Summary
Background
Acrylonitrile (ACN) is an extensively produced aliphatic nitrile. The gastrointestinal tract is an important target organ for ACN toxicity. The objective of the present study was to investigate the role of xanthine oxidase (XO) in ACN-induced gastric toxicity in rats.
Material/Methods
We assessed the effect of ACN on oxidative stress parameters as xanthine oxidase (XO) and total xanthine dehydrogenase (XD)/ XO activity, superoxide anion (O2·−) production, reduced glutathione (GSH) levels and lipid peroxidation in gastric tissues.
Results
A single oral dose of ACN (25 mg/kg) caused a significant enhancement in XO activity. ACN also caused a significant depletion of GSH levels, enhanced O2·− production and increased lipid peroxidation in the time-course experiment. In the dose-response experiment, ACN accelerated the conversion of XD to XO, with a significant depletion of gastric GSH in a dose-related manner. A strong negative correlation existed between the levels of GSH and the percentage enhancement in XO activity (r =−0.997). (O2·−) production and malondialdehyde (MDA) formation were significantly elevated in a dose-related manner. Pretreatment with allopurinol (50 mg/kg) significantly protected against ACN-induced rise in XO activity, depletion of GSH, and elevated production of (O2·−). However, pretreatment with diethyl maleate (DEM; 100 mg/kg) significantly aggravated the ACN-induced GSH depletion and rise in XO activity. Furthermore, DEM significantly enhanced (O2·−) and MDA production.
Conclusions
The present study indicates that enhancement of XO activity could be implicated in ACN-induced gastric damage in rats.
Keywords: acrylonitrile, xanthine oxidase, oxidative stress, gastric damage
Bacground
ACN is an extensively produced aliphatic nitrile [1] used in the synthesis of acrylic fibers, resins and plastics [2,3]. ACN is detected in acrylonitrile-styrene copolymers used in manufacture of kitchen utensils and nitrile-butadiene rubber gloves [4]. It is also used in coating membranes for Langerhans islets implants [5] and high permeable dialysis tubing [6]. ACN has been detected in drinking water [7], food products [8] and car exhaust [9]. ACN has been measured in the vapor phase of mainstream tobacco smoke at a concentration of 18.5 μg per cigarette [10]. In 2007, 94 facilities released a total of about 7 million pounds of ACN, most of which was released by 2 facilities to on-site hazardous waste underground injection wells [11]. ACN is possibly carcinogenic to humans [Group 2B] [2]. Animal studies indicated that ACN possesses mutagenic [12], carcinogenic [13], immunotoxic [14], embryotoxic [15] and neurotoxic properties [16]. Clinical studies indicated increased risk of lung cancer, brain cancer and renal cell carcinoma in some exposure categories 17,18].
The gastrointestinal tract (GIT) represents an important target site for ACN toxicity. ACN was reported to induce hemorrhagic focal superficial gastric mucosal necrosis and erosions in rats [19]. The mechanism of such toxicity was suggested to involve induction of oxidative stress [13]. Initial metabolic activation of ACN was found to be essential for the production of toxic effects [20]. It was reported that CYP 2E1 is the main enzyme in the cytochromes P450 (CYP450) family involved in the bioactivation of ACN [21]. However, the levels of CYP 450 in the GIT are much lower than those present in the liver, which is not the main target for ACN toxicity [22]. Therefore, other metabolic enzymatic and non-enzymatic pathways were suggested to explain the extra-hepatic metabolism of ACN and other nitriles. These include reactive oxygen species (ROS) [23], myeloperoxidase [24] and lactoperoxidase [25]. Reactive oxygen species generated by the xanthine oxidase(XO)/xanthine(X)/iron (Fe) system have been shown to activate the structurally related compound dibromoacetonitrile (DBAN) to cyanide [26].
Xanthine oxidase is highly localized in the GIT [27]. In addition, XO is considered one of the important sources of ROS in the GIT [28]. This enzyme is usually present in the dehydrogenase form, xanthine dehydrogenase (XD), which uses nicotinamide adenine dinucleotide (NAD+) instead of O2 as the electron acceptor. Alternatively, oxidation of substrates by XO results in the generation of superoxide anions (O2·−) and H2O2[29]. It is well recognized that conversion of XD to XO is an important pathway for the production of (O2·−) under some pathological conditions [30]. Several reports have indicated that XO is important in ischemic injury of the intestines, hypovolemic shock, renal transplantation and skin grafts [31,32]. In addition, XO activity increases in hypoxic conditions [33]. Furthermore, several studies have revealed that XO inhibitors such as allopurinol can protect against chemically induced injury in the GIT [34]. This suggests that enhancement of XO activity might be an important determinant in the gastrointestinal toxicity of many chemicals. Therefore, the objective of the present study was to evaluate the role of XO in ACN-induced gastric toxicity in rats.
Material and Methods
Chemicals
ACN, GSH, 5,5Ndithio-bis(2-nitrobenzoicacid), NAD, dithiothreitol (DTT), xanthine, lactate dehydrogenase (LDH), pyruvate, superoxide dismutase (SOD), cytochrome C, allopurinol, diethyl maleate (DEM), 2-thiobarbituric acid, 1,1,3,3-tetraethoxypmpane, phenylmethylsulfonylfluoride (PMSF) and crystalline bovine serum albumin were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All other chemicals were of analytical grade and of the highest purity commercially available.
Animals and animal treatment
Male Sprague-Dawley rats (150–200 g) were obtained from the animal facility of King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. The animals were provided with standard pellet diet and water ad libitum. They were kept at standard living conditions (room temperature of 25±2°C, 45–55% relative humidity and 12 h dark/light cycle). Procedures involving animals and their care were conducted in conformity with the institutional guidelines of King Abdulaziz University, Jeddah, Saudi Arabia.
For the time-course study, 50 rats were randomly assigned, 10 for each time point (6 rats subjected to ACN treatment and 4 rats as a control). Animals were fasted overnight (16 h) prior to receiving any treatment. Treated animals received a single oral dose of ACN (25 mg/kg, dissolved in distilled water). This dose is approximately equivalent to the oral LD12.5 of ACN in rats [35]. Control rats received distilled water. Dosing volume was always 5 ml/kg body weight. Rats were sacrificed by cervical dislocation, at 1, 2, 4, 6 and 12 h after treatment. An abdominal incision was made in each rat and the stomach was immediately dissected out. Tissues were immediately frozen on dry ice and stored at −70°C for subsequent biochemical analyses. For the dose-response study, the same above-mentioned precautions were applied. Twenty-four rats were randomly divided into 4 groups (6 rats in each group). Control animals received distilled water, while treated groups received a single dose of ACN (10, 25 or 50 mg/kg). At 2 h after dosing, animals were sacrificed by cervical dislocation and stomachs were immediately collected and handled as previously described. Based on the results obtained from the time-course and dose-response experiments, the effects of pretreatment with allopurinol or DEM were investigated. Thirty-six rats were equally divided into 6 groups. Control rats were given distilled water. The other rats represented the treated groups. The first treated group received ACN (25 mg/kg), the second group received allopurinol (50 mg/kg) and the third group received allopurinol (50 mg/kg) 1 h prior to ACN (25 mg/kg). The fourth group received DEM (100 mg/kg) and the fifth group was given DEM (100 mg/kg) 1 h prior to ACN (25 mg/kg). All animals wert sacrificed 2 h after the last treatment by cervical dislocation and stomachs were immediately collected. The same aforementioned precautions and handling procedures were applied. Doses of ACN, allopurinol and DEM were chosen based on preliminary experimental trials and were consistent with those in the literature [36,37].
Preparation of tissues
Stomachs were minced into small pieces and divided into 2 portions. The first portion was used for determination of XO and total XO/XD activity. Gastric tissues were homogenized in ice-cold potassium phosphate-EDTA buffer (potassium phosphate 0.05 M, EDTA 0.1 M; pH 7.8) containing 10 mM DTT and 1 mM PMSF. DTT and PMSF were added to prevent any artificial conversion of XD to XO during the homogenization procedure. Homogenization was achieved by 3 5-second bursts over a 5-min period to a fine suspension, using an Ultramax homogenizer. The homogenate was centrifuged at 27000 g for 45 min at 4°C. The supernatant was passed through a Sephadex G-25 (Sigma Chemical Co., MO, USA) to remove any endogenous inhibitors.
The second portion of the minced stomachs was homogenized with ice-cold potassium phosphate-EDTA buffer (potassium phosphate 0.05 M, EDTA 0.1 M; pH 7.8). Homogenization was achieved by 3 5-second bursts over a 5-min period to a fine suspension, using an Ultramax homogenizer. The homogenate was centrifuged at 2500 g for 5 min at 4°C. The supernatant was used for determination of GSH content, (O2·−) production and thiobarbituric acid reactive substances (TBARS) formation. Protein concentration was always determined parallel to any estimation in either type of supernatants.
Assay of XO and total XD/XO activity
Briefly, 400 μl of each sample was incubated at 37°C for 3 h with xanthine (100 μM) to measure XO, or with xanthine and NAD+ (300 μM), LDH (70 U) and pyruvate (1.75 mM) to measure total XO and XD activity. The reactions were stopped by adding perchloric acid and the precipitate was removed by centrifugation. Uric acid content of the supernatant was determined spectrophotometrically at 295 nm. XO activity was expressed either as mU/mg protein or as percentage of the total XO/XD activity. One unit of XO is defined as the enzyme activity required for the formation of 1 μmol of uric acid per min [38].
Assay of (O2·−)
Generation of (O2·−) was measured by the SOD-inhibited reduction of ferricytochrome C [39]. The supernatant (200 μI) of each sample was incubated with xanthine (50 μM) and cytochrome C (10 μM) at 37°C for 15 min. Allopurinol (1 mM) was added to stop the reaction. The generation of (O2·−) was detected by measuring the reduction of cytochrome C at 550 nm. The reaction was terminated by centrifugation at 12 000 × g at 4°C for 4 min. The difference in absorbance of the supernatant fluid in the presence or absence of SOD was determined in a spectrophotometer at 550 nm and the amount of reduced ferricytochrome C was calculated based on an extinction coefficient of 21.0 mM/cm.
Determination of reduced glutathione (GSH)
The levels of GSH in gastric tissues were determined by measuring total soluble sulfhydryl content, using 5,5′-dithio-bis(2-nitrobenzoic acid). Samples were prepared for non-protein fraction using equal volumes of 10% trichloroacetic acid solution. The protein precipitate was removed by centrifugation at 10 000 × g at 4°C for 10 min. The supernatants were spectrophotometrically assayed for sulfhydryl concentration [40].
Determination of lipid peroxidation
Lipid peroxidation was determined colorimetrically by measuring the tissue content of TBARS [41]. Briefly, 1,1,3,3-tetraethoxypropane in water was used to produce different concentrations of malondialdehyde (MDA); these solutions were used as TBARS standards. Thiobarbituric acid (TBA) and 1% H3PO4 were added to homogenates and heated to boiling for 45 min. TBA adducts were extracted with n-butanol. The TBARS concentrations were determined spectrophotometrically by measuring the absorbance at 535 nm.
Determination of protein concentration
Protein concentration of enzyme samples was determined using bovine serum albumin as a standard [42].
Data analysis
The GraphPad Prism version 4 (GraphPad Software, Inc. La Jolla, CA, USA) computer program was used to conduct regression analysis and to plot collected data. Data are expressed as mean ±SD. Assessment of the results was performed using one-way ANOVA procedure followed by Bonferroni multiple comparison test using Software GraphPad InStat 3 (GraphPad Software, Inc. La Jolla, CA, USA). The 0.05 level of probability was used as the criterion for significance.
Results
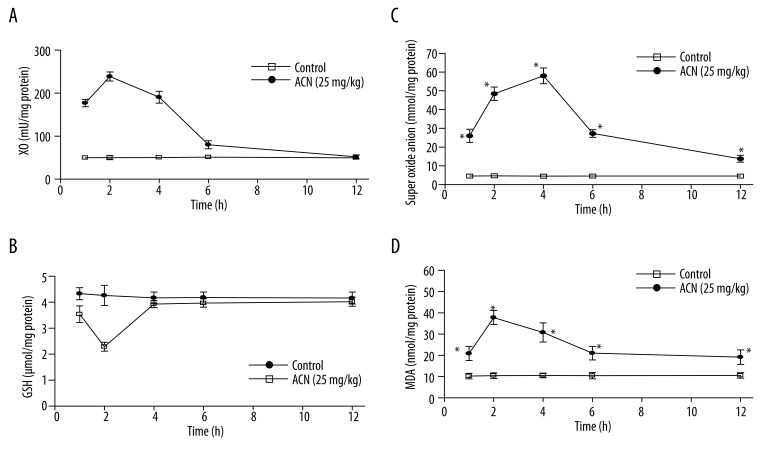
The results from the time-course study of the effect of ACN on the enzymatic activity of XO and the oxidative status of the gastric tissues are shown in Figure 1. Oral administration of ACN (25 mg/kg) caused a significant enhancement in XO activity in gastric tissues (Figure 1A). Maximum XO activity was observed at 2 h after ACN treatment and amounted to 476% of the control enzymatic activity. However, XO activity was almost normal at 12 h after ACN treatment. GSH levels throughout the time-course experiment are depicted in Figure 1B. It was obvious that ACN caused a statistically significant depletion in GSH levels at 1 and 2 h after exposure to ACN. Maximum depletion was observed at 2 h (53.7% of the control value). However, gastric GSH levels returned to control value at 6 and 12 h. (O2·−) production is illustrated in Figure 1C. It was significantly enhanced at all time points and reached its maximum value at 4 h (an approximately 13-fold increase compared to the corresponding control value). A similar pattern was observed when assessing lipid peroxidation in gastric tissues (Figure 1D). Lipid peroxidation was significantly increased at almost all time points. The highest level of TBARS (determined as MDA) was observed at 2 h (an approximately 4-fold increase compared to the corresponding control value).
Figure 1.
Time-course effect of ACN (25 mg/kg) on XO (A), GSH level (B), O2·− production (C) and lipid peroxidation (D) in rat stomach. * Significantly different from corresponding control group at p<0.05.
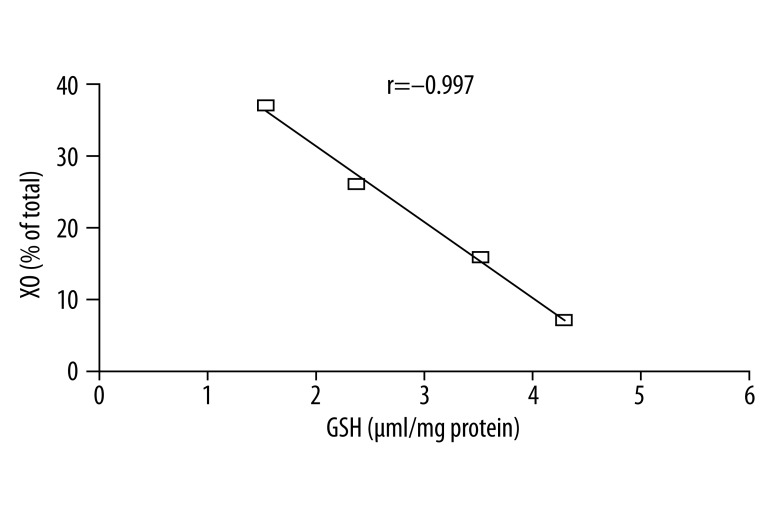
Based on the data obtained from the time-course experiment, a dose-response experiment was performed. ACN was tested at doses of 10, 25 and 50 mg/kg and animals were sacrificed at 2 h after ACN treatment. The results of the dose-response experiment are shown in Table 1. XO activity was significantly increased by administration of ACN. The highest dose of ACN (50 mg/kg) accelerated the conversion of XD to XO as the enzymatic activity of XO was elevated up to 36.99% compared with a value of 7.09% of the total XD/XO activity in control rats. ACN caused a significant depletion of gastric GSH in a dose-related manner. A strong negative correlation existed between the levels of GSH and the percentage enhancement in XO activity (Figure 2). The correlation coefficient was −0.997. (O2·−) production was drastically elevated in a dose-related manner. The highest dose of ACN caused an approximately 16-fold increase in (O2·−) production and a 5-fold increase in lipid peroxidation as compared to the control value.
Table 1.
Dose-response effects of ACN on XD-XO conversion, GSH, (O2·−) production and TBARS in rat stomach.
| XO % of total | GSH μmol/mg protein | (O2·−) nmol/mg protein | TBARS nmol/mg protein | |
|---|---|---|---|---|
| Control | 7.09±0.47 | 4.30±0.40 | 4.81±0.33 | 0.99±0.14 |
| ACN (10 mg/kg) | 15.86*±0.76 | 3.53*±0.35 | 22.21*±1.75 | 2.34*±0.27 |
| ACN (25 mg/kg) | 26.03*±1.18 | 2.39*±0.30 | 47.64*±2.96 | 3.36*±0.31 |
| ACN (50 mg/kg) | 36.99*±2.01 | 1.55*±0.27 | 75.62*±4.71 | 4.49*±0.29 |
Animals were sacrificed 2 h after treatment, n=6. Data were presented as mean ±SD.
Significantly different from the corresponding control group at p<0.05.
Figure 2.
Linear correlation between GSH depletion by different doses of ACN and XO activity in rat stomach.
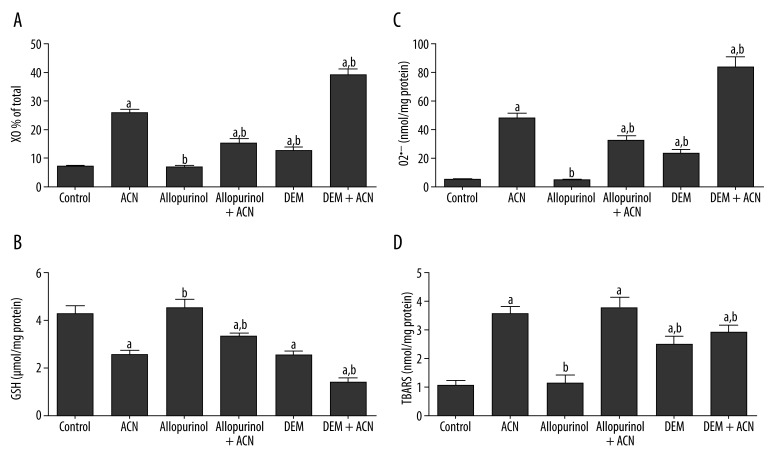
To substantiate the role of XO and GSH depletion in ACN-induced toxicity, a third experiment was performed. This experiment was an extension of the dose-response experiment. ACN was used as a single oral dose of 25 mg/kg and rats were sacrificed 2 h later. The data in Figure 3 indicate the impact of modulating XO activity or GSH level on ACN toxicity. XO activity was significantly inhibited by prior administration of allopurinol (50 mg/kg). Allopurinol alone did not affect any of the assessed parameters. It significantly protected against the rise in XO activity compared to the ACN alone-treated group. Allopurinol could also inhibit GSH depletion and (O2·−) production compared to the ACN alone-treated group. However, allopurinol did not exhibit any significant protection against ACN-induced increase in MDA formation. DEM (100 mg/kg) significantly enhanced XO activity, depleted GSH and increased (O2·−) and MDA production. Pretreatment with DEM significantly aggravated the toxic manifestations of ACN toxicity. Of special concern, XO activity was elevated from 26.04% (ACN-alone group) to 39.20% (DEM + ACN group) of the total XO/XD activity. Simultaneously, an almost 2-fold increase in (O2·−) production was observed in the DEM + ACN group compared to the can-alone group.
Figure 3.
Effects of pretreatment with allopurinol or DEM on ACN-induced changes in XD-XO conversion (A), GSH (B), (O2·−) production (C) and TBARS in rat stomach (D). Animals were sacrificed 2 h after treatment, n=6. Data were presented as mean ±SD In combined treatment groups, either allopurinol or DEM was given 1 h before CAN. a Significantly different from the corresponding control group at p<0.05; b significantly different from the corresponding ACN-treated group at p<0.05.
Discussion
Oxidative stress and generation of ROS mediate cellular injury in the GIT. One important source of the cytotoxic ROS is XO [43]. Previous studies have indicated that GIT is the primary target of ACN toxicity in experimental animals [13]. The mechanism of toxicity of the structurally related compound, DBAN, was shown to involve activation of XO [26]. Therefore, the present work was designed to evaluate the potential role of XO in ACN-induced gastric toxicity in rats.
The toxic insult of ACN in gastric tissues was examined in a time-course experiment and in a dose-response experiment. The obtained results suggest that ACN toxicity in the stomach is associated with a significant elevation in XO activity. It has been reported that ACN is bioactivated in vivo to CN−[2], causing a condition of cytotoxic hypoxia which eventually leads to decreased energy production. Hypoxia due to ischemia has been shown to be associated with rapid conversion of XD to XO in gastrointestinal tissues [30,44]. Under normal physiological conditions, this enzyme is present in the dehydrogenase form, which uses NAD+ instead of O2 as the electron acceptor [29]. Thus, the elevated XO activity can be explained based on the ACN-induced hypoxia in gastric tissues.
Gastrointestinal tissues, like other tissues, possess different protective antioxidant scavengers and enzymes. GSH represents one of the most important cellular defenses against oxidative insult [45]. The present study revealed that ACN treatment caused marked GSH depletion in rat stomach. This is in agreement with the known GSH-depleting properties of ACN [2]. ACN can be metabolized by glutathione transferase (GST)-mediated conjugation to GSH, where it is eliminated as mercapturic acid derivatives [46]. Previous studies with 14C ACN have shown that ACN covalently binds to thiol groups of proteins [47] and tissue macromolecules and nucleic acids [48]. Thus, GSH-depleting properties of ACN could be attributed to enzymatic conjugation and/or direct binding with thiol groups, which in turn resulted in enhanced lipid peroxidation. Importantly, a strong negative correlation was observed between XO activity and GSH level in the dose-response experiment, suggesting that GSH depletion may activate conversion of XD to XO in gastric tissues.
ACN toxicity was accompanied by increased generation of (O2·−). This finding can be attributed to the ACN-induced hypoxia and enhancement of XO activity. XO, in the presence of xanthine, reduces O2 to (O2·−) [49]. Other potent oxidants can also be generated as by-products of this reduction [50]. This process is greatly enhanced in hypoxia, with increased oxygen free radicals production [51]. In addition, the data indicate that ACN toxic insult resulted in significant enhancement of lipid peroxidation processes in gastric tissues. This is consistent with the well-known ability of ACN to increase lipid peroxidation in rat liver [52]. The observed ACN-induced GSH depletion may provide a satisfactory explanation of this finding. Thus, “vicious cycle” could be a better description of the mutual influence between GSH depletion and XO conversion.
The increase in XO activity could represent an important source of oxidants involved in ACN-induced toxicity in gastric tissues and this possibility was further substantiated. Allopurinol, a XO inhibitor [53], was given to animals prior to ACN in a dose-response experiment. Allopurinol provided significant protection against most of the toxic manifestations of ACN insult. However, allopurinol did not exhibit any significant protection against the increased lipid peroxidation induced by ACN. This suggests that lipid peroxidation is an early step in ACN toxicity and occurs before the enhanced XO activity. This suggestion is supported by results of previous studies reporting that changes in lipid peroxidation markers occur earlier than conversion of XD to XO in hypoxic cells [44].
The role of GSH depletion was further evaluated in the process of XO activation. Rats were pretreated with the GSH-depleting agent DEM. This dramatically enhanced the conversion of XD to XO and aggravated all the toxic manifestation of ACN. These data were supported by the observation that GSH depletion enhanced conversion of XD to XO in rat liver [37]. The exact mechanism of this conversion is still unclear; however, GSH depletion seems to be a critical step in ACN-induced conversion of gastric XD to XO. However, a previous in vitro study indicated that sulfhydryl compounds as NAC and GSH significantly enhanced the rate of CN− formation from a structurally related nitrile. This has been correlated with a significant increase in the production of •OH [54]. The enhancing effect of sulfhydryl compounds was attributed to their ability to reduce Fe3+ to Fe2+[55,56]. Further support for this idea was provided by Wefers and Sies [57], who investigated the superoxide-dependent oxidation of GSH in the xanthine-XO system. It was suggested that the interaction of O2·− with GSH results in the production of a glutathione thiol radical (GS•) and H2O2. This further suggests the “vicious cycle” concept.
Conclusions
In present study indicates that enhancement of XO activity is implicated in ACN-induced gastric damage in rats.
Footnotes
Source of support: Departmental sources
References
- 1.Reisch MS. Top 50 chemicals production stagnated last year. Chem Eng News. 1992;70:16–22. [Google Scholar]
- 2.IARC. Monographs on the evaluation of carcinogenic risks to humans. Re-evaluation of some organic chemicals, hydrazine, and hydrogen peroxide. Compounds reviewed in plenary sessions (comprehensive monographs) 1999;71(1):43. [PMC free article] [PubMed] [Google Scholar]
- 3.CEN. Facts & figures of the chemical industry. Chem Eng News. 2009;87:29–63. [Google Scholar]
- 4.Ohno H, Kawamura Y. Analysis of acrylonitrile, 1,3-butadiene, and related compounds in acrylonitrile-butadiene-styrene copolymers for kitchen utensils and children’s toys by headspace gas chromatography/mass spectrometry. J AOAC Int. 2010;93:1965–71. [PubMed] [Google Scholar]
- 5.Kessler L, et al. Diffusion properties of an artificial membrane used for Langerhans islets encapsulation: interest of an in vitro test. Transplant Proc. 1992;24:953–54. [PubMed] [Google Scholar]
- 6.Ward RA, et al. Biocompatibility of a new high-permeability modified cellulose membrane for haemodialysis. Nephrol Dial Transplant. 1993;8(1):47–53. doi: 10.1093/oxfordjournals.ndt.a092271. [DOI] [PubMed] [Google Scholar]
- 7.Rubio R, Galceran MT, Rauret G. Nitrites and isonitriles as interferents in cyanide determination in polluted water. Analyst. 1990;115(7):959–63. doi: 10.1039/an9901500959. [DOI] [PubMed] [Google Scholar]
- 8.Conacher HB, Page BD, Ryan JJ. Industrial chemical contamination of foods. Food Addit Contam. 1993;10(1):129–43. doi: 10.1080/02652039309374136. [DOI] [PubMed] [Google Scholar]
- 9.Kondakova LV, Fomina EI, Alekseeva OV. Composition of volatile thermo-destruction components of automobile brake straps. Gig Tr Prof Zabol. 1990;(3):18–21. [PubMed] [Google Scholar]
- 10.Laugesen M, Fowles J. Scope for regulation of cigarette smoke toxicity according to brand differences in toxicant emissions. N Z Med J. 2005;118:U1402. [PubMed] [Google Scholar]
- 11.TRI. TRI Explorer Chemical Report. U.S. Environmental Protection Agency; 2009. http://www.epa.gov/triexplorer and select Acrylonitrile. Last updated 2/09. [Google Scholar]
- 12.Duverger-Van Bogaert M, et al. Effect of several factors on the liver extract mediated mutagenicity of acrylonitrile and identification of four new in vitro metabolites. Toxicol Lett. 1981;7(4–5):311–19. doi: 10.1016/0378-4274(81)90054-0. [DOI] [PubMed] [Google Scholar]
- 13.Ghanayem BI, et al. Acrylonitrile is a multisite carcinogen in male and female B6C3F1 mice. Toxicol Sci. 2002;68:59–68. doi: 10.1093/toxsci/68.1.59. [DOI] [PubMed] [Google Scholar]
- 14.Hamada FM, et al. Possible functional immunotoxicity of acrylonitrile (VCN) Pharmacol Res. 1998;37(2):123–29. doi: 10.1006/phrs.1997.0264. [DOI] [PubMed] [Google Scholar]
- 15.Saillenfait AM, et al. Modulation of acrylonitrile-induced embryotoxicity in vitro by glutathione depletion. Arch Toxicol. 1993;67(3):164–72. doi: 10.1007/BF01973303. [DOI] [PubMed] [Google Scholar]
- 16.Schaffer CB. Conference Proceedings on Environmental Aspects of Chemical Use in Rubber Processing and Operation; 1975; US Environmental Protection Agency, Washington, DC. [Google Scholar]
- 17.Swaen GM, et al. Mortality update of workers exposed to acrylonitrile in The Netherlands. J Occup Environ Med. 2004;46:691–98. doi: 10.1097/01.jom.0000128161.17144.27. [DOI] [PubMed] [Google Scholar]
- 18.Karami S, et al. Renal cancer risk and occupational exposure to polycyclic aromatic hydrocarbons and plastics. J Occup Environ Med. 2011;53:218–23. doi: 10.1097/JOM.0b013e31820a40a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghanayem BI, Boor PJ, Ahmed AE. Acrylonitrile-induced gastric mucosal necrosis: role of gastric glutathione. J Pharmacol Exp Ther. 1985;232(2):570–77. [PubMed] [Google Scholar]
- 20.Léonard A, et al. Mutagenicity, carcinogenicity, and teratogenicity of acrylonitrile. Mutat Res. 1999;436:263–83. doi: 10.1016/s1383-5742(99)00006-x. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Chanas B, Ghanayem BI. Cytochrome P450 2E1 (CYP 2E1) is essential for acrylonitrile metabolism to cyanide: comparative studies using CYP2E1-null and wild-type mice. Drug Metab Dispos. 2002;30:911–17. doi: 10.1124/dmd.30.8.911. [DOI] [PubMed] [Google Scholar]
- 22.Park BK, Pirmohamed M, Kitteringham NR. The role of cytochrome P450 enzymes in hepatic and extrahepatic human drug toxicity. Pharmacol Ther. 1995;68:385–424. doi: 10.1016/0163-7258(95)02013-6. [DOI] [PubMed] [Google Scholar]
- 23.Mohamadin AM. Possible role of hydroxyl radicals in the oxidation of dichloroacetonitrile by Fenton-like reaction. J Inorg Biochem. 2001;84(1–2):97–105. doi: 10.1016/s0162-0134(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Naim AB, Mohamadin AM. Myeloperoxidase-catalyzed oxidation of chloroacetonitrile to cyanide. Toxicol Lett. 2004;146:249–57. doi: 10.1016/j.toxlet.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Nasralla SN, et al. Lactoperoxidase catalyzes in vitro activation of acrylonitrile to cyanide. Toxicol Lett. 2009;191:347–52. doi: 10.1016/j.toxlet.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Mohamadin AM, Abdel-Naim AB. In vitro activation of dibromoacetonitrile to cyanide: role of xanthine oxidase. Arch Toxicol. 2003;77(2):86–93. doi: 10.1007/s00204-002-0418-7. [DOI] [PubMed] [Google Scholar]
- 27.Moriwaki Y, et al. Immunohistochemical localization of aldehyde and xanthine oxidase in rat tissues using polyclonal antibodies. Histochem Cell Biol. 1996;105(1):71–79. doi: 10.1007/BF01450880. [DOI] [PubMed] [Google Scholar]
- 28.Grisham MB, Granger DN. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988;33(3 Suppl):6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- 29.Parks DA, Granger DN. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- 30.Wakabayashi Y, et al. Conversion of xanthine dehydrogenase to xanthine oxidase in bovine carotid artery endothelial cells induced by activated neutrophils: involvement of adhesion molecules. Biochim Biophys Acta. 1995;1265(2–3):103–9. doi: 10.1016/0167-4889(94)00202-p. [DOI] [PubMed] [Google Scholar]
- 31.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. New Engl J Med. 1985;312:159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 32.Hakgüder G, et al. Short-term intestinal ischemia-reperfusion alters intestinal motility that can be preserved by xanthine oxidase inhibition. Dig Dis Sci. 2002;47:1279–83. doi: 10.1023/a:1015314312730. [DOI] [PubMed] [Google Scholar]
- 33.Terada LS, et al. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc Natl Acad Sci USA. 1992;89:3362–66. doi: 10.1073/pnas.89.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamada FMA, et al. A new therapeutic use of allopurinol: the drug protects the gastric mucosa from the ulcerogenic effect of alcohol. Arab J Lab Med. 1992;18(2):283–92. [Google Scholar]
- 35.Woutersen RA. Toxicologic profile of acrylonitrile. Scand J Work Environ Health. 1998;24(Suppl 2):5–9. [PubMed] [Google Scholar]
- 36.Shaw S, Jayatilleke E. The role of aldehyde oxidase in ethanol-induced hepatic lipid peroxidation in the rat. Biochem J. 1990;268(3):579–83. doi: 10.1042/bj2680579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cighetti G, Debiasi S, Paroni R. Effect of glutathione depletion on the conversion of xanthine dehydrogenase to oxidase in rat liver. Biochem Pharmacol. 1993;45(11):2359–61. doi: 10.1016/0006-2952(93)90213-g. [DOI] [PubMed] [Google Scholar]
- 38.Waud WR, Rajagopalan KV. Purification and properties of the NAD+-dependent (type D) and O2-dependent (type O) forms of rat liver xanthine dehydrogenase. Arch Biochem Biophys. 1976;172(2):354–64. doi: 10.1016/0003-9861(76)90087-4. [DOI] [PubMed] [Google Scholar]
- 39.Johnston RBJ. Measurement of Or secreted by monocytes and macrophages. Methods Enzymol. 1984;105:365–69. doi: 10.1016/s0076-6879(84)05049-7. [DOI] [PubMed] [Google Scholar]
- 40.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 41.Uchiyama M, Mihara M. Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–78. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 42.Lowry OH, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75. [PubMed] [Google Scholar]
- 43.Smith SM, et al. Gastric mucosal injury in the rat. Role of iron and xanthine oxidase. Gastroenterology. 1987;92(4):950–56. doi: 10.1016/0016-5085(87)90969-3. [DOI] [PubMed] [Google Scholar]
- 44.Hasan NM, Cundall RB, Adams GE. Effects of hypoxia and reoxygenation on the conversion of xanthine dehydrogenase to oxidase in Chinese hamster V79 cells. Free Radic Biol Med. 1991;11(2):179–85. doi: 10.1016/0891-5849(91)90169-4. [DOI] [PubMed] [Google Scholar]
- 45.Miester A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 46.Fennell TR, Kedderis GL, Sumner SC. Urinary metabolites of [1,2,3-13C]acrylonitrile in rats and mice detected by 13C nuclear magnetic resonance spectroscopy. Chem Res Toxicol. 1991;4(6):678–87. doi: 10.1021/tx00024a013. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed AE, et al. Distribution and covalent interactions of [1–14C]acrylonitrile in the rat. Toxicology. 1982;23(2–3):159–75. doi: 10.1016/0300-483x(82)90095-6. [DOI] [PubMed] [Google Scholar]
- 48.Pilon D, Roberts AE, Rickert DE. Effect of glutathione depletion on the irreversible association of acrylonitrile with tissue macromolecules after oral administration to rats. Toxicol Appl Pharmacol. 1988;95(2):311–20. doi: 10.1016/0041-008x(88)90167-6. [DOI] [PubMed] [Google Scholar]
- 49.McCord JM, Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968;243(21):5753–60. [PubMed] [Google Scholar]
- 50.Winterbourn CC, Sutton HC. Iron and xanthine oxidase catalyze formation of an oxidant species distinguishable from OH.: comparison with the Haber-Weiss reaction. Arch Biochem Biophys. 1986;244(1):27–34. doi: 10.1016/0003-9861(86)90090-1. [DOI] [PubMed] [Google Scholar]
- 51.Nilsson UA, et al. Detection of oxygen radicals during reperfusion of intestinal cells in vitro. Free Radic Biol Med. 1989;6(3):251–59. doi: 10.1016/0891-5849(89)90052-x. [DOI] [PubMed] [Google Scholar]
- 52.Guangwei X, et al. Curcumin pretreatment protects against acute acrylonitrile-induced oxidative damage in rats. Toxicology. 2010;267(1–3):140–46. doi: 10.1016/j.tox.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Granger DN, et al. Xanthine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterology. 1986;90(1):80–84. doi: 10.1016/0016-5085(86)90078-8. [DOI] [PubMed] [Google Scholar]
- 54.Mohamadin AM, Abdel-Naim AB. In vitro activation of dibromoacetonitrile to cyanide: role of xanthine oxidase. Arch Toxicol. 2003;77:86–93. doi: 10.1007/s00204-002-0418-7. [DOI] [PubMed] [Google Scholar]
- 55.Reed CJ, Douglas KT. Chemical cleavage of plasmid DNA by glutathione in the presence of Cu(II) ions. The Cu(II)-thiol system for DNA strand scission. Biochem J. 1991;275:601–8. doi: 10.1042/bj2750601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujimoto S, Kawakami N, Ohara A. Hydroxylation of phenylalanine and salicylate by stimulated polymorphonuclear leukocytes and the accelerating effect of glutathione on their hydroxylation. Biol Pharm Bull. 1997;17:767–72. doi: 10.1248/bpb.17.767. [DOI] [PubMed] [Google Scholar]
- 57.Wefers H, Sies H. Oxidation of glutathione by the superoxide radical to the disulfide and the sulfonate yielding singlet oxygen. Eur J Biochem. 1983;137:29–36. doi: 10.1111/j.1432-1033.1983.tb07791.x. [DOI] [PubMed] [Google Scholar]