Abstract
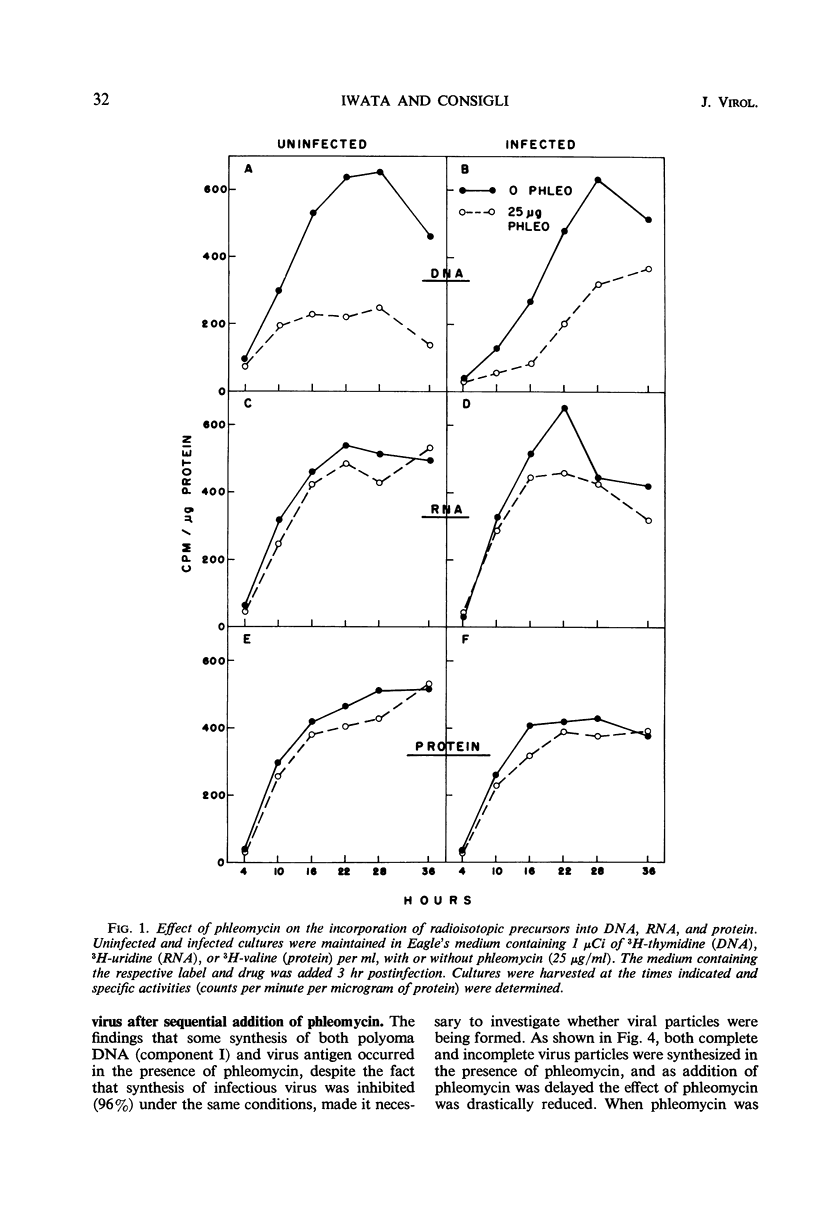
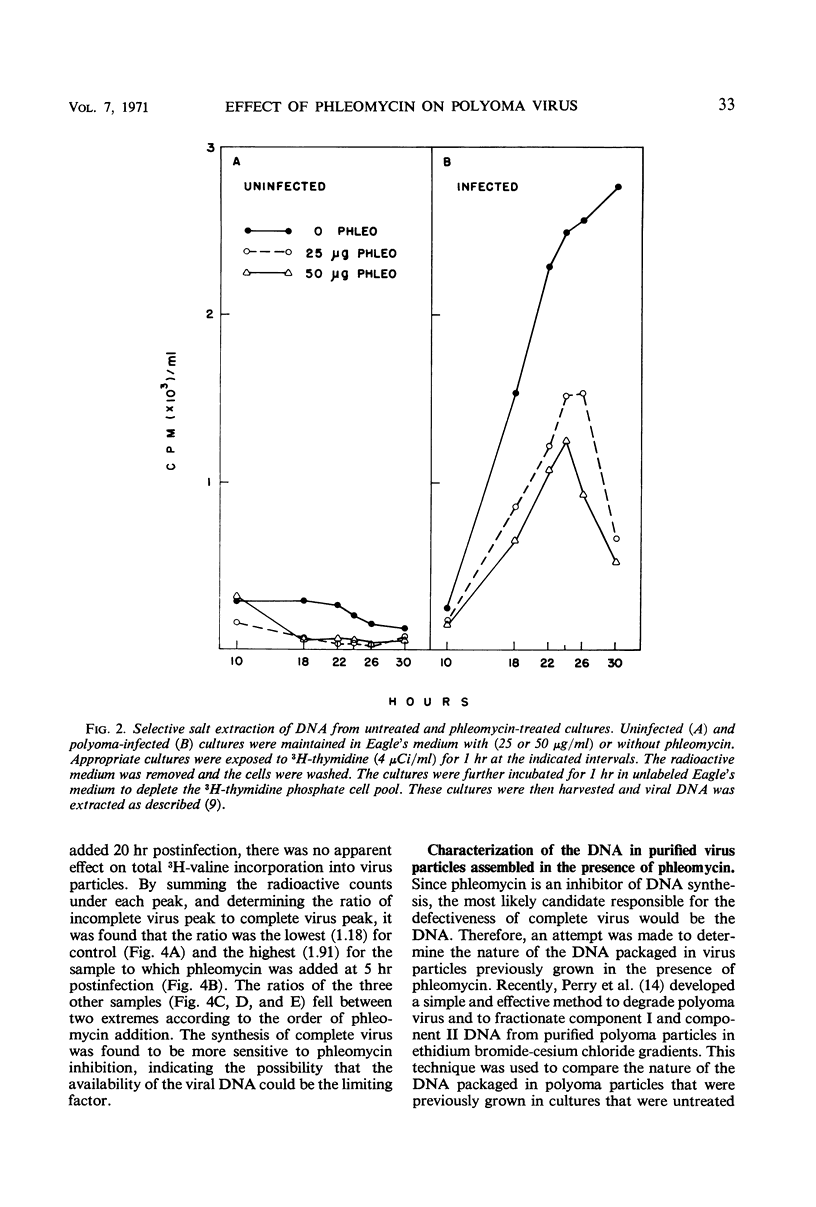
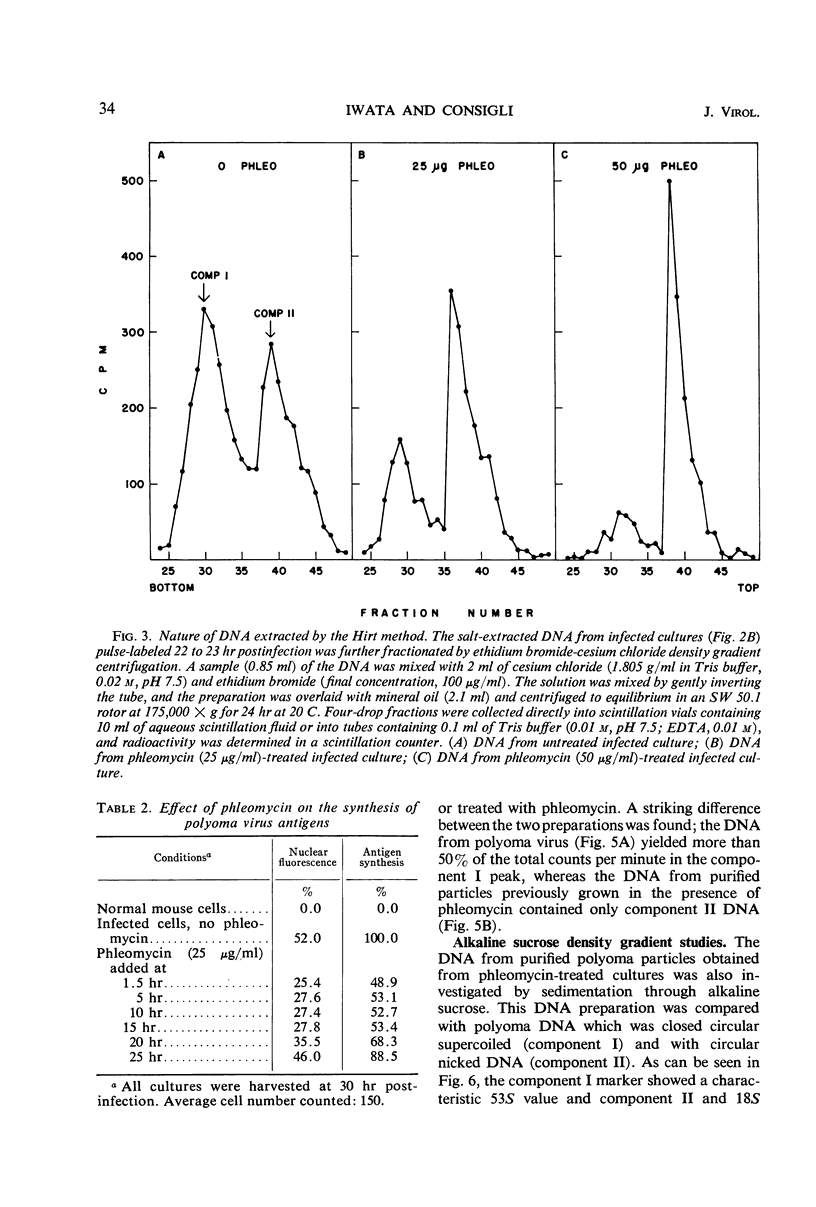
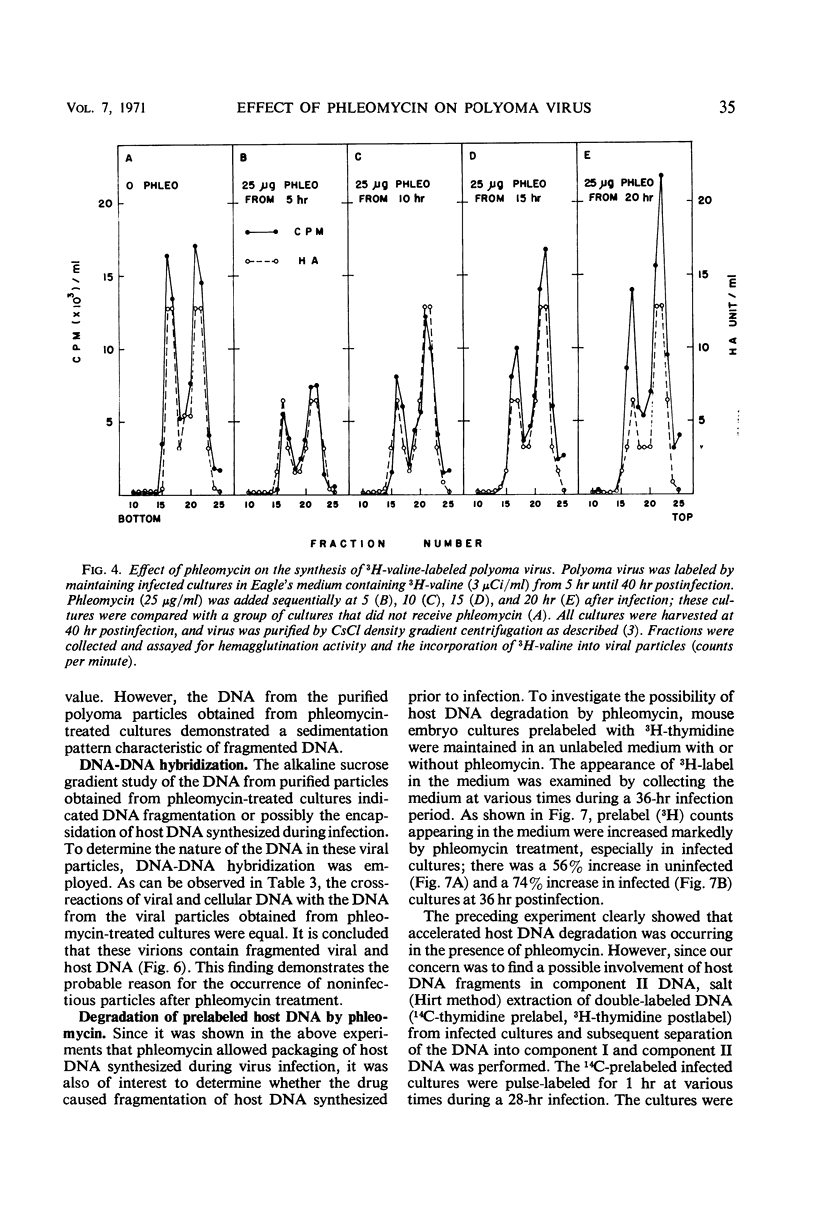
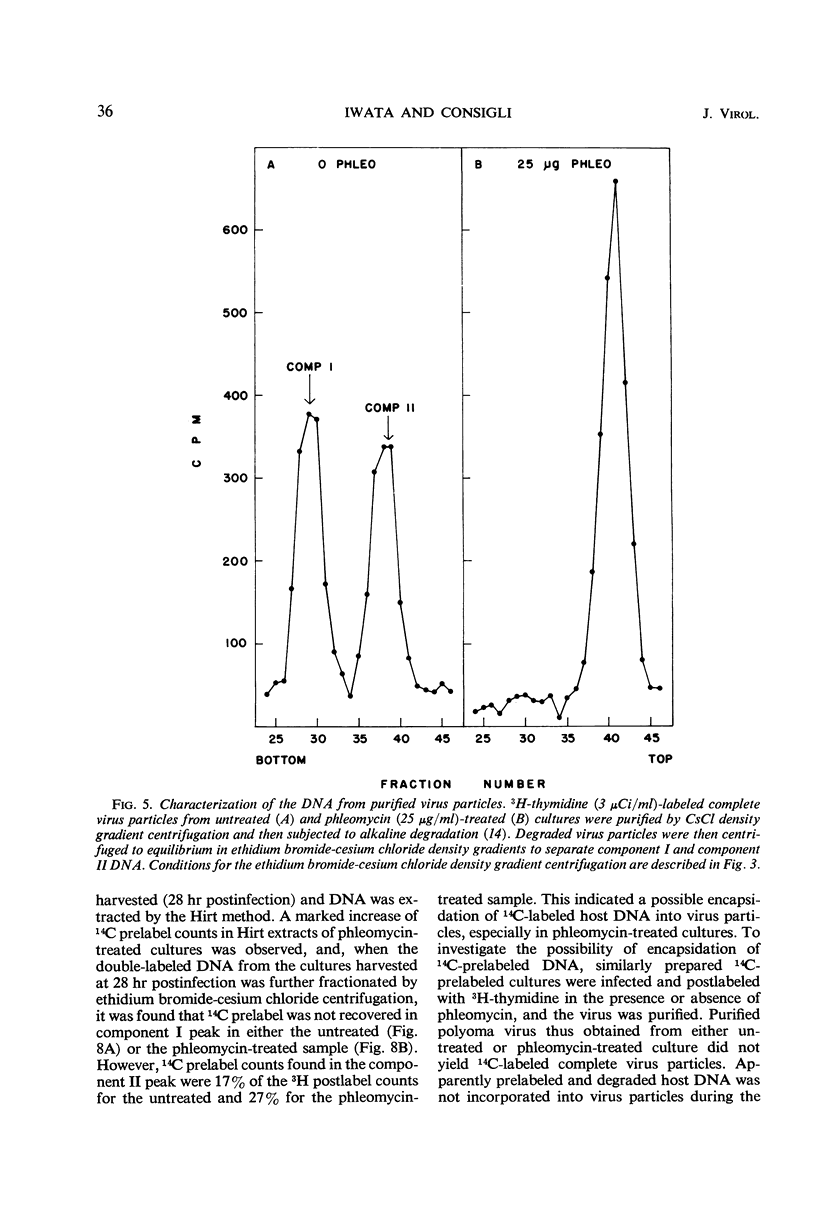
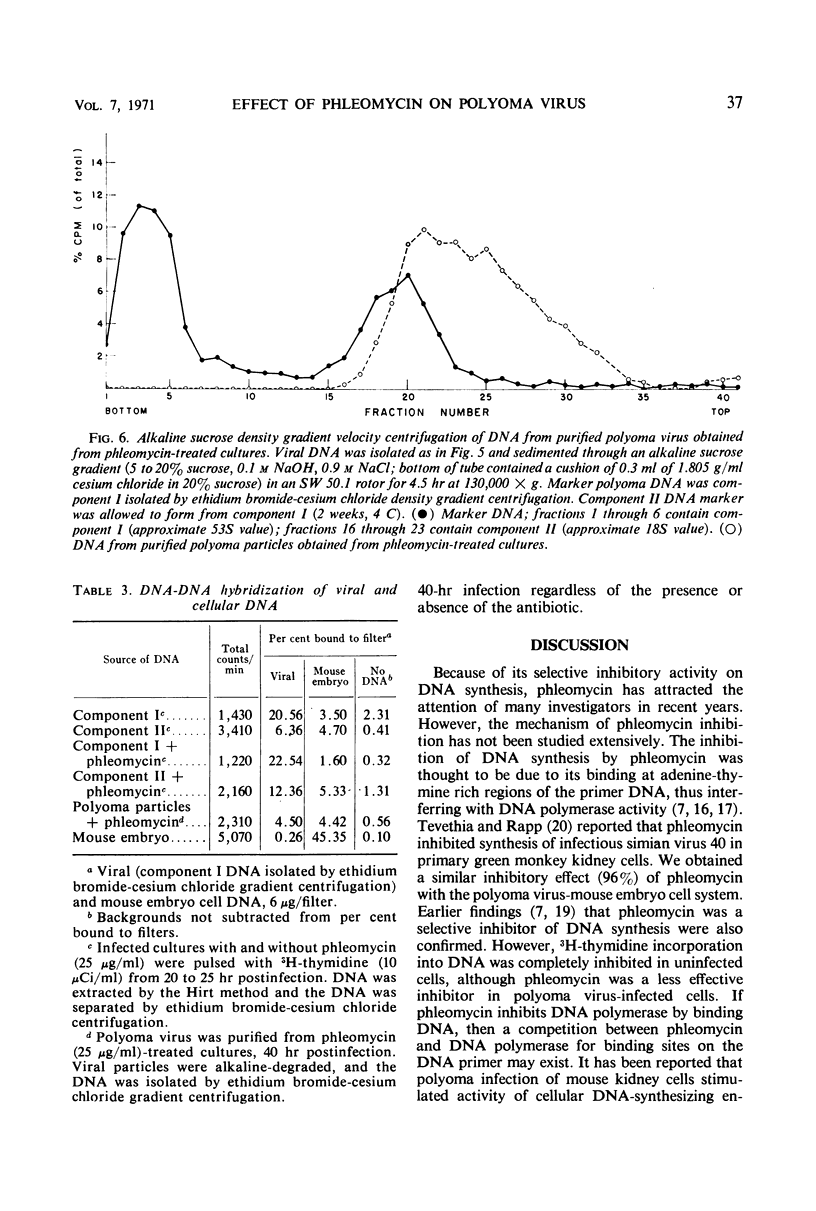
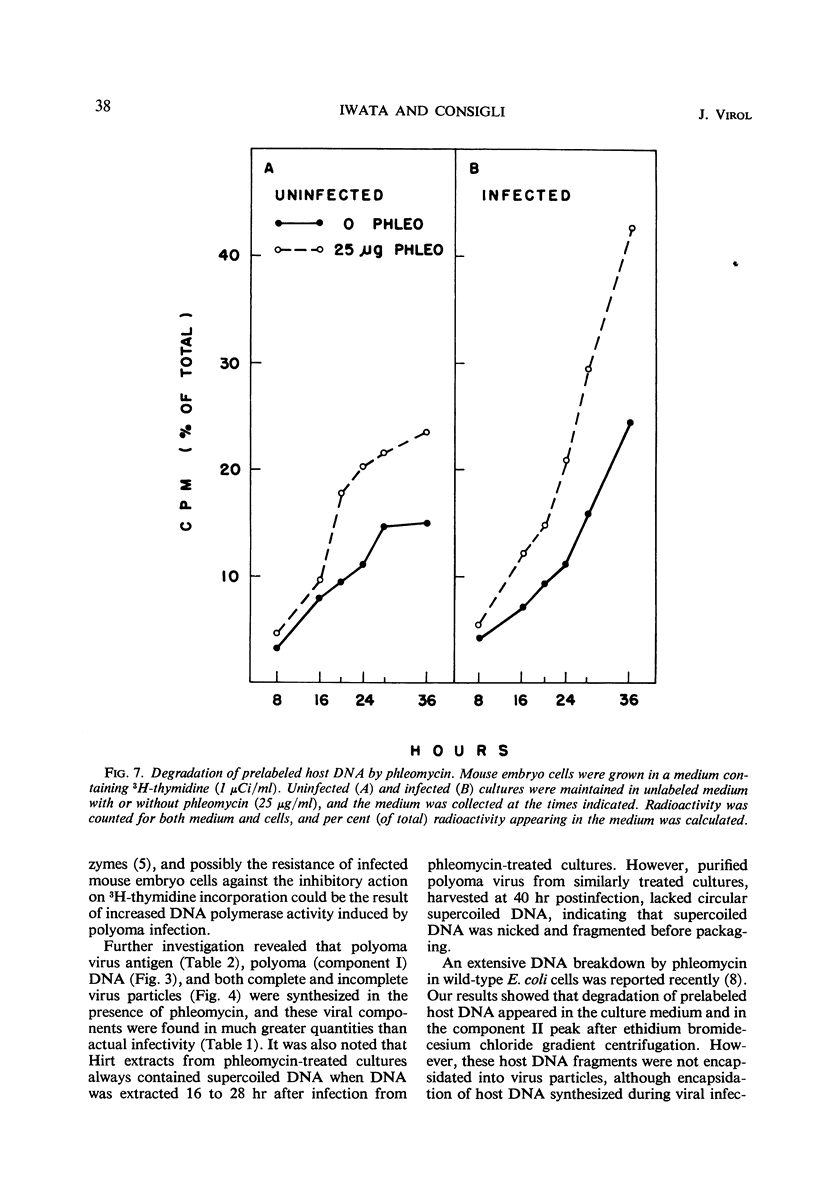
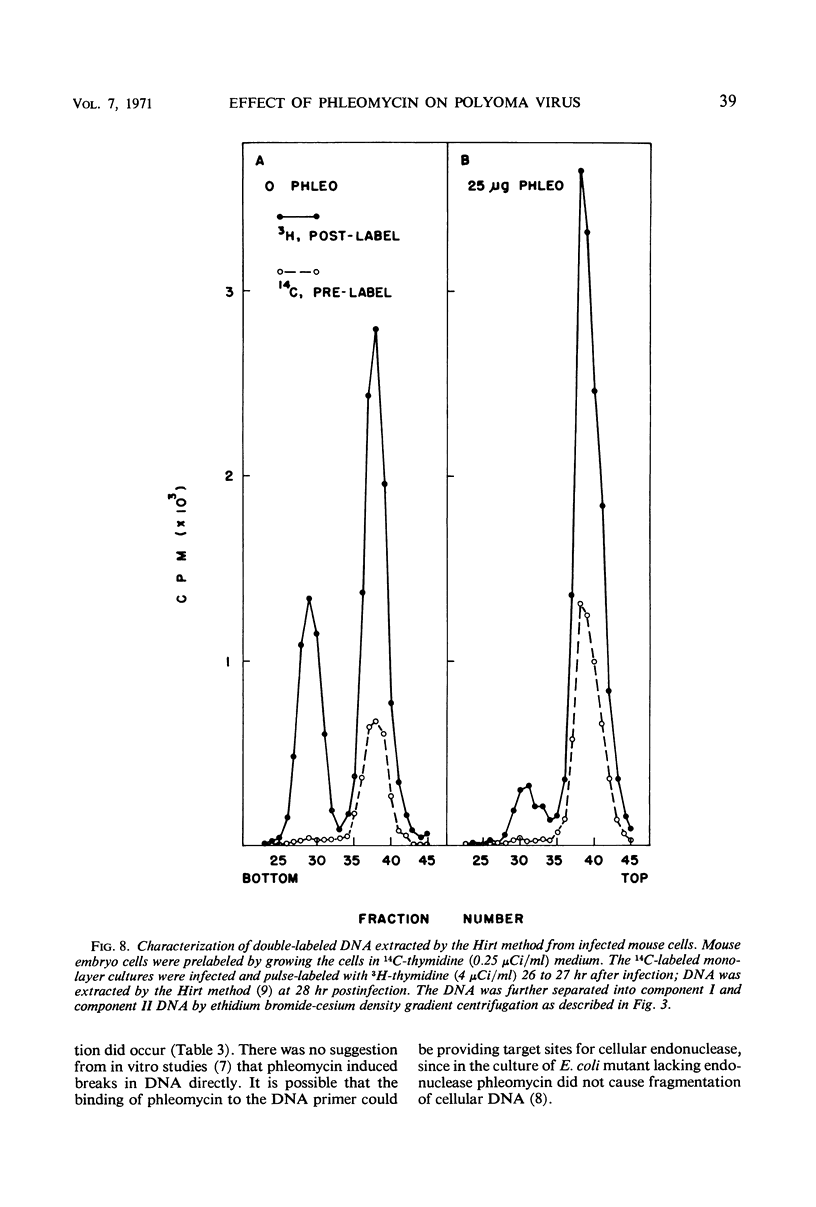
The addition of phleomycin (25 μg) to primary mouse embryo cells infected with polyoma virus was found to cause 96% inhibition of the synthesis of infectious virus. When ribonucleic acid and protein synthesis was investigated in these cells by use of isotope incorporation, it was found that neither was inhibited drastically. Immunofluorescent staining studies with the use of antibody directed to the viral structural proteins showed that proteins were synthesized in the presence of the antibiotic. However, when deoxyribonucleic acid (DNA) synthesis was investigated, it was found that DNA synthesis in uninfected cells was completely inhibited within the initial 10 hr of phleomycin addition, whereas DNA synthesis in infected cells proceeded at a reduced rate. Selective DNA extraction (Hirt method) of phleomycin-treated infected cells demonstrated that synthesized viral DNA was salt-extractable, similar to that in infected control cells lacking phleomycin. This extracted DNA was further fractionated by ethidium bromide-cesium chloride density gradient equilibrium centrifugation. The phleomycin-treated preparations revealed twice as much component II (circular nicked and linear) as component I (supercoiled) DNA, whereas the DNA from normally infected control cells showed the reverse picture. It was also demonstrated that viral particles synthesized in the presence of phleomycin did not contain component I DNA. This packaged DNA was found to consist of fragments of both the host and viral types. Cells that were prelabeled with 3H-thymidine and then treated with phleomycin demonstrated host DNA degradation. However, fragments formed from prelabeled host DNA were not encapsidated into viral particles.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgaux P., Bourgaux-Ramoisy D., Dulbecco R. The replication of the ring-shaped DNA of polyoma virus. I. Identification of the replicative intermediate. Proc Natl Acad Sci U S A. 1969 Oct;64(2):701–708. doi: 10.1073/pnas.64.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consigli R. A., Minocha H. C., Abo-Ahmed H. Multiplication of polyoma virus. II. Source of constituents for viral deoxyribonucleic acid and protein synthesis. J Bacteriol. 1966 Sep;92(3):789–791. doi: 10.1128/jb.92.3.789-791.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzin F., Vogt M., Dieckmann M., Berg P. Induction of virus multiplication in 3T3 cells transformed by a thermosensitive mutant of polyoma virus. II. Formation of oligometric polyoma DNA molecules. J Mol Biol. 1970 Feb 14;47(3):317–333. doi: 10.1016/0022-2836(70)90305-0. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., HARTWELL L. H., VOGT M. INDUCTION OF CELLULAR DNA SYNTHESIS BY POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Feb;53:403–410. doi: 10.1073/pnas.53.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- FALASCHI A., KORNBERG A. ANTIMETABOLITES AFFECTING PROTEIN OR NUCLEIC ACID SYNTHESIS. PHLEOMYCIN, AN INHIBITOR OF DNA POLYMERASE. Fed Proc. 1964 Sep-Oct;23:940–945. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ishizuka M., Takayama H., Takeuchi T., Umezawa H. Studies on antitumor activity, antimicrobial activity and toxicity of phleomycin. J Antibiot (Tokyo) 1966 Nov;19(6):260–271. [PubMed] [Google Scholar]
- Khare G. P., Consigli R. A. Cytologic studies in polyoma virus-infected mouse embryo cells. Am J Vet Res. 1967 Sep;28(126):1527–1536. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAEDA K., KOSAKA H., YAGISHITA K., UMEZAWA H. A new antibiotic, phleomycin. J Antibiot (Tokyo) 1956 Mar;9(2):82–85. [PubMed] [Google Scholar]
- Perry J. L., To C. M., Consigli R. A. Alkaline degradation of polyoma virus. J Gen Virol. 1969 Apr;4(3):403–411. doi: 10.1099/0022-1317-4-3-403. [DOI] [PubMed] [Google Scholar]
- Pietsch P., Corbett C. Competitive effects of phleomycin and mercuric chloride in vivo. Nature. 1968 Aug 31;219(5157):933–934. doi: 10.1038/219933a0. [DOI] [PubMed] [Google Scholar]
- Pitts J. D., Sinsheimer R. L. Effect of phleomycin upon replication of bacteriophage phiX174. J Mol Biol. 1966 Feb;15(2):676–680. doi: 10.1016/s0022-2836(66)80136-5. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]



