12*,*** | 34.9±3.19* | 44.0±3.40 |
CE – celecoxib group, n(3rd month)=9, n(6th month)=8, n(9 th month)=8; IBP – ibuprofen group, n(3M)=8, n(6 th month)=7, n(9 th month=6; IN – indomethacin group, n(3 rd month)=7, n(6 th month)=5, n(9 th month)=0; NS – normal saline group, n(3 rd month)=9, n(6 th month)=9, n(9 th month)=8;
p<0.01, compared with NS group;
p<0.01, compared with IN group;
p<0.01, compared with IBP group.
The change of chondrocyte ultra-microstructure
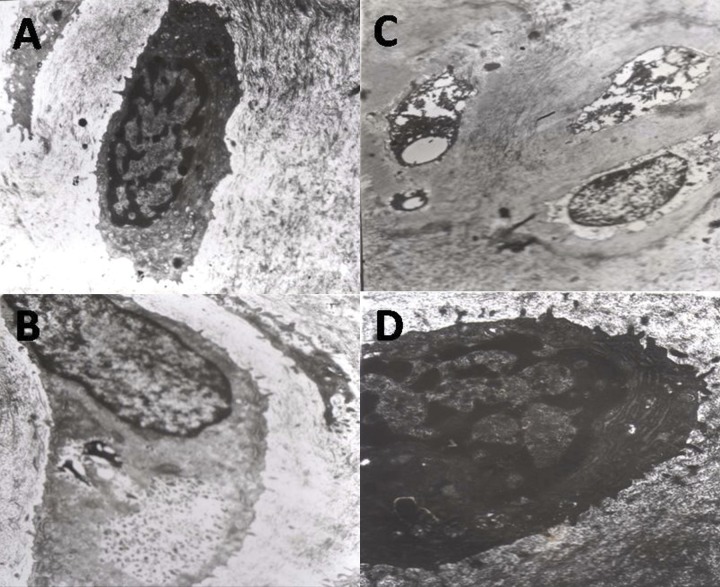
In the NS and CE groups, the chondrocytes of articular cartilage appeared similar. The shape of cells was normal, with integrated nuclear membrane, slight aggregated chromatin and abundant cytoplasm. In the CE group, there were a large number of rough endoplasmic reticulum (RER), mitochondria and glycogen in the chondrocytes. In the IBP group, the perinuclear halo gradually disappeared, cell shape was irregular, chromatin structure was obscure, the number of Golgi complex and rough endoplasmic reticulum reduced and they shrank, and the structure of mitochondria was unclear. There are many microfilament and lysosome-related organelles in the cytoplasm. In the IN group, the electron density of chondrocytes significantly increased, and many had lytic necrosis. It was difficult to recognize the structure of the cytoplasm and nucleus. The structure of collagen fiber in the matrix also became unclear (Figure 3).
Figure 3.
The change of chondrocyte ultra-microstructure in celecoxib group (A), ibuprofen group (B), indomethacin group (C), normal saline group (D).
Discussion
The pathological changes and the chemical indicators of cartilage in rat OA models which are noted in human OA have been widely used in the OA research. We adopted the resection of the tendon of the rats, which decreased the cartilage stress of the same side, and induced the instability of the joint and the irregularity of load conduction, resulting in the degeneration of the articular cartilage and then inducing OA. Two months after surgery, the early degenerative change appeared in the articular cartilage. The pathological changes in the 3rd, 6th and 9th months were similar to the slow pathological development of human OA [16,17]. Surgically-induced OA, which largely mimics the pathological process of OA, represents an ideal platform to study the early pathological changes associated with the disease, as well as being of use in the study on cartilage metabolism response to the drugs.
Recent in vitro and in vivo data on celecoxib have shown positive effects on cartilage of OA [18–21]. These studies showed that celecoxib has favorable effects on the turnover of collagen metabolism of OA cartilage. Type II collagen is the most abundant in articular cartilage. The expression of type II collagen significantly increases in early OA [22,23]. Late in OA, the balance between synthesis and degradation of type II collagen is destroyed and the amount of type II collagen in the cartilage matrix markedly decreases [24]. In our study, at the 3rd, 6th and 9th months of treatment, CE had no effect on the expression of the type II collagen in OA chondrocytes, which indicates CE possibly does not interfere with the metabolism of type II collagen in articular cartilage when it plays a role in anti-inflammatory and analgesics. In contrast, IBP promotes the expression of type II collagen in chondrocytes and increase the synthesis of type II collagen, which compensates for the loss of type II collagen during OA. Moreover, IN inhibits the expression of type II collagen in chondrocytes, decreases the synthesis of type II collagen, and restrains the metabolism of type II collagen in degenerative cartilage.
Accumulating evidences indicates chondrocyte apoptosis may represent an important component in the pathogenesis of OA [3–6]. In our study, a high frequency of chondrocyte apoptosis existed in articular cartilage during OA. Chondrocyte apoptosis is positively associated with degree of cartilage matrix damage, which is consistent with previous reports [25–27]. Moreover, CE suppressed chondrocyte apoptosis and retained the degeneration of articular cartilage, while IN and IBP promoted chondrocyte apoptosis and aggravated the degeneration of articular cartilage. The mechanism by which cell apoptosis is inhibited by CE remains unclear. Mastbergen et al. recently showed a beneficial effect of celecoxib in normal cartilage under the influence of IL-1 and TNF-α, but no effects in normal healthy cartilage [18]. Human articular chondrocytes stimulated with cytokines such as IL-1 or TNF produce high levels of NO [28]. Celecoxib may inhibit chondrocyte apoptosis though decreasing IL-1 or TNF-α-induced NO elevation.
Chondrocytes are the cellular components of cartilage metabolic activity, which is associated with the synthesis and degradation of cartilage matrix. The morphology of chondrocytes is related with metabolic activity and the synthesis of proteoglycan. In our study, CE promoted the number of Golgi complex and rough endoplasmic reticulum, and enlarged their size. The ability of the synthesis of cartilage matrix in chondrocytes was enhanced. The content of proteoglycan increased. IN induced chondrocyte shrinkage and lytic necrosis, and aggravated the degeneration of cartilage. IBP induced the change of chondrocyte ultra microstructure between CE and IN.
Conclusions
In the long-term use of NSAIDs in treatment of OA, CE displays better therapy and toleration, and retained the degeneration of OA cartilage, and may be the ideal choice for treatment of chronic destructive joint disease.
Footnotes
Conflict of interest
The authors all declare they have no conflict of interest.
Source of support: Departmental sources
Reference
- 1.Creamer P, Hochberg MC. Osteoarthritis. Lancet. 1997;350:503–9. doi: 10.1016/S0140-6736(97)07226-7. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Naimark A, Anderson J, et al. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis & Rheumatism. 1987;30(8):914–18. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 3.Blanco FJ, Guitian R, Vazquez-Martul E, et al. Osteoarthritis chondrocytes die by apoptosis: a possible pathway for osteoarthritis pathology. Arthritis & Rheumatism. 1987;42(2):914–18. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto S, Takahashi K, Amiel D, et al. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis & Rheumatism. 1998;41(7):1266–74. doi: 10.1002/1529-0131(199807)41:7<1266::AID-ART18>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Yatsugi N, Tsukazaki T, Osaki M, et al. Apoptosis of articular chondrocytes in rheumatoid arthritis and osteoarthritis: correlation of apoptosis with degree of cartilage destruction and expression of apoptosis-related proteins of p53 and c-myc. J Orthop Sci. 2000;5(2):150–6. doi: 10.1007/s007760050142. [DOI] [PubMed] [Google Scholar]
- 6.Kirscha T, Swobodab B, Nah HD. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Arthritis & Rheumatism. 2000;8(4):294–302. doi: 10.1053/joca.1999.0304. [DOI] [PubMed] [Google Scholar]
- 7.Hamel MB, Toth M, Legedza A, et al. Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee: decision making, postoperative recovery, and clinical outcomes. Arch Intern Med. 2008;168(13):1430–40. doi: 10.1001/archinte.168.13.1430. [DOI] [PubMed] [Google Scholar]
- 8.Ding C. Do NSAIDs affect the progression of osteoarthritis? Inflammation. 2002;26:139–42. doi: 10.1023/a:1015504632021. [DOI] [PubMed] [Google Scholar]
- 9.Olszewska-Słonina DM, Matewski D, Drewa G, et al. Oxidative equilibrium in the prophylaxis of degenerative joint changes: an analysis of pre- and postoperative activity of antioxidant enzymes in patients with hip and knee osteoarthritis. Med Sci Monit. 2010;16(5):CR238–45. [PubMed] [Google Scholar]
- 10.Mastbergen SC, Marijnissen AC, Vianen ME, et al. Inhibition of COX-2 by celecoxib in the canine groove model of osteoarthritis. Rheumatology. 2006;45(4):405–13. doi: 10.1093/rheumatology/kei187. [DOI] [PubMed] [Google Scholar]
- 11.Mastbergen SC, Bijlsma JW, Lafeber FP. Selective COX-2 inhibition is favorable to human early and late-stage osteoarthritic cartilage: a human in vitro study. Osteoarthritis Cartilage. 2005;13(6):519–26. doi: 10.1016/j.joca.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Zweers MC, de Boer TN, van Roon J, et al. Celecoxib: considerations regarding its potential disease-modifying properties in osteoarthritis. Arthritis Res Ther. 2011;13:239–50. doi: 10.1186/ar3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goggs R, Carter SD, Schulze-Tanzil G, et al. Apoptosis and the loss of chondrocyte survival signals contribute to articular cartilage degradation in osteoarthritis. Vet J. 2003;166:140–58. doi: 10.1016/s1090-0233(02)00331-3. [DOI] [PubMed] [Google Scholar]
- 14.Gu Y, Dai KW, Qiu SJ, et al. A morphological study of degenerative mechanism of articular cartilage induced by low stress. Chin J Orthop. 1995;15(9):631–33. [PubMed] [Google Scholar]
- 15.Xu SY, Pian RL, Chen X. The methodology of pharmacological experiment [M] People’s Medical Publishing House; Beijing, China: 2005. p. 406. [Google Scholar]
- 16.Squives GR, OKouneff S, Ionescu M, et al. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthritis Rheum. 2003;48(5):1261–70. doi: 10.1002/art.10976. [DOI] [PubMed] [Google Scholar]
- 17.Lavigne P, Benderdour M, Lajeunesse D, et al. Subchondral and trabecular bone metabolism regulation in canine experimental knee osteoarthritis. Osteoarthritis Cartilage. 2005;13(4):310–17. doi: 10.1016/j.joca.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Hajjaji H, Marcelis A, Devogelaer JP, et al. Celecoxib has a positive effect on the overall metabolism of hyaluronan and proteoglycans in human osteoarthritic cartilage. J Rheumatol. 2003;30:2444–51. [PubMed] [Google Scholar]
- 19.Mastbergen SC, Bijlsma JW, Lafeber FP. Selective COX-2 inhibition is favorable to human early and late-stage osteoarthritic cartilage: a human in vitro study. Osteoarthritis Cartilage. 2005;13:519–26. doi: 10.1016/j.joca.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Mastbergen SC, Lafeber FP, Bijlsma JW. Selective COX-2 inhibition prevents proinflammatory cytokine-induced cartilage damage. Rheumatology. 2002;41:801–8. doi: 10.1093/rheumatology/41.7.801. [DOI] [PubMed] [Google Scholar]
- 21.de Boer TN, Huisman AM, Polak AA, et al. The chondroprotective effect of selective COX-2 inhibition in osteoarthritis: ex vivo evaluation of human cartilage tissue after in vivo treatment. Osteoarthritis Cartilage. 2009;17:482–88. doi: 10.1016/j.joca.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Garnero P, Ayral X, Rousseau JC, et al. Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum. 2002;46(10):2613–24. doi: 10.1002/art.10576. [DOI] [PubMed] [Google Scholar]
- 23.Nelson F, Dahlberg L, Laverty S, et al. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J Clin Invest. 1998;102:2115–25. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen SW, Wei XC, Xiang C, et al. Experimental study of collagen I, II and III in osteoarthritic articular cartilage. Journal of Shanxi Medical University. 2006;37(3):248–51. [Google Scholar]
- 25.Thomas CM, Fuller CJ, Whittles CE, et al. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage. 2007;15(1):27–34. doi: 10.1016/j.joca.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Mistry D, Oue Y, Chambers MG, et al. Chondrocyte death during murine osteoarthritis. Osteoarthritis Cartilage. 2004;12(2):131–41. doi: 10.1016/j.joca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Kusunoki N, Yamazaki R, Kawai S. Induction of Apoptosis in Rheumatoid Synovial Fibroblasts by Celecoxib, but Not by Other Selective Cyclooxygenase 2 Inhibitors. Arthritis Rheumatism. 2002;12:3159–67. doi: 10.1002/art.10692. [DOI] [PubMed] [Google Scholar]
- 28.Mastbergen SC, Lafeber FP, Bijlsma JW. Selective COX-2 inhibition prevents proinflammatory cytokine induced cartilage damage. Rheumatolgy. 2002;41:801–8. doi: 10.1093/rheumatology/41.7.801. [DOI] [PubMed] [Google Scholar]
- 29.Lotz M, Hashimoto S, Kuhn K. Mechanisms of chondrocyte apoptosis. Osteoarthritis Cartilage. 1999;7:389–91. doi: 10.1053/joca.1998.0220. [DOI] [PubMed] [Google Scholar]