Summary
Background
Recent studies show that molecular hydrogen (dihydrogen, H2) has potential as an effective and safe radioprotective agent through reducing oxidative stress. The aim of this study was to investigate whether H2 is able to protect spermatogenesis and hematopoiesis from radiation-induced injuries.
Material/Methods
H2 was dissolved in physiological saline using an apparatus produced by our department. 60Co-gamma rays in the irradiation centre were used for irradiation. Spermatid head counts and histological analysis were used to evaluate spermatogenesis. Endogenous hematopoietic spleen colony formation (endoCFUs), bone marrow nucleated cells (BMNC) and peripheral blood (PB) leukocytes were used to evaluate hemopoiesis.
Results
This study demonstrates that treating mice with H2 before ionizing radiation (IR) can increase the spermatid head count and protect seminiferous epithelium from IR. This study also demonstrates that H2 could significantly increase the number of endoCFUs, BMNC and PB leukocyte.
Conclusions
This study suggests that hydrogen-rich saline could partially protect spermatogenesis and hematopoiesis in irradiated mice.
Keywords: ionizing radiation, radioprotection, spermatogenesis, hematopoiesis, hydrogen
Background
Spermatogenesis is a compound process of the germ cell of male proliferation and maturation from spermatogonia to spermatozoa [1]. Spermatogenesis is especially sensitive to ionizing radiation (IR); doses as low as 0.1 Gy are known to cause damage to spermatogonia [2–4]. Less than about 2% of men who received total-body IR are able to father children later in life [5]. The hematopoietic system is also known to be sensitive to IR, and myelosuppression is a critical issue for individuals exposed to IR [6]. Hemorrhage and infection are serious complications in hematopoietic syndrome. Hematopoietic transplantation is often performed in irradiated victims but has resulted in low efficacy in treatment of the syndrome [7,8].
In major part, detrimental effects of IR on biological tissue are mediated via increased production of hydroxyl radicals (•OH). Ohsawa et al. [9] found that hydrogen (H2) could selectively reduce •OH and peroxynitrite radicals (ONOO−) in vitro and exert therapeutic antioxidant activity in a rat middle cerebral artery occlusion model. We demonstrated that H2 treatment could protect cultured human cells and the gastrointestinal tract from gamma radiation in mice [10,11]. Terasaki et al. [12] demonstrated H2 treatment could attenuate radiation pneumonitis in mice. These encouraging results prompted us to study if H2 would be able to protect spermatogenesis and hematopoiesis from IR.
Material and Methods
Hydrogen-rich saline production
H2 was dissolved in physiological saline for 6 h under high pressure (0.4 MPa) to a supersaturated level using a hydrogen-rich water-producing apparatus produced by our department. The saturated H2 saline was stored under atmospheric pressure at 4°C in an aluminium bag with no dead volume. Hydrogen-rich saline was freshly prepared every week, which ensured that a concentration of more than 0.6 mmol/L was maintained. Gas chromatography was used to confirm the content of H2 in saline by the method described by Ohsawa et al. [9].
Irradiation
60Co-gamma rays in the irradiation centre (Faculty of Naval Medicine, Second Military Medical University, China) were used for irradiation. Mice (with or without H2 pre-treatment) were exposed to different doses of radiation, depending upon the requirement of the present study.
Mice and treatment
All protocols were approved by the Second Military Medical University, China in accordance with the Guide for Care and Use of Laboratory Animals published by the US NIH (publication No. 96-01). Male BALB/c mice (8 weeks old, weighing 22±1 g) were used in the experiments. The animals were housed in individual cages in a temperature-controlled room with a 12 h light/dark cycle and food and water were provided ad libitum. For experiments, mice were treated intraperitoneally (IP) with physiological saline or hydrogen-rich saline (5 ml/kg) 5 min before radiation. Mice were irradiated in a holder designed to immobilize anaesthetized mice such that the abdomens were presented to the beam.
Sample retrieval for evaluation of spermatogenesis
For evaluating spermatogenesis and detecting dose-response effects of testicular γ-rays [1,13,14], mice were sacrificed by cervical dislocation under isoflurane anaesthesia at 29 d after irradiation. Body weight and testis weight were recorded. The left testis was stored at −20°C for spermatid head counts. The right testis was fixed in Bouin’s fluid for histological analysis.
Spermatid head counts
For testicular spermatid head counting, the testes were thawed at room temperature and then the tunica albuginea was removed. The testicular parenchyma was homogenized in 2 ml of 0.9% NaCl solution containing 0.1% Triton X-100, and the homogenized tissue was sonicated. After homogenization and sonication, an aliquot of the cell suspension was loaded on a hemocytometer and sonication-resistant spermatid heads were counted [15]. Number of spermatid heads in the testis was expressed as testicular spermatid number per gram testis (TSN).
Histological analysis
For histological analysis, the right testis was embedded in paraffin, sectioned to 4-μm-thick and stained with hematoxylin and eosin. The quality of the seminiferous epithelium was evaluated in each sample on the basis of the Johnsen score (JS), which gives a score of 1–10 according to the presence or absence of the spermatogenic cell types [16]. JS for assessing the spermatogenesis depends on scoring each seminiferous tubule cross-section. The criteria are as follows: 10, complete spermatogenesis; 9, many spermatozoa present but disorganized spermatogenesis; 8, only a few spermatozoa present; 7, no spermatozoa but many spermatids present; 6, only a few spermatids present; 5, no spermatozoa or spermatids present but many spermatocytes present; 4, only a few spermatocytes present; 3, only spermatogonia present; 2, no germ cell present; and 1, no germ cell or Sertoli cell present. Mean score count was given to each sample. The tubule diameter was measured using a micrometer scale, the smallest diameter of 25 different, randomly selected round cross-sections of seminiferous tubules were measured for each sample. The average tubule diameter for each sample was calculated as a mean of these 25 values [17].
Endogenous hematopoietic spleen colony formation
For detecting endogenous hematopoietic spleen colony formation (endoCFUs), mice were killed 11 d after exposure to 7Gy of irradiation as described previously [18]. Body weight and spleen weight were recorded. Spleens were fixed in Bouin’s fluid for 24 h, and then the colonies on the surface of the spleens were scored.
Bone marrow nucleated cells
After exposure to 4Gy of irradiation, bone marrow (BM) cells were obtained from anesthetized mice by aseptic isolation of the femurs followed by a flushing of the marrow with RPMI 1640 medium, using a 25-gauge needle, and single cell suspensions were made as described previously [19]. BM cells were counted immediately using a hemocytometer.
Leukocyte counts
Peripheral blood (PB) samples were obtained from the tail vein of mice at different days after irradiation as described previously [19]. Numbers of PB leukocyte were determined with a hemocytometer.
Statistical analysis
Data are expressed as means ±SEM for each experiment. The number of samples is indicated in the description of each experiment. Statistical analysis was performed by using an analysis of variance (ANOVA). Between groups, variance was determined using the Student-Newman-Keuls post-hoc test. A p-value of less than 0.05 was considered to be statistically significant.
Results
Organ index after irradiation
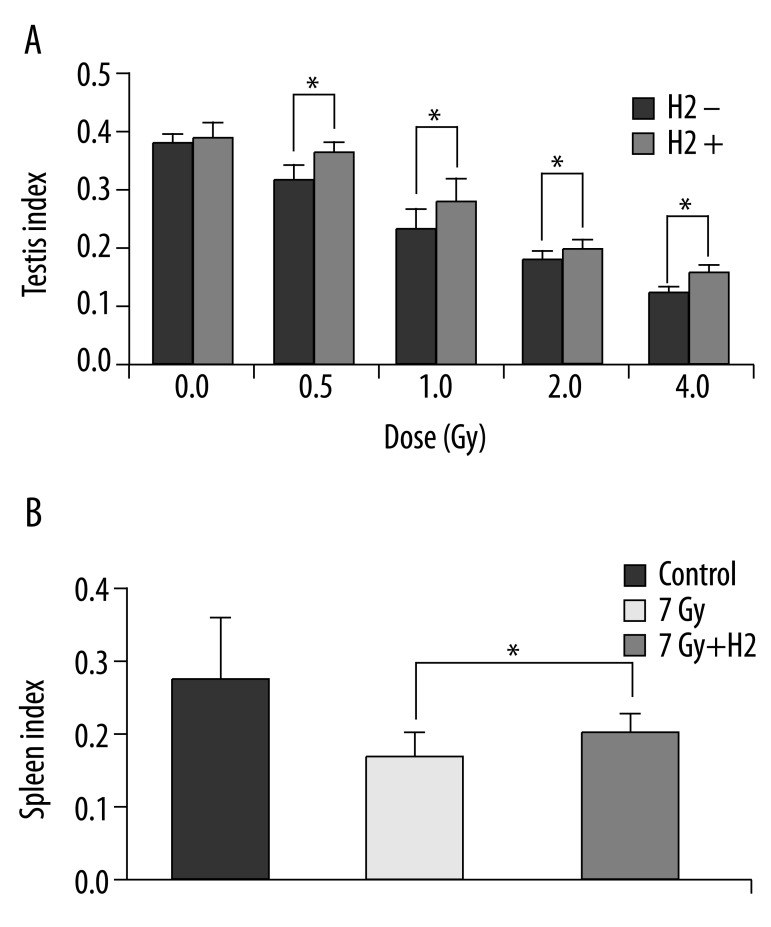
Body weight and organ weight were recorded at sacrifice, and organ index was calculated (organ index=organ weight/body weight ×100). Organ indices of testis/spleen in the H2 groups were significantly higher than those of the non-H2 groups (Figure 1A, B).
Figure 1.
Testis index after irradiation (A). Spleen index after irradiation (B). Organ index = organ weight/body weight ×100. The data were expressed as means ±SEM (n=8), *p<0.05.
Spermatid head counts
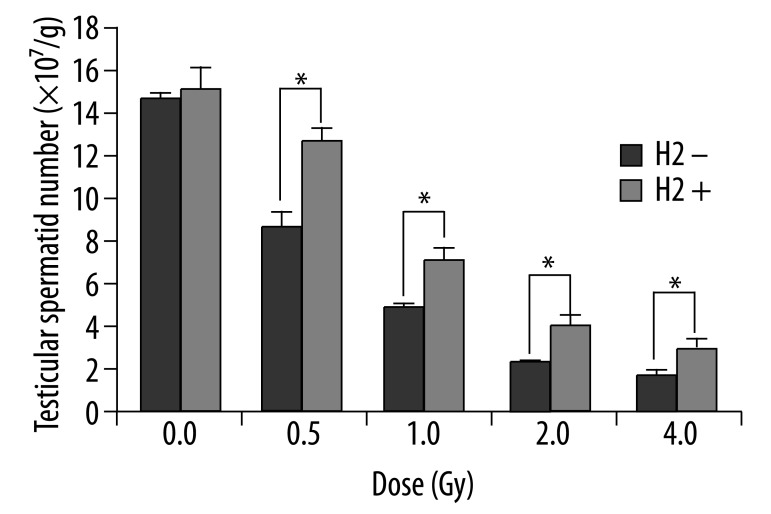
Testicular spermatid head counts were evaluated 29 d after irradiation to examine the ability of A1 through B spermatogonia to survive and differentiate into late spermatids. As predicted, after exposure of mice to 0.5 Gy, 1.0 Gy, 2.0 Gy and 4.0 Gy radiation, spermatid head counts were 11.8×107/g, 7.0×107/g, 4.1×107/g and 2.9×107/g in the H2 groups, respectively. These were 1.4-fold, 1.5-fold, 1.6-fold and 1.7-fold higher than those of the non-H2 groups, which were 77.7%, 45.9%, 27.1% and 18.8% of normal nonirradiated control level (Figure 2).
Figure 2.
Testicular spermatid heads number per gram testis (TSN, ×107/g) 29 d after irradiation. The data were expressed as means ±SEM (n=8), *p<0.05.
Testicular histology
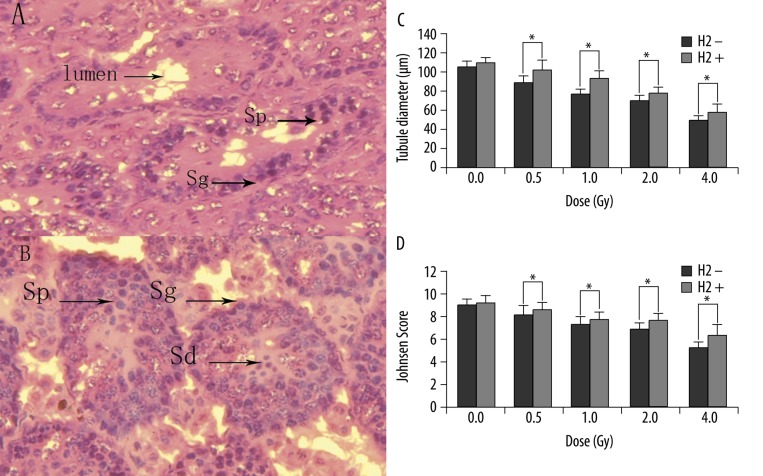
At 29 d after irradiation, spermatozoa and spermatids were decreased or absent in some tubules, and disordered and shrinking tubules were observed (Figure 3A). All these changes were ameliorated by H2 pre-treatment (Figure 3B), (Figure 3C and D show tubule diameter and JS). As shown, H2 pre-treatment significantly reduced the seminiferous epithelium injury caused by IR.
Figure 3.
Morphologic observation of the testicular tissue in γ-irradiated and H2 pre-treated mice (400×). Note the cells lost, disordered and shrinking tubules after 4Gy γ-irradiation (A). All these changes were ameliorated by H2 (B). The tubule diameter of mice 29 d after irradiation (C). JS of mice 29 d after irradiation (D). Sg, spermatogonia; Sp, spermatocytes; Sd, spermatids. The data were expressed as means ±SEM (n=8), *p<0.05.
Endogenous hematopoietic spleen colony formation
The numbers of endoCFUs/spleen, by group, were the normal control group, 1.5; the H2 group, 6.4; the non-H2 group, 2.1 (Figure 4A, B). The number of endoCFUs in mice of the H2 group was approximately 3.1-fold higher than that in the non-H2 group.
Figure 4.
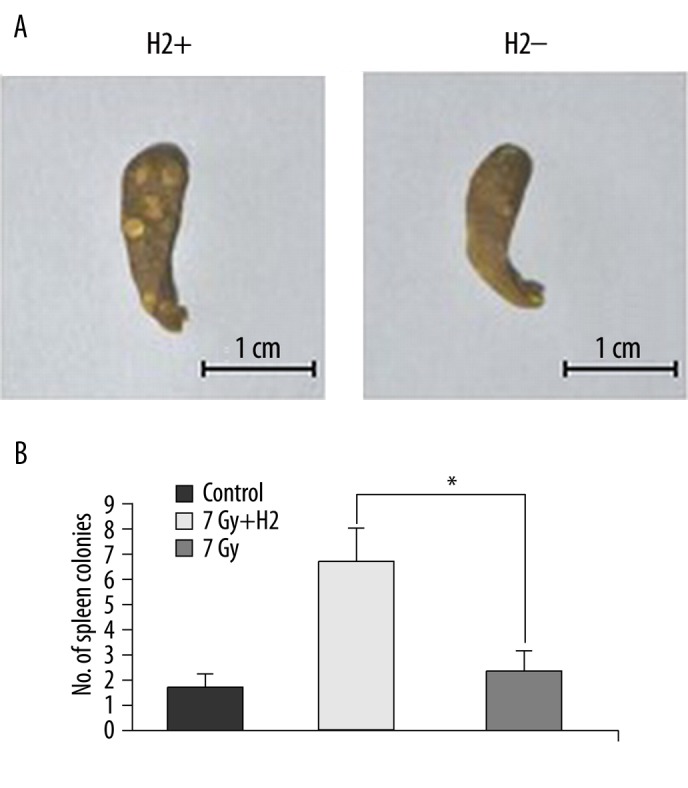
Effect of H2 administration on endogenous hematopoietic spleen colony formation in irradiated mice. Spleens in different groups (A). Number of spleen colonies in different groups (B). The data were expressed as means ±SEM (n=8), *p<0.05.
Bone marrow nucleated cells
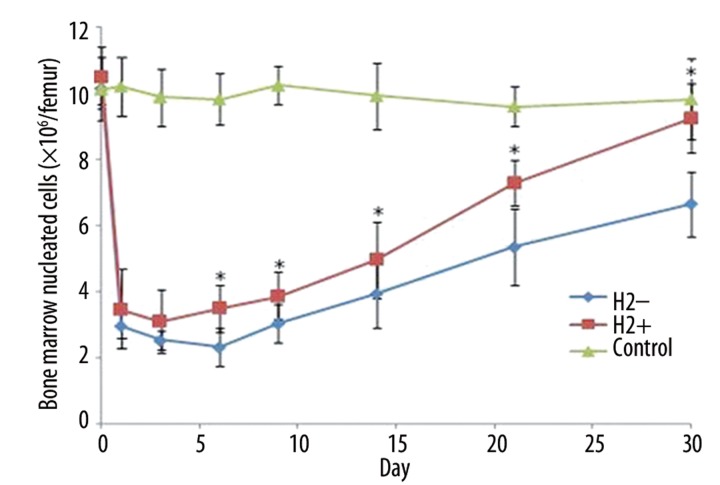
Radiation clearly decreased numbers of bone marrow nucleated cells (BMNC) and induced hematopoiesis suppression in both irradiated groups, and recovery of nucleated cells started at day 6 after irradiation. Compared to the non-H2 group, H2 pre-treatment clearly accelerated hematopoietic recovery by the increase of BMNC numbers (Figure 5). At day 30 after 4Gy irradiation, the number of nucleated cells in the H2 group returned to 9.2×106/femur, as compared to 6.6×106/femur in the non-H2 group.
Figure 5.
Number of bone marrow nucleated cells on different days after 4Gy irradiation (×106/femur). The data were expressed as means ±SEM (n=3), *p<0.05.
Leukocyte counts
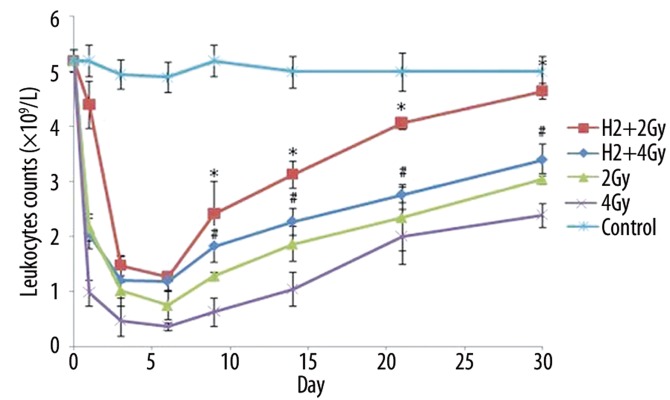
Leukocyte counts declined rapidly and elevated gradually from day 9 following irradiation. Within the whole post-irradiation period, the recovery of leukocytes in the H2 groups was significantly faster than the non-H2 groups (Figure 6). At day 30 after 2Gy irradiation, leukocyte counts in the H2 group returned to 4.65×109/L, as compared to 3.05×109/L in the non-H2 group. At day 30 after 4Gy irradiation, leukocyte counts in the H2 group returned to 3.40×109/L, as compared to 2.39×109/L in the non-H2 group.
Figure 6.
Leukocyte counts on different days after irradiation in different groups (×109/L). The data were expressed as means ±SEM (n=3), *p<0.05, compared with group 2Gy; #p<0.05, compared with group 4Gy.
Discussion
To our knowledge, 60–70% of the ionizing radiation-induced tissue damage was caused by •OH [20]. •OH can easily react with cellular macromolecules such as DNA, proteins and lipids, to exert strong cytotoxic effects. It has been reported that free radical scavengers could effectively ameliorate oxidative injuries due to IR [21–23]. Ohsawa et al. [9] found that H2 could effectively neutralize •OH in living cells. H2 will penetrate biomembranes, and then diffuse into the cytosol, mitochondria and nucleus. Its rapid gaseous diffusion might make it highly effective for reducing cytotoxic radicals, unlike most known antioxidants which are unable to successfully target organelles [24]. H2 will react with only the strongest oxidants (•OH and ONOO−) and is mild enough not to disturb metabolic oxidation-reduction reactions or to disturb reactive oxygen species (ROS) in cell signaling, unlike some antioxidants with strong reductive reactivity which might increase mortality by affecting vital defensive mechanisms [25]. H2 is continuously produced by colonic bacteria in the body, normally circulates in the blood [26] and reacts with •OH to produce water [27]. It is physiologically safe for humans to ingest H2 at a relatively low concentration [28]. Dissolving H2 in physiological saline has no risk of flammability or explosion and is easy to apply.
Our previous study showed that H2 pre-treatment could protect gastrointestinal endothelia from IR [10], and Terasaki et al. [12] showed that H2 pre-treatment could prevent radiation pneumonitis from IR. The radioprotective effect results from H2, which could decrease the attack of •OH, prevent DNA damage and decrease lipid peroxidation, thereby decreasing the deleterious effects of radiation. The aims of this study were to investigate whether H2 is able to protect spermatogenesis and hematopoiesis from radiation-induced injuries.
IR exposure of the testes mainly targets actively dividing germ cells without causing significant injury to Sertoli cells [14]. The decrease of testis weight after irradiation reflects elimination of germ cell populations [29]. Differentiating spermatogonia were the cell types most vulnerable to IR. Cell identification is difficult, and the presence of a cell does not guarantee its subsequent function and viability [30]. Based on the kinetics of spermatogenesis, spermatid head counts were evaluated 29 d after irradiation to examine the ability of A1 through B spermatogonia to survive and differentiate into late spermatids. The spermatid head count is a sensitive indicator in detecting adverse effects on spermatogenesis; however, it should be followed by histological analysis in detecting integrated effects on spermatogenesis. The tubule diameter and JS were simple and clear methods to quantify the seminiferous epithelium. In our study, we observed significant decrease of testis index, spermatid head count, the tubule diameter and JS in irradiated mice. However, pre-treatment of H2 prior to radiation exposure increased the levels of testis index, spermatid head count, the tubule diameter and JS.
Myelosuppression is the major syndrome of hematopoietic system damaged by total-body exposure to IR. The spleen, as a part of hematopoietic system, involves the regulatory mechanism of BM hemopoiesis; spleen index reflects its function status [31]. It is generally agreed that impairment of BM hematopoietic function and PB cells ultimately lead to hemorrhage and infection [19]. Accordingly, endoCFUs counts, BMNC counts and PB leukocyte counts were used to evaluate hemopoiesis. The endoCFUs is a good indicator of stem cell viability and/or the stimulation, proliferation and survival of cells recovering from exposure to IR [19]. The quantity of BMNC is a good indicator of the hemopoietic function of marrow after irradiation. Additional indicator of hemopoietic injury of BM induced by IR was the changes in the numbers of PB leukocytes. In our study, pre-treatment of H2 prior to radiation exposure increased the levels of spleen index, endoCFUs, BMNC and PB leukocytes in irradiated mice compared with mice irradiated without H2.
Conclusions
In conclusion, we showed that hydrogen-rich saline could partially protect spermatogenesis and hematopoiesis in irradiated mice (the protective effects by H2 have been shown to be statistically different from non-H2 group, but the extent of the protective effects seem to be limited). Although our investigations might provide some quantitative basis for the possible use of H2 as a radioprotector, further studies are necessary to determine the exact mechanism.
Footnotes
Conflict of interest
The author has no conflict of interest to disclose.
Source of support: This work was supported by a grant from the National Natural Science Foundation of China (Grant No. 81072241)
References
- 1.Otala M, Suomalainen L, Pentikainen MO, et al. Protection from Radiation-Induced Male Germ Cell Loss by Sphingosine-1-Phosphate. Biol Reprod. 2004;70:759–67. doi: 10.1095/biolreprod.103.021840. [DOI] [PubMed] [Google Scholar]
- 2.Oakberg EF. Sensitivity and time of degeneration of spermatogenic cells irradiated in various stages of maturation in the mouse. Rad Res. 1955;2:369–91. [PubMed] [Google Scholar]
- 3.Oakberg EF. Degeneration of spermatogonia of the mouse following exposure to X rays, and stages in the mitotic cycle at which cell death occurs. J Morphol. 1955;97:39–54. [Google Scholar]
- 4.Chuai Y, Gao F, Li B, et al. Hydrogen-rich saline attenuates radiation-induced male germ cell loss in mice through reducing hydroxyl radicals. Biochem J. 2012;442(1):49–56. doi: 10.1042/BJ20111786. [DOI] [PubMed] [Google Scholar]
- 5.Costabile RA. The effects of cancer and cancer therapy on male reproductive function. J Urol. 1993;149:1327–30. doi: 10.1016/s0022-5347(17)36384-x. [DOI] [PubMed] [Google Scholar]
- 6.Grande T, Bueren JA. The mobilization of hematopoietic progenitors to peripheral blood is predictive of the hematopoietic syndrome after total or partial body irradiation of mice. Int J Radiation Oncology Biol Phys. 2006;64:612–18. doi: 10.1016/j.ijrobp.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Baranov A, Gale RP, Guskova A, et al. Bone marrow transplantation after the Chernobyl nuclear accident. N Engl J Med. 1989;321:205–12. doi: 10.1056/NEJM198907273210401. [DOI] [PubMed] [Google Scholar]
- 8.Normile D. Nuclear accident. Special treatment set for radiation victim. Science. 1999;286:207–9. doi: 10.1126/science.286.5438.207b. [DOI] [PubMed] [Google Scholar]
- 9.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–94. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 10.Qian L, Cao F, Cui J. Radioprotective effect of hydrogen in cultured cells and mice. Free Radic Res. 2010;44:275–82. doi: 10.3109/10715760903468758. [DOI] [PubMed] [Google Scholar]
- 11.Qian L, Li B, Cao F, et al. Hydrogen-rich PBS protects cultured human cells from ionizing radiation-induced cellular damage. Nuclear Technology & Radiation Protection. 2010;25:23–29. [Google Scholar]
- 12.Terasaki Y, Dedong K, Kuwahara N, Ohsawa Y, et al. Fukuda: Hydrogen therapy attenuates radiation-induced pulmonary injury in C57/Bl6 mice through reducing oxidative stress. Am J Respir Crit Care Med. 2010;181:A6356. [Google Scholar]
- 13.Haines GA, Hendry JH, Daniel CP, Morris ID. Germ cell and dose-dependent DNA damage measured by the comet assay in murine spermatozoaa after testicular X-irradiation. Biol Reprod. 2002;67:854–61. doi: 10.1095/biolreprod.102.004382. [DOI] [PubMed] [Google Scholar]
- 14.Rasoulpour T, DiPalma K, Kolvek B, Hixon M. Akt1 suppresses radiation-induced germ cell apoptosis in vivo. Endocrinology. 2006;147:4213–21. doi: 10.1210/en.2006-0174. [DOI] [PubMed] [Google Scholar]
- 15.Meistrich ML, van Beek MEAB. Spermatogonial stem cells: Assessing their survival and ability to produce differentiated cells. In: Chapin RE, Heindel J, editors. Methods in Toxicology. 3A. Academic Press; New York: 1993. pp. 106–23. [Google Scholar]
- 16.Johnsen SG. Testicular biopsy count, a method for registration of spermatogenesis in human testis, normal values and results in 355 hypogonadal males. Hormone. 1970;1:1–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 17.Ehmcke J, Joshi B, Hergenrother SD, Schlatt S. Aging does not affect spermatogenic recovery after experimentally induced injury in mice. Reproduction. 2007;133:75–83. doi: 10.1530/REP-06-0148. [DOI] [PubMed] [Google Scholar]
- 18.Park E, Ahn G-n, Lee NH, et al. Radioprotective properties of eckol against ionizing radiation in mice. FEBS Letters. 2008;582:925–30. doi: 10.1016/j.febslet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Grande T, Bueren JA. The mobilization of hematopoietic progenitors to peripheral blood is predictive of the hematopoietic syndrome after total or partial body irradiation of mice. Int J Radiation Oncology Biol Phys. 2006;64:612–18. doi: 10.1016/j.ijrobp.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 20.Chuai Y, Zhao L, Ni J, et al. A possible prevention strategy of radiation pneumonitis: combine radiotherapy with aerosol inhalation of hydrogen-rich solution. Med Sci Monit. 2011;17(4):HY1–4. doi: 10.12659/MSM.881698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirazi A, Ghobadi G, Ghazi-Khansari M. A radiobiological review on melatonin: a novel radioprotector. J Radiat Res. 2007;48:263–72. doi: 10.1269/jrr.06070. [DOI] [PubMed] [Google Scholar]
- 22.Yan SX, Hong XY, Hu Y, Liao KH. Tempol, one of nitroxides, is a novel ultraviolet-A1 radiation protector for human dermal fibroblasts. J Dermatol Sci. 2005;37:137–43. doi: 10.1016/j.jdermsci.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Mihailovic M, Milosevic V, Grigorov I, et al. The radioprotective effect of alpha2-macroglobulin: A morphological study of rat liver. Med Sci Monit. 2009;15(7):BR188–93. [PubMed] [Google Scholar]
- 24.Yang Y, Li B, Liu C, Chuai Y, et al. Hydrogen-rich saline protects immunocytes from radiation-induced apoptosis. Med Sci Monit. 2012 doi: 10.12659/MSM.882616. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjelakovic G, Nikolova D, Gluud LL, et al. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. J Am Med Assoc. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 26.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3:1129–34. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 27.Labiche LA, Grotta JC. Clinical trials for cytoprotection in stroke. NeuroRx. 2004;1:46–70. doi: 10.1602/neurorx.1.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975;190:152–54. doi: 10.1126/science.1166304. [DOI] [PubMed] [Google Scholar]
- 29.Shah FJ, Tanaka M, Nielsen JE, et al. Gene expression profiles of mouse spermatogenesis during recovery from irradiation. Reprod Biol Endocrinol. 2009;7:130. doi: 10.1186/1477-7827-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meistrich ML. Critical components of testicular function and sensitivity to disruption. Biol Reprod. 1986;34:17–28. doi: 10.1095/biolreprod34.1.17. [DOI] [PubMed] [Google Scholar]
- 31.Jieping Z, Guang W, Jianghua S, et al. Protective Effects of Squid Ink Extract Towards Hemopoietic Injuries Induced by Cyclophosphamine. Mar Drugs. 2009;7:9–18. doi: 10.3390/md7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]