Summary
Background
The purpose of the current study is to evaluate the effects of systemic ornidazole (SO) and systemic and local compound ornidazole and pefloxacin mesylate (SCOPM/LCOMP) on the inflammatory response associated with rat experimental chronic periodontitis (ECP) in sites with subgingival debridement.
Material/Methods
Periodontitis was induced in male Sprague-Dawley rats by placing a thin steel ligature around the upper first molars and inoculating them with Porphyromonas gingivalis 381. After the successful induction of the rat ECP, the periodontitis rats were randomly divided into 3 different combined treatment groups: (A) SO with scaling and root planing (SRP); (B) SCOMP with SRP; and (C) LCOMP with SRP. After 2 weeks the effects of the treatments were evaluated based on gingivitis, plaque index, probing pocket depth, aspartate aminotransferase, alveolar bone loss, and hematoxylin-eosin staining of the region around the first molars.
Results
After treatment, comparison with ECP was performed. The mean percentage reductions of SBI in SO, SCOPM, and LCOPM were 27.73%, 33.61%, and 58.82%, respectively. Those of PI were 33.20%, 42.80%, and 60.00%; those of PPD were 48.66%, 55.70%, and 72.48%; those of GCF-AST were 41.64%, 49.03%, and 66.42%; and those of ABL were 41.19%, 43.63%, and 54.47%, respectively. The inflammatory score of H&E showed median scores of 2.5, 1.75, 1.63, and 0.95 for ECP, SO, SCOMP, and LCOMP, respectively. All 3 treatment groups exhibited significantly reduced inflammation indicators (P<0.05). Of the 3, group C was the most effective (P<0.05).
Conclusions
Although all the combined treatment groups responded to therapy with significant resolution of the infection, adjunctive LCOMP therapy is more effective for periodontitis.
Keywords: periodontitis, antimicrobial agents, local drug delivery, ornidazole, pefloxacin mesylate
Background
Periodontitis is one of the most common infections and is a major cause of tooth loss. It is a polymicrobial infection, and thus, the complex interaction among microorganisms makes the disease challenging to understand and treat. These pathogens include anaerobes, such as Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nudeatum and Peptostreptococcus anaerobius, as well as facultative anaerobes, including Staphylococcus aureus and Staphylococcus epidermidis, among others [1,2]. Bacterial plaque is recognized as the primary agent for the initiation and progression of periodontitis [3]. Plaque microorganisms can damage the periodontium by releasing their proteolytic and noxious waste products and by stimulating the host cells to produce pro-inflammatory cytokines, inducing connective tissue and alveolar bone destruction [4].
Therefore, a successful treatment depends on the elimination or control of the pathogens, together with a microbial shift toward a microbial population that is typically present in healthy individuals. Treatments include mechanical and drug therapy; mechanical therapy involves nonsurgical scaling and root planing (SRP) and drug therapy is adjunctive to SRP [5,6].
Drug therapy includes local and systemic administration. Although systemic administration has some benefits, an inadequate concentration in the periodontium and a high plasma concentration may be associated with bacterial resistance and adverse effects. An important advantage of local administration is the higher therapeutic concentration in the lesion location, leaving residual parts unaffected.
Ornidazole is a member of the nitroimidazole family that is widely used in the treatment of the oral disease and which has better activity against anaerobes than do the quinolones [7–9]. Pefloxacin mesylate is a member of the quinolones, and these have better activity against facultative anaerobes than the nitroimidazoles [10]. As previously mentioned, the dominant microbial population is anaerobes, but facultative anaerobes also play a part in break-down of deep periodontal tissues. To generate significant antimicrobial activity, we designed the compound ornidazole and pefloxacin mesylate (COPM) and the local COPM. The carrier of the local COPM consists of ethylcellulose and hydroxypropyl methylcellulose, which are widely used as framework materials in sustained release [11].
In the current study, a rat experimental model of chronic periodontitis (ECP) was induced by a ligature [12]. In addition, the treatment effects of systemic and local administration of COPM after SRP were investigated.
To gain a better understanding of the effects, we evaluated several end-points of the inflammatory process: the sulcus bleeding index (SBI), plaque index (PI), probing pocket depth (PPD), gingival crevicular fluid-aspartate aminotransferase (GCF-AST), alveolar bone loss (ABL), and HE staining.
Material and Methods
Animals
Experiments were performed on male Sprague-Dawley rats (180±20 g), housed in standard conditions (12 h light/dark cycle and 22±2°C), with free access to commercial laboratory food (standard rodent chow) and tap water before the surgical procedure.
A total of 60 rats were used in the current study. Of these, ECP was induced to 48 rats, while the remaining 12 rats were used in the normal group.
The use of animals, ethical clearance, and the study protocol were duly approved by the Institutional Animal Ethics and User Committee of The Third Military Medical University (Chongqing, China).
Induction of Rat ECP
A total of 48 rats were subjected to ligature-induced chronic periodontitis. The rats were lightly anaesthetized with surgical doses of 3% sodium pentobarbitone (35 mg·kg−1). A sterile, thin steel ligature was placed around the cervix of the maxillary right first molar. Once the rats recovered from the anesthetic, they were allowed to eat a high-sugar diet and drink 10% water with sucrose ad libitum.
About 1 week after induction, 0.1 ml P. gingivalis 381 (1010 cells/ml) (standard strain purchased from the R&D Department of P&G Co.) was injected into the gingival crevice every other day, 5 times in all, to improve the efficiency [13].
Identification of Rat ECP
After 8 weeks, 12 rats were randomly selected from the 48 to evaluate the success of the rat ECP. All the following indicators were analyzed by a histologist in single-blind fashion [14–16]. The 12 normal rats were used as part of the control group.
Various indicators (live)
SBI
The periodontal pockets or gingival crevices of the selected tooth were probed for 10 s every SIT and graded as follows:
Score 0: Gingival margin and gingival papilla (GM&P) are healthy, and no bleeding is observed after slight probe.
Score 1: GM&P are mildly inflamed, and no bleeding is observed after slight probe.
Score 2: GM&P are mildly inflamed; changes in color, absence of edema, and occurrence of punctate hemorrhage after slight probe are observed.
Score 3: GM&P are moderately inflamed; changes in color, mild edema, and bleeding after slight probe while the blood is still in the gingival crevice are observed.
Score 4: GM&P are severely inflamed; changes in color, severe edema, and bleeding after slight probe while the blood flows out of the gingival crevice are observed.
Score 5: GM&P are severely inflamed; changes in color, severe edema, ulcer, and bleeding after slight probe or spontaneous bleeding, and blood is observed flowing out of the gingival crevice.
PI
The selected tooth was smeared with 2% basic fuchsin for 30 seconds and then washed. The size and depth of the purple stains on the tooth were observed (score 0–5).
PPD
PPD was taken from the gingival margin to the bottom of the pocket using a round-ended probe tip 0.4 mm in diameter. Three values were averaged (mesiobuccal, distobuccal, and midbuccal).
GCF-AST
The gingival margin was dried with air and cotton swabs. The GCF samples were obtained from the distobuccal, mesiobuccal, and midbuccal sites. A standard volume of 1.0 μl crevicular fluid was collected in a Hirschman volumetric micropipette (Sigma Chemical Co., USA). When plaque or debris clogged the micropipette or blood contaminated the GCF, the GCF collection was repeated. The samples were immediately sent to the laboratory for AST enzyme analysis. The Erba SGOT (AST) kit (Transasia Biomedical Co., Ltd., Taiwan, China) was used for the quantitative determination of AST activity.
Various indicators (sacrificial)
After the evaluation of the aforementioned parameters, the animals were euthanized by cervical dislocation.
A total of 8 rats were randomly selected from the 12 rats for measurement of ABL. The last 4 rats were used for hematoxylin-eosin (HE) staining.
ABL
The excised maxillae were fixed in 10% neutral-buffered formalin for 24 hours. The total ABL was obtained by measuring the distance from the cementoenamel junction to the alveolar crest. Measurements were made along the axis of each root surface. Three recordings (3 roots) were made.
HE staining
The specimens were fixed into 10% neutral-buffered formalin for 48 hours and demineralized in 5% nitric acid for 24 hours. The specimens were then dehydrated, embedded in paraffin, and sectioned along the molars in a mesiodistal plane for HE staining. Sections of 6 ìm thickness were evaluated using light microscopy. Indicators such as inflammatory cell influx and cementum integrity were analyzed and graded as follows:
Score 0: absence of or only discrete cellular infiltration, preserved cementum;
Score 1: moderate cellular infiltration, some but minor cementum;
Score 2: accentuated cellular infiltration, and partial destruction of cementum;
Score 3: accentuated cellular infiltrate, and severe destruction of cementum.
Treatment of rat ECP
After the successful induction of the rat ECP, the remaining 36 ECP rats were randomly assigned to 3 drug treatment groups with 12 rats for each group.
Drugs
Ornidazole (Bodyguard Pharmaceutical Co., Ltd., China)
Pefloxacin mesylate (North China Pharmaceutical Group Co., Ltd., China)
Local COMP
LCOMP is a sustained-release periodontal suppository administered into the periodontal pocket (Dental Research Centre of Daping Hospital, Third Military Medical University, China).
The precursor of LCOPM is composed of EC (Sinopharm Chemical Reagent Beijing Co., Ltd., China), HPMC (Sinopharm Chemical Reagent Beijing Co., Ltd., China), ornidazole, pefloxacin mesylate, 95% ethanol, glycerine, and water.
According to periodontal pocket morphology, the lengths of the suppositories range from 2 to 4 mm, whereas the average width, thickness, taper angle, and weight are 3 mm, 350 μm, 55°, and 10 mg, respectively. An average suppository contains 0.8 mg ornidazole, 1.2 mg pefloxacin mesylate, 3.5 mg HPMC, and 3.5 mg EC.
Treatment groups
Group A, systemic administration of ornidazole (SO): the rats subjected to ECP received ornidazole (100 mg·kg−1 body weight/day in drinking water for 2 weeks) after SRP.
Group B, systemic administration of COPM (SCOPM): the rats subjected to ECP received COPM (40 mg·kg−1 ornidazole with 60 mg·kg−1 pefloxacin, body weight/day in drinking water for 2 weeks) after SRP.
Group C, LCOMP: the rats subjected to ECP received a local application of COPM (The sustained-release periodontal suppository is held by forceps and gently administrated into the periodontal pocket, every other day for 2 weeks) after SRP.
After 2 weeks, the following end-points of the inflammatory process were evaluated: SBI, PI, PPD, GCF-AST, ABL, and HE staining.
Control Groups: the normal rats and the rats subjected to ECP used for the identification of ECP comprised the negative control group and positive control group, respectively.
Statistical analysis
Data were expressed as mean ±SD. Mean values were compared by single-factor analysis of variance (ANOVA) and a paired t-test using the SPSS13.0 statistical software. The Kruskal-Wallis and Dunn’ tests were used for histopathological analysis. P<0.05 was considered significant.
Results
Effect of drug treatments on SBI, PI, PPD, GCF-AST
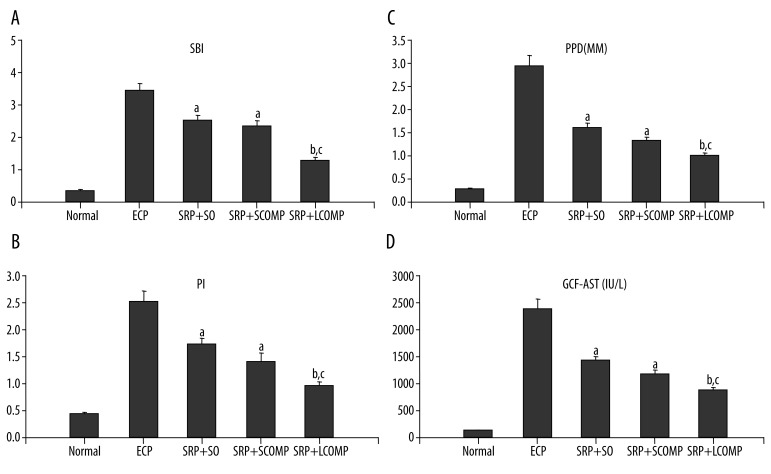
In normal rats, the gum was pink, and the gingival margin was thin and tightly affixed to the tooth surface. After 8 weeks of induction, a large number of chyme surrounded the upper right first molar, and the rat periodontal tissue showed erosion, ulceration, and atrophy; a significant increase was observed in the indices in ECP group compared to normal rats. Compared with the ECP group, similar reductions were observed after SRP+SO and SRP+SCOPM, whereas further reduction was observed after SRP+LCOPM. More specifically, the mean value of SBI in normal rats was 0.43, which increased to 3.57 in ECP rats; after treatment, it was reduced to 2.58, 2.37, and 1.47 in SO, SCOPM, and LCOPM, with mean percentage reductions of 27.73%, 33.61% and 58.82%, respectively (Figure 1A). The mean value of PI in normal rats was 0.40, which increased to 2.50 in ECP rats; after treatment, it was reduced to 1.67, 1.43, and 1.00 in SO, SCOPM, and LCOPM, with mean percentage reductions of 33.20%, 42.80%, and 60.00%, respectively (Figure 1B). The mean value of PPD (mm) in normal rats was 0.29, which increased to 2.98 in ECP rats; after treatment, it was reduced to 1.53, 1.32, and 0.82 in SO, SCOPM, and LCOPM, with mean percentage reductions of 48.66%, 55.70%, and 72.48%, respectively (Figure 1C). The mean value of GCF-AST (IU/L) in normal rats was 121.55, which increased to 2346.70 in ECP rats; after treatment, it was reduced to 1369.50, 1196.21, and 788.00 in SO, SCOPM, and LCOPM, with mean percentage reductions of 41.64%, 49.03%, and 66.42%, respectively (Figure 1D).
Figure 1.
Effects of the SRP+antibiotics on various parameters (live) in rats subjected to ECP. n=12. a: vs. NORMAL P<0.05, b: vs. SRP+SO P<0.05, c: vs. SRP+SCOMP P<0.05 (ANOVA & Paired t-test).
Effect of drug treatments on ABL
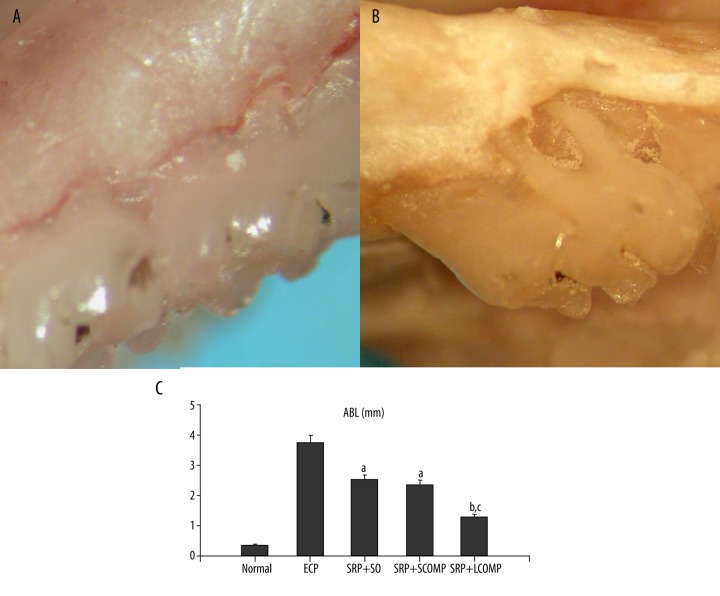
There was no evidence of pathology in normal first molars (Figure 2A); after induction, the macroscopic aspects of periodontium revealed bone matrix resorption in the first molar region (Figure 2B). A significant ABL between the ECP group and normal group was observed (Figure 2C). Compared with the ECP group, similar reductions were observed after SRP+SO and SRP+SCOPM, whereas further reduction was observed after SRP+LCOPM (Figure 2C). More specifically, the ABL (mm) in normal rats was 0.46, which increased to 3.69 in ECP rats; after treatment, it was reduced to 2.17, 2.18, and 1.68 in SO, SCOPM, and LCOPM, with mean percentage reductions of 41.19%, 43.63%, and 54.47%, respectively (Figure 2C).
Figure 2.
Macroscopic aspects of periodontia of rats submitted to ECP and the effects of SRP+antibiotics on ABL in rats subjected to ECP. The normal periodontia exhibited no resorption of the alveolar bone (A, original magnification ×50). In contrast, The periodontia from ECP rats demonstrated severe resorption of the alveolar bone with root exposure (B, original magnification ×50). The periodontia from ECP rats demonstrated severe resorption of the alveolar bone with root exposure (A, original magnification ×50). In contrast, normal periodontia exhibited no resorption of the alveolar bone (B, original magnification ×50). Compared with the normal group, a significant increase in the distance between cemento-enamel injunction and alveolar crest was observed in the ECP group (C). After treatment, the increase was significantly reduced (C). Among those treatment groups, SPR+LCOMP was most effective (C). n=8. a: vs. NORMAL P<0.05, b: vs. SRP+SO P<0.05, c: vs. SRP+SCOMP P<0.05 (ANOVA & Paired t-test).
Histopathological analysis of periodontium after drug treatments
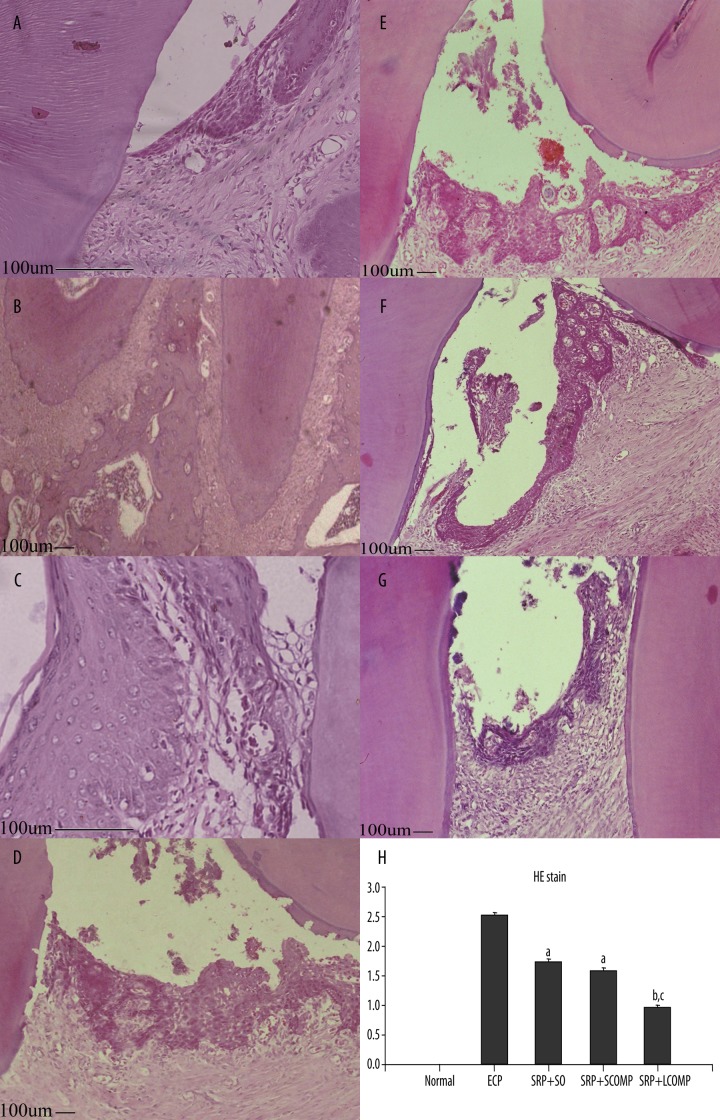
When compared with tissue sections taken from the normal group and ECP group, the histology of the region around the first molars of normal rats showed only a minimal number of polymorphonuclear cells in tissue, preserved cementum, and normal attachment of the junctional epithelium (Figure 3A,B). The histopathology of the periodontium of the rats subjected to ECP revealed edema, a large number of infiltrating polymorphonuclear cell coupled with severe destruction of cementum and apical migration of the junctional epithelium (Figure 3C,D). After treatment, the histopathology of the periodontium of the SO (Figure 3E), SCOMP (Figure 3F), and LCOMP (Figure 3G) groups after SRP revealed that all the treatment groups had a significant reduction in the numbers of inflammatory cell infiltration, as well as an inhibition destruction of cementum and apical migration of the junctional epithelium compared with ECP, whereas a more significant reduction in SRP+LCOPM was observed. Inflammatory score showed the Normal group, ECP group, SRP +SO, SRP +SCOMP, SRP +LCOMP received a median score of 0.0, 2.5, 1.75, 1.63, 0.95, respectively (Figure 3H).
Figure 3.
HE staining of the rat periodontia subjected to ECP and treated with SRP+antibiotics. Photomicrographs (A and B) showing the region around the first molars of normal rats; these indicate that the gingival, periodontal ligament, and cementum are normal. Rat periodontia subjected to ECP (C and D) showed inflammatory cell infiltration with apical migration of the junctional epithelium, as well as severe cementum destruction. SRP +SO (E), SRP +SCOMP (F), and SRP +LCOMP (G) all resulted in a significant reduction in the number of inflammatory cell infiltration and cementum destruction. Among those, SPR+LCOMP was most effective (HE stain; original magnification A, C ×400 B, D, E, F, G ×100). According to the evaluation standard, the scores ranged from a scale of 0 to 3. Data are medians with the range within the parentheses (H); n=4. a: vs. NORMAL P<0.05, b: vs. SRP+SO P<0.05, c: vs. SRP+SCOMP P<0.05 (Kruskal-Wallis and Dunn’ tests).
Discussion
Periodontitis was induced in rats by placing a thin steel ligature around the upper first molars [17,18] and inoculating them with Porphyromonas gingivalis 381 [19,20].
After 8 weeks, the periodontal health indicators in ECP rats, such as SBI, PI and PPD, showed statistically significant differences compared with normal rats. The disorders of these indicators indicated the success of using ECP. In order to further identify the reliable animal model, the analyses of GCF-AST, ABL and HE stain were conducted.
AST is particularly important in the transport of reducing equivalents across the mitochondrial membrane via the malate aspartate shuttle and is a sensitive indicator of necrosis in a number of tissues. During inflammation and cell death this enzyme is not utilized and liberated in extracellular fluids such as the cerebrospinal fluid, serum, joint fluid, and GCF where it can be detected [21]. In GCF, AST levels can be a useful adjunct as a biomarker in the assessment of periodontitis to distinguish between active and inactive disease sites. Thus, GCF-AST is predicted to increase during periodontitis. In the current study, the mean GCF-AST increased significantly in the ECP group compared with the normal group. This is consistent with the results presented in a previous study [22].
Morphometric measurements demonstrated that the rats subjected to ECP exhibited significantly more ABL than did normal rats. This agrees well with the result presented in a previous study [23].The histologic changes have been reported in periodontitis rats [16]. In our study, the histological analysis of the region around the first molars of normal rats shows the structure of the normal periodontium, where cementum and healthy gingival and periodontal ligaments can be observed. Histopathology observation in ECP rats revealed inflammatory cell infiltration, apical migration of the junctional epithelium, and severe cementum destruction resembling a periodontitis lesion.
Thus, a rat model of ECP, which can provide an important basis for follow-up treatment experiments, was successfully established.
The treatment of periodontitis should be primarily based on the mechanical debridement of subgingival plaque, because subgingival plaque and plaque-retaining factors in periodontal pockets (e.g., calculus or irregularities and endotoxin deposits of cementum) are associated with the destructive diseases [24]. However, mechanical debridement alone is unlikely to be sufficient to control periodontitis because of the location of the pathogenic bacteria within the gingival and dental tissues or in other areas inaccessible to mechanical instruments. Several studies have been performed to evaluate the efficacy of the combined mechanical treatment with drug administration. Bansalet et al. [25] used satranidazole-containing mucoadhesive gel as an adjunctive to mechanical treatment, getting an additional effect on the tested parameters, such as SBI, PI and PPD, as well as probing attachment levels. Studies have also been conducted to assess the efficacy of systemic administration after mechanical debridement [26,27]. Although the use of antibiotics alone, either locally or systemically, produced some beneficial effects on clinical parameters, it cannot be used as an alternative to mechanical treatment. Several studies compared the ability of pure systemic or local metronidazole to SRP to alter clinical indicators [28,29]. Their results suggest that the differences between the improvements were too small to justify the use of antibiotics instead of SRP. Thus, in the current study, we focused our attention on the treatment effects of SO, SCOPM and LCOPM as an adjunctive to mechanical therapy on the damaged tissue of rats subjected to ECP.
Nitroimidazoles (which include metronidazole, ornidazole, and tinidazole) are effective against anaerobic bacteria, rendering them specifically helpful for periodontal therapy. Among these nitroimidazoles, ornidazole is comparable to or has better antibacterial properties than metronidazole. Comparative pharmacokinetic studies have shown that ornidazole has a higher level of half-life elimination from plasma (14.4 h) than metronidazole (8.4 h); thus frequency of intake is reduced [30]. Kamma et al suggested that ornidazole, when combined with subgingival debridement, shows the ability for substantial clinical improvement due to the favorable alterations detected in the subgingival microflora. This indicates the effectiveness of ornidazole in the treatment of early-onset periodontitis, where anaerobic bacteria are predominant [7]. Pefloxacin mesylate is a quinolone with better antimicrobial activity against facultative anaerobes than nitroimidazole [10]. In periodontitis, anaerobes are the dominant microbial population, but facultative anaerobes also play a part in the breakdown of deep periodontal tissues [2]. To provide a significant antimicrobial activity, we designed COPM, which consists of ornidazole and pefloxacin mesylate.
Two ways to administer COMP, SCOMP and LCOMP are available. Systemic administration may be associated with bacterial resistance and adverse effects. Systemic pefloxacin is associated with irreversible peripheral neuropathy [31]. To minimize the adverse effects, we chose the sustained-release periodontal suppository as the LCOMP. The suppository was developed through the cold compression method in the Dental Research Centre of Daping Hospital, Third Military Medical University (Chongqing, China).
The precursor of LCOPM comprises EC, HPMC, ornidazole, pefloxacin mesylate, 95% ethanol, glycerine, and water. The flexibility of HPMC, a hydrophilic polymer matrix system, was used to obtain a desirable drug-release profile and broad regulatory acceptance [32]. However, for a free water-soluble drug, using a hydrophilic matrix system alone is restricted because of the rapid diffusion of the dissolved drug through the hydrophilic gel network. Therefore, EC, a hydrophobic polymer, along with HPMC, is suitable for developing sustained-release dosage forms. Incorporation of a high concentration of EC provides better control of the drug release, which could be attributed to the decreased penetration of the solvent molecules in the presence of hydrophobic polymer. Consequently, decreased diffusion of the drug from the matrix is achieved [33]. Furthermore, because of the presence of EC, which is generally responsible for the hardness of the suppository, the suppository is not easily cracked when held by forceps.
Two ways to administer COMP, SCOMP, and LCOMP are available. We chose the sustained-release periodontal suppository as the LCOMP. The suppository was developed through the cold compression method in the Dental Research Centre of Daping Hospital, the Third Military Medical University (Chongqing, China).
After treatment, compared with ECP, all the combined treatments produced beneficial changes in the clinical indicators on a short-term basis; SRP+LCOMP seems to be more effective in producing clinical improvements.
The histopathology of the periodontium of the SO, SCOMP, and LCOMP groups after SRP revealed that compared with ECP, SRP+SO, SRP+SCOMP, and SRP+LCOMP all resulted in a significant reduction in the numbers of inflammatory cell infiltration as well as in the inhibition of destruction of cementum, whereas a more significant reduction following SRP+LCOPM was observed.
Our findings are in accordance with several studies. In previous studies, Pradeep et al [34] reported that the adjunctive use of 0.5% azithromycin as a controlled drug-delivery system enhanced the clinical and microbiologic results of periodontitis. Similarly, a previous study [35] found a significant decrease in PPD, no further loss of attachment, and a decrease in SBI after treating severe chronic periodontitis (PPD ≥5 mm) with SRP and a controlled-release chlorhexidine chip. Another study [7] found that an adjunctive ornidazole is more effective in the treatment of early-onset periodontitis patients.
Therefore, the concentration of ornidazole and pefloxacin mesylate in the study may have reached levels above the threshold needed to suppress the pathogens. We noticed the more rapid improvement in clinical indicators and suggest that the combination of mechanical and LCOMP therapy accelerates the rate of healing of periodontal tissues and leads to a more favorable healing process.
Future studies must focus on 2 aspects: conducting a comparison with other similar local drug delivery systems in the treatment, and identifying the action mechanism of the COMP in periodontitis, such as in the areas of microbiology and immunohistochemistry.
Conclusions
In summary, during the treatment of periodontitis, SO, SCOMP, and LCOMP were adjuvant to SRP. All 3 treatment groups exerted potent anti-inflammatory effects, significantly inhibiting inflammation; among these, LCOMP was most effective.
Acknowledgements
We thank the Department of Stomatology of Daping Hospital for providing the sustained-release periodontal suppository, “Enpapers” and Miss Na Liu for editorial assistance with the manuscript.
Abbreviations
- ABL
alveolar bone loss
- AST
aspartate minotransferase
- EC
ethylcellulose
- ECP
experimental model of chronic periodontitis
- GCF
gingival crevicular fluid
- GM&P
gingival margin and gingival papilla
- HE
hematoxylin and eosin
- HPMC
hydroxypropyl methylcellulose
- LCOMP
local administration of compound ornidazole and pefloxacin mesylate
- PPD
probing pocket depth
- PI
plaque index
- SBI
sulcus bleeding index
- SCOPM
systemic administration of compound ornidazole and pefloxacin mesylate
- SO
systemic administration of ornidazole
- SRP
scaling and root planing
Footnotes
Source of support: This study was supported by a Grant from the Science and Technology Foundation of Chongqing, China (Project No.2004BB5065 and No.2009AC5019)
References
- 1.Sanz M, Lau L, Herrera D, et al. Methods of detection of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythensis in periodontal microbiology, with special emphasis on advanced molecular techniques: a review. J Clin Periodontol. 2004;31(12):1034–47. doi: 10.1111/j.1600-051X.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 2.Fujii R, Saito Y, Tokura Y, et al. Characterization of bacterial flora in persistent apical periodontitis lesions. Oral Microbiol Immunol. 2009;24(6):502–5. doi: 10.1111/j.1399-302X.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 3.Pizzo G, Guiglia R, Licata ME, et al. Effect of hormone replacement therapy (HRT) on periodontal status of postmenopausal women. Med Sci Monit. 2011;17(4):PH23–27. doi: 10.12659/MSM.881700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haffajee AD, Socransky SS. Relationship of cigarette smoking to the subgingival microbiota. J Clin Periodontol. 2001;28(5):377–88. doi: 10.1034/j.1600-051x.2001.028005377.x. [DOI] [PubMed] [Google Scholar]
- 5.von Troil B, Needleman I, Sanz M. A systematic review of the prevalence of root sensitivity following periodontal therapy. J Clin Periodontol. 2002;29(Suppl 3):173–77. doi: 10.1034/j.1600-051x.29.s3.10.x. discussion 195–96. [DOI] [PubMed] [Google Scholar]
- 6.Chung DT, Bogle G, Bernardini M, et al. Pain experienced by patients during periodontal maintenance. J Periodontol. 2003;74(9):1293–301. doi: 10.1902/jop.2003.74.9.1293. [DOI] [PubMed] [Google Scholar]
- 7.Kamma JJ, Nakou M, Mitsis FJ. The clinical and microbiological effects of systemic ornidazole in sites with and without subgingival debridement in early-onset periodontitis patients. J Periodontol. 2000;71(12):1862–73. doi: 10.1902/jop.2000.71.12.1862. [DOI] [PubMed] [Google Scholar]
- 8.Granizo JJ, Pia Rodicio M, Manso FJ, Gimenez MJ. Tinidazole: a classical anaerobical drug with multiple potential uses nowadays. Rev Esp Quimioter. 2009;22(2):106–14. [PubMed] [Google Scholar]
- 9.Poulet PP, Duffaut D, Barthet P, Brumpt I. Concentrations and in vivo antibacterial activity of spiramycin and metronidazole in patients with periodontitis treated with high-dose metronidazole and the spiramycin/metronidazole combination. J Antimicrob Chemother. 2005;55(3):347–51. doi: 10.1093/jac/dki013. [DOI] [PubMed] [Google Scholar]
- 10.Gascon AR, Gutierrez-Aragon G, Hernandez RM, Errasti J, Pedraz JL. Pharmacokinetics and tissue penetration of pefloxacin plus metronidazole after administration as surgical prophylaxis in colorectal surgery. Int J Clin Pharmacol Ther. 2003;41(6):267–74. doi: 10.5414/cpp41267. [DOI] [PubMed] [Google Scholar]
- 11.Ye ZW, Rombout P, Remon JP, et al. Correlation between the permeability of metoprolol tartrate through plasticized isolated ethylcellulose/hydroxypropyl methylcellulose films and drug release from reservoir pellets. Eur J Pharm Biopharm. 2007;67(2):485–90. doi: 10.1016/j.ejpb.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Keremi B, Lohinai Z, Komora P, et al. Antiinflammatory effect of BPC 157 on experimental periodontitis in rats. J Physiol Pharmacol. 2009;60(Suppl 7):115–22. [PubMed] [Google Scholar]
- 13.Kesavalu L, Sathishkumar S, Bakthavatchalu V, et al. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect Immun. 2007;75(4):1704–12. doi: 10.1128/IAI.00733-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loe H, Silness J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 15.Kamma JJ, Nakou M, Persson RG. Association of early onset periodontitis microbiota with aspartate aminotransferase activity in gingival crevicular fluid. J Clin Periodontol. 2001;28(12):1096–105. doi: 10.1034/j.1600-051x.2001.281203.x. [DOI] [PubMed] [Google Scholar]
- 16.Ridgeway EE. Periodontal disease: diagnosis and management. J Am Acad Nurse Pract. 2000;12(3):79–84. doi: 10.1111/j.1745-7599.2000.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 17.Struillou X, Boutigny H, Soueidan A, Layrolle P. Experimental animal models in periodontology: a review. Open Dent J. 2010;29(4):37–47. doi: 10.2174/1874210601004010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bezerra MM, Brito GA, Ribeiro RA, Rocha FA. Low-dose doxycycline prevents inflammatory bone resorption in rats. Braz J Med Biol Res. 2002;35(5):613–16. doi: 10.1590/s0100-879x2002000500015. [DOI] [PubMed] [Google Scholar]
- 19.Graves DT, Fine D, Teng YT, et al. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35(2):89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalla E, Lamster IB, Hofmann MA, et al. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2003;23(8):1405–11. doi: 10.1161/01.ATV.0000082462.26258.FE. [DOI] [PubMed] [Google Scholar]
- 21.Satoh H, Yamato O, Asano T, et al. Cerebrospinal fluid biomarkers showing neurodegeneration in dogs with GM1 gangliosidosis: possible use for assessment of a therapeutic regimen. Brain Res. 2007;1133(1):200–8. doi: 10.1016/j.brainres.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 22.Miller CS, Foley JD, Bailey AL, Campell CL. Current developments in salivary diagnostics. Biomark Med. 2010;4(1):171–89. doi: 10.2217/bmm.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinney JS, Ramseier CA, Giannobile WV. Oral fluid-based biomarkers of alveolar bone loss in periodontitis. Ann N Y Acad Sci. 2007;1098:230–51. doi: 10.1196/annals.1384.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niedzielska I, Janic T, Cierpka S, Swietochowska E. The effect of chronic periodontitis on the development of atherosclerosis: review of the literature. Med Sci Monit. 2008;14(7):RA103–6. [PubMed] [Google Scholar]
- 25.Bansal K, Rawat MK, Jain A, et al. Development of satranidazole mucoadhesive gel for the treatment of periodontitis. AAPS PharmSciTech. 2009;10(3):716–23. doi: 10.1208/s12249-009-9260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haasn AN, de Castro GD, Moreno T, et al. Azithromycin as an adjunctive treatment of aggressive periodontitis: 12-months randomized clinical trial. J Clin Periodontol. 2008;35(8):696–704. doi: 10.1111/j.1600-051X.2008.01254.x. [DOI] [PubMed] [Google Scholar]
- 27.Gapski R, Barr JL, Sarment DP, et al. Effect of systemic matrix metalloproteinase inhibition on periodontal wound repair: a proof of concept trial. J Periodontol. 2004;75(3):441–52. doi: 10.1902/jop.2004.75.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McColl E, Patel K, Dahlen G, et al. Supportive periodontal therapy using mechanical instrumentation or 2% minocycline gel: a 12 month randomized, controlled, single masked pilot study. J Clin Periodontol. 2006;33(2):141–50. doi: 10.1111/j.1600-051X.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- 29.Guarnelli ME, Farina R, Cucchi A, Trombelli L. Clinical and microbiological effects of mechanical instrumentation and local antimicrobials during periodontal supportive therapy in aggressive periodontitis patients: smoker versus non-smoker patients. J Clin Periodontol. 2010;37(11):998–1004. doi: 10.1111/j.1600-051X.2010.01623.x. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Cao G, Hu X, et al. Chiral separation of rac-Ornidazole and detection of the impurity of (R)-Ornidazole in (S)-Ornidazole injection and raw material. Chirality. 2006;18(8):587–91. doi: 10.1002/chir.20292. [DOI] [PubMed] [Google Scholar]
- 31.Leone R, Venegoni M, Motola D, et al. Adverse drug reactions related to the use of fluoroquinolone antimicrobials: an analysis of spontaneous reports and fluoroquinolone consumption data from three italian regions. Drug Saf. 2003;26(2):109–20. doi: 10.2165/00002018-200326020-00004. [DOI] [PubMed] [Google Scholar]
- 32.Michailova V, Titeva S, Kotsilkova R, et al. Water uptake and relaxation processes in mixed unlimited swelling hydrogels. Int J Pharm. 2000;209(1–2):45–56. doi: 10.1016/s0378-5173(00)00536-6. [DOI] [PubMed] [Google Scholar]
- 33.Reddy KR, Mutalik S, Reddy S. Once-daily sustained-release matrix tablets of nicorandil: formulation and in vitro evaluation. AAPS Pharm Sci Tech. 2003;4(4):61–69. doi: 10.1208/pt040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pradeep AR, Sagar SV, Daisy H. Clinical and microbiologic effects of subgingivally delivered 0.5% azithromycin in the treatment of chronic periodontitis. J Periodontol. 2008;79(11):2125–35. doi: 10.1902/jop.2008.070589. [DOI] [PubMed] [Google Scholar]
- 35.Kasaj A, Chiriachide A, Willershausen B. The adjunctive use of a controlled-release chlorhexidine chip following treatment with a new ultrasonic device in supportive periodontal therapy: a prospective, controlled clinical study. Int J Dent Hyg. 2007;5(4):225–31. doi: 10.1111/j.1601-5037.2007.00255.x. [DOI] [PubMed] [Google Scholar]