Summary
Background
A number of studies have evaluated exercise interventions compared with other treatment strategies for subjects with recurrent low back pain (LBP); however, subject pain level and balance were not carefully considered. The purpose of this study was to investigate the effectiveness of spinal stabilization exercises (SSE) for managing pain and increasing balance strategy changes following unexpected perturbations in patients diagnosed with recurrent LBP.
Material/Methods
Twenty-one age- and gender-matched patients participated in a supervised SSE or control exercise program 5 times a week over a 4-week period. The Million Visual Analogue Scale (MVAS) and Oswestry Disability Index (ODI) were used to measure each patient’s level of pain and disability. Balance measurements were derived from recordings of the anterior-posterior (A/P) and medio-lateral (M/L) center of pressure (COP) displacements during 3 consecutive, unexpected random perturbations.
Results
The level of reported pain and disability significantly decreased following treatment for both groups. Although the M/L sway was not significantly different in either group (p=0.86), there was a significant difference between group and measurement time during A/P sway (p=0.04). The A/P displacement of the SSE group significantly decreased compared with the control group. The decreased A/P displacement can be linked to the SSE intervention, which helps prevent further injury by limiting an individual’s response rate to external perturbations.
Conclusions
Clinicians might consider SSE for LBP patients as a possible rehabilitation strategy to reduce A/P displacement.
Keywords: balance of body, low back pain, stabilization exercises, center of pressure
Background
Exercise therapy is a widely used treatment for low back pain (LBP) and has been shown to be more effective than the usual care provided by general practitioners [1–3]. However, it is still unclear whether any specific type of exercise is more effective for pain relief and balance strategy than other exercise interventions. Recently, evidence-based practice has led to the need to provide evidence for which treatment, if any, is optimal for patients with chronic LBP [4]. However, to date, LBP studies lack evidence regarding both a valid diagnosis and the appropriate treatment [5].
Previous investigations that have attempted to identify the incidence of LBP include delayed response times in trunk muscle stabilizers, balance sway, and pain reduction [5–7]. These factors have resulted in increased muscular fatigue and decreased spinal stability [8–10]. In this regard, stabilization training might provide stability of the pelvic girdle and relieve pain for those who have recurrent LBP. However, there is limited and mostly inconclusive evidence for the effectiveness of specific exercises. An increasingly common approach utilized in the physical therapy management of LBP has been low load, high repetition training of the abdominal and trunk muscles for increasing stabilization or muscle imbalance training [11]. These practices were developed partially in response to evidence indicating specific neuromuscular alterations in the control and activation of the back and abdominal muscles in the presence of back pain conditions. However, there have been only a limited number of studies of balance changes in subjects with recurrent LBP. It is difficult to define precisely when pain becomes chronic, but as many as 70% to 80% of individuals continue to have LBP for 1 year after the initial onset [12]. This episodic nature of LBP also affects the individual’s ability to function in both work and personal life. Indeed, the need for further clinical studies on specific treatments such as spinal stabilization exercise (SSE) is imperative to understanding the mechanism of improvement for chronic back pain.
Postural sway is associated with postural alignment, postural strategies, and abnormal patterns of postural responses [13–15]. However, little evidence exists regarding balance and the level of pain changes in patients with LBP. Assessment of balance sway using center of pressure (COP) provides the characteristics of complex balance sway, since postural control is an integral part of motor control. It was reported that subjects with LBP demonstrated increased postural sway along the anterior-posterior (A/P) axis [16]. Other studies indicated that there was a significant increase in balance sway in the medio-lateral (M/L) direction in chronic LBP subjects [13]. However, a recent study contradicted these results by stating that individuals with LBP demonstrated reduced and delayed COP responses compared to healthy subjects [17]. This particular study’s results revealed that subjects with recurrent LBP might possess altered automatic postural coordination in terms of magnitude of responses, indicating alterations in neuromuscular control. It is also possible that the unbalanced trunk musculature in subjects with LBP may reduce kinematic displacement and create increased back muscle stiffness associated with co-contraction to avoid further injuries during daily activities [18,19].
Therefore, the purpose of this study was to determine the effectiveness of SSE in improving the level of pain and balance sway in patients with recurrent LBP following treatment. It was hypothesized that those who were treated with SSE would decrease their postural sway compared to the control group.
Material and Methods
Subjects
Subjects were recruited from the greater city Seoul, Korea. Subjects who expressed interest in the study became eligible for the study. Those subjects who met study inclusion criteria received information regarding the purpose and methods of the study and signed a copy of the Institutional Review Board approved consent form. In this study, patients with recurrent LBP were defined as those who met study inclusion criteria and experienced a disturbing impairment or abnormality in the functioning of the low back [20]. The patients with recurrent LBP were defined as those who previously experienced at least 1 episode of work-related back pain. Current diagnoses and prior injury data were based on both a physician’s history and physical exam results, which were obtained from the patients’ records. Subjects were eligible to participate if they: 1) were 21 years of age or older, 2) had at least 1 episode of work-related back pain without referred pain into the lower extremities, and 3) indicated a willingness to participate in a daily exercise program and in supervised exercise sessions 5 times a week for 4 weeks during the intervention period. Subjects were excluded from participation if they: 1) had a diagnosed mental illness that might interfere with the study protocol, 2) had difficulty in understanding written/spoken English that precluded them from completing questionnaires, 3) had overt neurological signs (sensory deficits or motor paralysis), or 4) were pregnant. Patient hand preference was also considered as previously reported [21], and hand dominance was determined by using a modified Edinburgh Handedness inventory [22] based on the performance of 10 everyday tasks such as writing, drawing, and throwing.
Participants were withdrawn from the study if they requested to withdraw. Those patients with LBP who met study inclusion criteria received information regarding the purpose and methods of the study and signed a copy of the Institutional Review Board-approved consent form.
Outcome measures
Patient pain was determined from self-reported scores on the Million Visual Analogue Scale (MVAS). The scale was administered to each patient at the initial and final testing sessions. Despite its relative lack of use in studies, the MVAS was primarily selected for its psychometric properties and was also chosen secondary to the promising initial investigations of its test-retest reliability [23,24]. The 15 items of this instrument are scored using an anchored visual analog scale to allow responses to range from best- to worst-case scenarios. Such scales inherently increase the response categories available for subjects and rely less on verbal skills. In addition, the visual analog scale is more sensitive to measured changes. Also, patient disability was inferred from self-reported scores on the Oswestry Disability Index (ODI), which was also given to each patient during the initial and final testing sessions. The ODI [25] is one of the most frequently used tools for measuring chronic disability. A sum is calculated and presented as a percentage, where 0% represents no disability and 100% represents the worst possible disability.
The balance sway range based on ground reaction forces was collected using a 6-channel force platform (Advanced Mechanical Technology, Watertown, MA) for 5 seconds during the task, with a sampling rate at 1000 Hz. The collected ground reaction force was filtered using zero-lag, fifth- order Butterworth with the cutoff frequency of 10 Hz during 3 consecutive, random perturbations. Before the experiment, data were collected from the unloaded platform to determine the zero offset. The COP excursion was obtained from force plate flexion-extension and lateral bend moments normalized by vertical force. The axes of the coordinate system were labeled x, y, and z. There were 3 components of force (Fx, Fy, Fz) and 3 components of moment (Mx, My, Mz). The amount of COP excursion was computed by the equation COPx=(−Fx−Fy)/Fz,, which is for the A/P direction, and COPy=(−Fy+Mx)/Fz, which is for the M/L displacement. The average A/P and M/L ranges were calculated to quantify cumulative postural sway over the full 5 seconds of the data collection period during each sudden perturbation.
For each perturbation, the sudden load protocol was applied for this study. Patients stood with knees extended, feet approximately shoulder-width apart, holding a pan with both hands, while maintaining 90 degrees of elbow flexion (Figure 1). Each patient was blindfolded and stood on top of the AMTI force platform. The COP displacement was computed in the A/P and M/L directions and was measured randomly during 3 occasions in response to sudden loads (dropping a 6.4 Newton weight approximately 1.8 meters onto a load cell mounted pan). The patients experienced the first ball drop without previous understanding of the characteristics of the ball; therefore, continuation of the learning effects was observed from these 3 trials. Data was collected using a customized software program written in LabVIEW V7.1 (National Instruments, Austin, TX). A greater neuromuscular demand to maintain standing balance is inferred if a larger displacement occurs. This method of testing was repeated following the completion of the 4-week exercise program.
Figure 1.
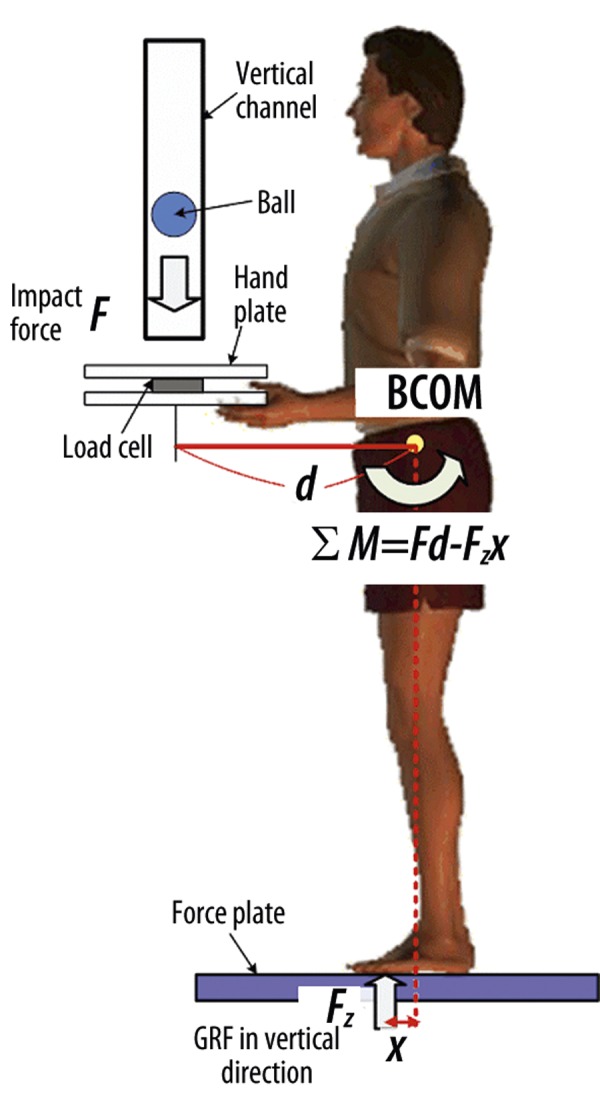
The set-up for the sudden load apparatus. A weighted tennis ball (6.4 N) is dropped onto a platform equipped with a load cell, which indicates the instant the ball hits the platform. (F: Force, BCOM: Body center of mass, GRF: Ground reaction force)
Randomization and treatment procedures
A randomization list was provided, with patients having an equal chance of being allocated to the intervention or control group. The coordinator ensured anonymity of allocation with respect to randomization. The randomization schedule was prepared prior to the beginning of the trial, and the coordinator was given a sealed envelope for each patient before the assessment. In addition to performing home exercises, the patients performed the 20-minute exercise session in the lab (supervised by the research coordinator) 3 times per week for 4 weeks to ensure that the exercises were being performed correctly. Patients kept an exercise log, and phone calls were made to ensure compliance with the exercise protocol.
Intervention and patient management
The SSE protocol was designed to improve spinal stabilization through core muscle strengthening rather than to improve spinal stabilization through low back muscles endurance or strengthening [3]. The SSE group performed specific localized exercises aimed at restoring the stabilizing protective function of the spinal muscles around the spinal joint. As applied by several authors, the exercises were designed specifically to activate and train the isometric holding function of the spinal muscle at the affected vertebral segment (in co-contraction with the transversus abdominis muscle); this rehabilitation approach is described in detail [3,5]. Patients from the SSE group were seen 3 times per week, but performed the exercises 5 times per week at home. The control group received a hard copy of medical management techniques, which included advice regarding bed rest, absence from work, prescription medications, and resuming normal activity as tolerated [26].
Statistical analysis
Statistical analyses were completed using SPSS 16 (SPSS, Chicago, IL). Normality was assessed for each of the dependent variables. The independent variables included age, hand dominance, gender, and number of months since initial onset of pain. The Kolmogorov-Smirnov test was conducted for the dependent variables (pain/disability scores, A/P and M/L balance sways). A power analysis was also conducted to ensure an appropriate sample size. We inspected descriptive statistics for sample characteristics and scatter plots of the data to ensure that no outliers existed in the data set.
The level of pain/disability scores was evaluated by independent t-test in order to detect any differences between groups. The mixed repeated measure analysis of variance (ANOVA) was used based on A/P and M/L balance sway changes during sudden perturbations. A Bonferroni post-hoc test was employed to determine which perturbations were significantly different for the A/P and M/L balance sways.
To take into account correlated error structure, a general estimating equation (GEE) with the Hubar-White correction for robust standard errors was performed to analyze balance sways. The GEE approach has important advantages over fixed and random effects models and requires only first and second moments of the dependent variable. Therefore, by implementing the GEE approach, parametric assumptions about unknown distributions and the correlation structure of observations can be avoided [27,28]. In addition, GEE produces estimators even when the arbitrarily chosen correlation structure among observations is not overtly specified.
Preliminary statistical power analyses associated with comparing the two independent treatment groups, conducted with 2-tailed testing and assuming effect sizes of 0.2, 0.4, 0.6, 0.7, 0.8, and 0.9, produced estimated power values of 0.10, 0.26, 0.50, 0.63, 0.75, and 0.84, respectively. Since these power estimates would be associated with follow-up tests of simple effects, they are potentially conservative. For all statistical tests, type I error rate was set at 0.05. In addition, possible effects of age, gender, and length of time since initial onset of pain were also considered by adding these continuous variables to the model using ANCOVA procedures.
Results
As shown in Table 1, 42 patients enrolled in the study, and the average patient age was 52.0±7.37 years, ranging from 37 to 64 years of age. Treatment assignment did not demonstrate significant differences based on patient age, with the average age being 53.09±9.04 years for the SSE and 50.90±5.24 years of age for the control (p=0.34) groups. Assignment to treatment was not significantly different across genders (p=0.75). The number of months since pain onset averaged 11.1±6.25 months and ranged from 2 to 24 months. Age and number of months since initial pain episode were not significantly correlated, with Pearson r=−0.11 (p=0.47). The mean times since pain onset between the SSE intervention group (10.09±7.0 months; range=4 to 24 months) and the control group (11.23±5.3 months; range=2 to 24 months) were not significantly different (p=0.55). The body mass index (BMI) did not differ significantly between groups (26.83±3.61 vs. 25.04±3.02, respectively, p=0.62).
Table 1.
Summary of subject demographics and bivariate relationship of treatment group with selected demographics.
| Variable | Total | SSE group | Control group | p |
|---|---|---|---|---|
| N | 42 | 21 | 21 | |
|
| ||||
| Gender | ||||
| Male | 21 | 10 | 11 | 0.75 |
| Female | 21 | 11 | 10 | |
|
| ||||
| Body Mass Index | 25.94±3.31 | 26.83±3.61 | 25.04±3.02 | 0.62 |
|
| ||||
| Age (mean ±SD) | 50.19±9.28 | 53.09±9.04 | 50.90±5.24 | 0.34 |
|
| ||||
| Number of months since initial pain onset (mean ±SD) | 11.06±6.25 | 10.09±7.00 | 11.23±5.33 | 0.55 |
Data are given as mean (SD) except where noted;
SSE – spinal stabilization exercise; p – probability; N – number of cases; Age – years for age.
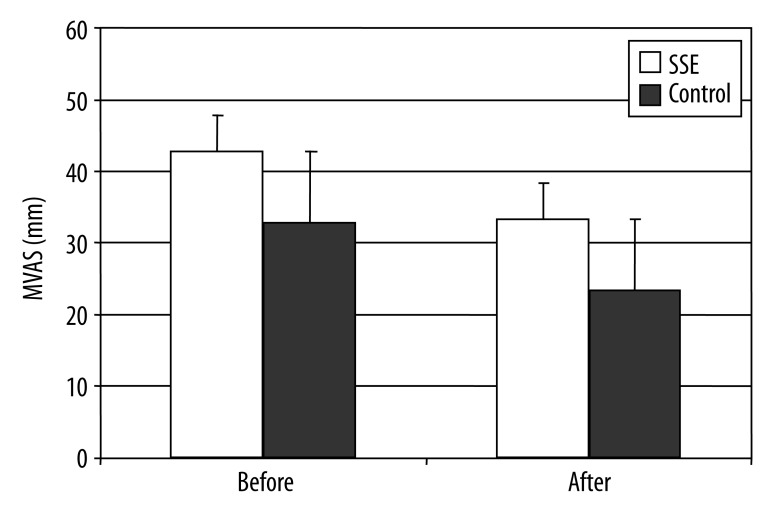
Where higher MVAS scores indicate worse health status, the level of pain significantly decreased before and after treatment intervention (42.70±13.80 vs. 33.26±15.27, respectively) for the SSE group (Figure 2). For the control group, the scores also significantly decreased before and after treatment intervention (32.81±10.85 vs. 23.42±13.43, respectively). Although both groups reported decreased or improved levels of pain from pre- to post-treatment, the SSE group demonstrated higher pain reduction compared to the control group (p<0.01).
Figure 2.
The level of pain changes based on MVAS following intervention (F=7.38, p<0.01).
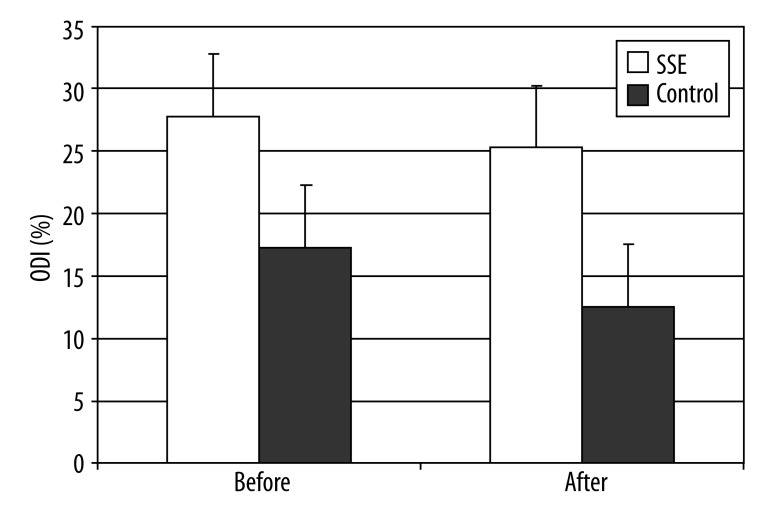
For the ODI, where high scores indicate worse health status, pre-treatment assessment scores were 17.29±9.15 vs. 12.52±8.50 for the SSE group and control groups, respectively, p<0.001. Accordingly, post-treatment assessment scores were 27.76±12.11 vs. 25.29±12.59 for the SSE and control groups, respectively (Figure 3). Overall, the SSE group demonstrated higher pain reduction scores when compared to the control group (p<0.001). The ODI post-treatment outcome was significantly related to the pre-treatment ODI score (p<0.001) and unrelated to age (p<0.92), gender (p<0.67), and weight (p<0.50) covariates. A modest trend for a treatment effect (p<0.092) was also apparent.
Figure 3.
The level of pain changes based on ODI following intervention (F=14.18, p<0.001).
Balance sway changes in quiet standing were evaluated based on sway in both the A/P and M/L directions of COP displacement. A preliminary evaluation to examine pre-treatment differences in the outcomes indicated no significant effects following treatment. The A/P COP sway decreased in both groups following intervention. As indicated in Tables 2 and 3, the A/P balance sway changes were significantly different in the SSE group compared to the control group (p=0.04), while the M/L sway displacement did not differ among groups (p=0.86).
Table 2.
The A/P displacement changes following intervention during repeated perturbations.
| Coef. | Std. err. | z | P>|z| | [95% Conf. Interval] | ||
|---|---|---|---|---|---|---|
| Age | −0.0012 | 0.0013 | −0.92 | 0.359 | −0.0038 | 0.0013 |
| Dominance | 0.0116 | 0.0266 | 0.44 | 0.663 | −0.0406 | 0.0639 |
| Gender | −0.0110 | 0.0236 | −0.47 | 0.640 | −0.0573 | 0.0352 |
| Duration | 0.0028 | 0.0020 | 1.40 | 0.161 | −0.0011 | 0.0068 |
| Treatment | −0.0559 | 0.0275 | −2.03 | 0.042* | −0.1098 | −0.0020 |
| ODI | 0.1272 | 0.156 | 0.81 | 0.416 | −0.1793 | 0.4339 |
| MVAS | −0.0112 | 0.0155 | −0.72 | 0.470 | −0.0416 | 0.0192 |
| Constant | 0.0952 | 0.1025 | 0.93 | 0.353 | −0.1058 | 0.2962 |
p<0.05; A/P, ODI, MVAS abbreviations.
Table 3.
The M/L displacement changes following intervention during repeated perturbations.
| Coef. | Std. err. | z | P>|z| | [95% Conf. Interval] | ||
|---|---|---|---|---|---|---|
| Age | 0.0005 | 0.0013 | 0.40 | 0.687 | −0.0021 | 0.0032 |
| Dominance | 0.0317 | 0.03452 | 0.92 | 0.359 | −0.0360 | 0.0994 |
| Gender | −0.0180 | 0.0227 | −0.79 | 0.429 | −0.0627 | 0.0266 |
| Duration | 0.0023 | 0.0027 | 0.88 | 0.380 | −0.0029 | 0.0077 |
| Treatment | −0.0040 | 0.02317 | −0.18 | 0.861 | −0.0494 | 0.0413 |
| ODI | 0.1241 | 0.1386 | 0.90 | 0.371 | −0.1475 | 0.3957 |
| MVAS | −0.0144 | 0.0121 | −1.19 | 0.233 | −0.0382 | 0.0092 |
| Constant | 0.0060 | 0.1048 | 0.06 | 0.954 | −0.1995 | 0.2115 |
p<0.05; M/L, ODI, MVAS abbreviations.
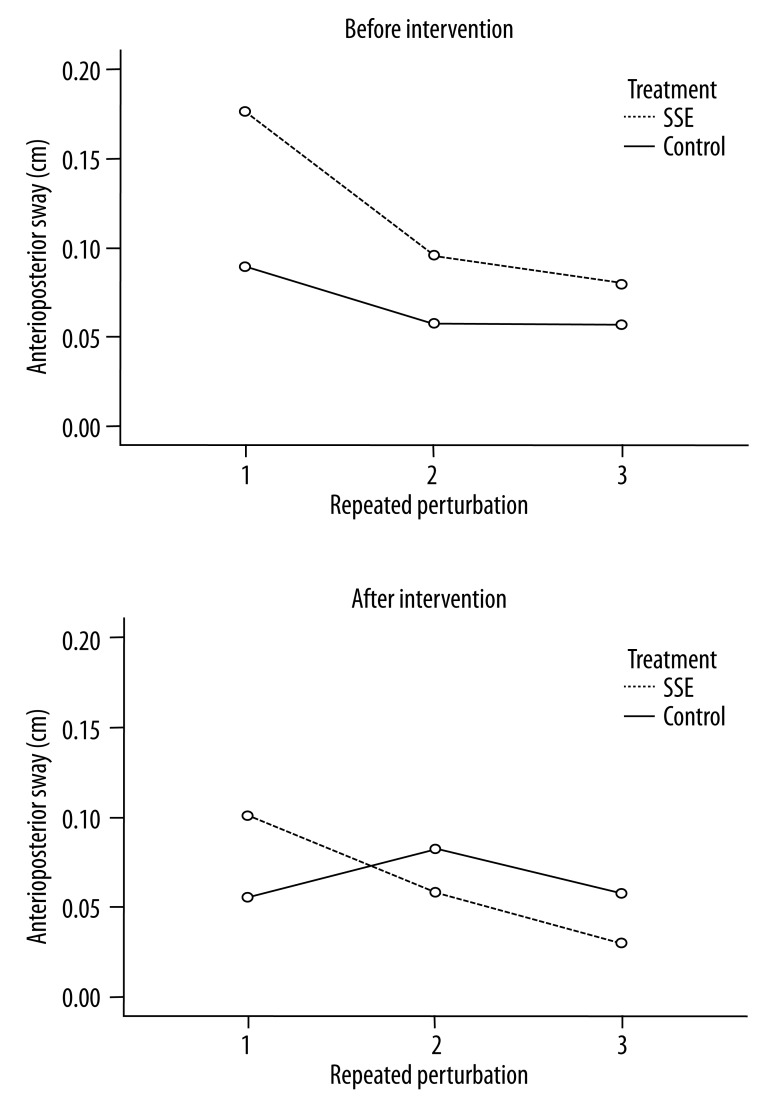
For the A/P displacement of balance sway, age, hand dominance, gender, length of duration since pain onset, ODI, and MVAS were not statistically significant. These results also indicated that the A/P sway in the SSE group decreased following intervention over time (Figure 4). The A/P sway changes in the group following SSE decreased over time, especially in the A/P direction during the second perturbation, compared to the control group. There were interactions in group × perturbation (p=0.01), and there was a significant difference following treatment (p<0.001) and repeated perturbations (p<0.001). The Bonferroni post-hoc pairwise comparison test indicated that postural sway significantly increased during the first perturbation compared to the third perturbation; however, the first perturbation was not statistically different from the second perturbation.
Figure 4.
The A/P displacement changes before and after intervention during repeated perturbations. The A/P sway changes in the group following SSE decreased compared to the control group over time, especially following the first perturbation. The pairwise comparison test indicated that the sway from the first perturbation significantly increased compared to the third perturbation.
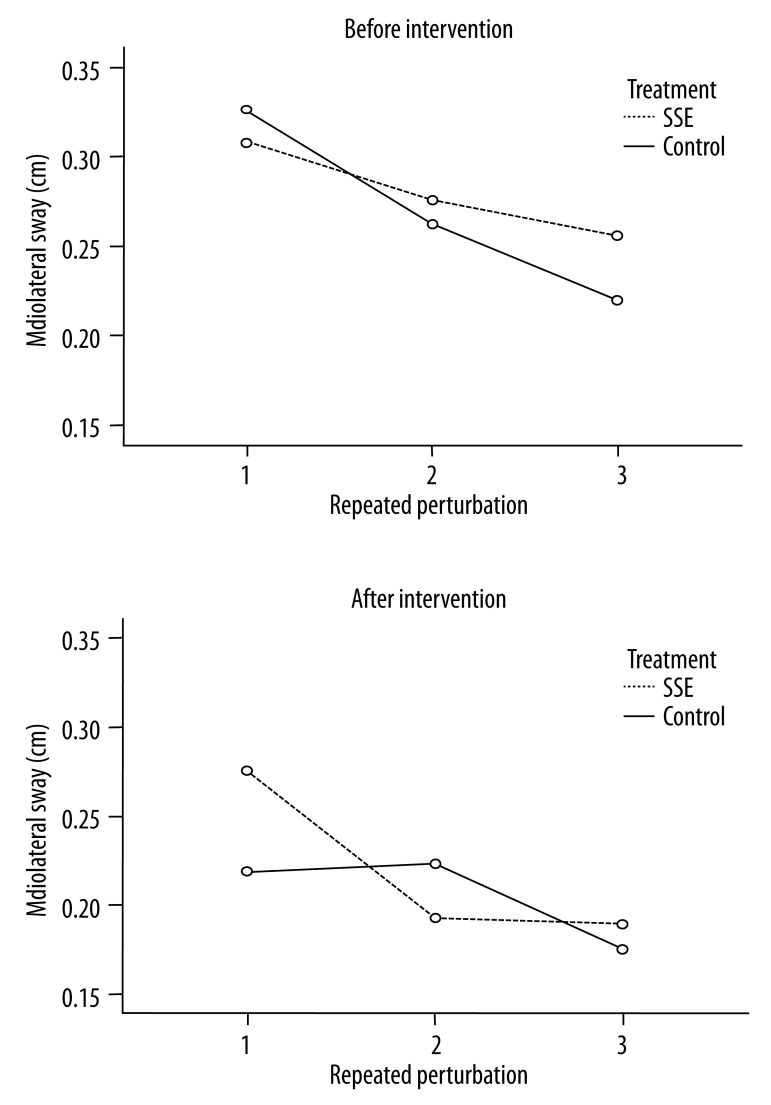
In Figure 5, the M/L COP sway displacement also changed slightly following intervention in group × perturbation (p=0.49). There was a significant difference following treatment (p<0.04) and repeated perturbations (p<0.001). The Bonferroni pairwise comparison test indicated that the first perturbation was significantly greater than both the second and third perturbations.
Figure 5.
The M/L displacement changes before and after intervention during repeated perturbations. The M/L sway changes in the group following SSE decreased compared to the control group over time, especially following the first perturbation.
Discussion
This study investigated the differences between SSE and control treatments for patients with LBP. The patients in both groups reported decreased pain levels following treatment. Overall, A/P and M/L COP sway also improved in both groups, and the A/P balance sway significantly decreased in the SSE group compared to the control group. Although the M/L sway was not significantly different in either group, there was a significant interaction between group and measurement time in the A/P displacement.
The post-treatment MVAS scores, adjusted for all covariates, indicated that the SSE group scores were slightly higher than the control group scores. The decreased level of pain in the SSE group changed from a moderate to a mild range in accordance with the guidelines [29]. This improvement is important since the minimum clinically significant difference in MVAS pain scores was found to be 9 mm [30]. Therefore, the amount of pain reduction reported by patients in the SSE group is important to consider since the MVAS systematically assesses the level of pain and potentially predicts treatment outcomes in patients with LBP.
The results also indicated that there was no difference in pain based on age, gender, and BMI; however, previous studies have indicated that men and women can differ in self-reported levels of pain and the relative importance of pain to each gender [30,31]. Female patients report more frequent use of several coping strategies, which are unrelated to their appraisal of pain [31]. Appraisal of pain may have important implications for coping as well as contributing to the overall well-being of women and men. In our study, we hypothesized that it might be related to a dose-response problem, where significant changes in the level of pain may take longer than 4 weeks to become detectable [3]. The fact that the level of pain improved across time, but not necessarily across treatments, suggests that overall improvement in pain scores may lag behind improvements in the aspects of overall health status. As Ward and Leigh noted, it is possible that pain was a larger contributor to the measurement of overall health status than individual physical disability [32]. As they indicated, disability appeared to be more important for males, while other reports indicated that the presence of pain appears to predict the progression of disability.
The COP displacement, which has been used to characterize a neurological disorder during standing, represents the distribution of the total force applied to the feet [15]. The balance impairments in patients with LBP were thought to be secondary to a limitation in using appropriate balance strategies caused by the adoption of a pain-relieving hyper-lordotic lumbar posture in standing [15]. Although changes in trunk musculature control may lead to pain, it may also cause excitability changes in the motor pathway. It has been reported that the central nervous system does not simply stiffen the spine, but actively controls movements to maintain posture equilibrium [33]. The sudden perturbation events are not an actual situation, and possible learning effects may affect the results. However, this perturbation was applied to a controlled situation without any practice for both groups. In addition, the covariates were analyzed during data analyses to control initial differences between groups. Specific motor control dysfunctions might result in faulty movement strategies, which could be corrected by properly coordinating abdominal and back musculature in patients with chronic LBP.
The effects of SSE for subjects with LBP have not been investigated with regard to pain level and postural changes. In our study, the displacement of the interaction effect indicated that the SSE group revealed decreased balance sway compared to the control group, especially in the A/P direction during the third perturbation. However, the balance sway results of this study are inconsistent with other studies [13,34]. Another study compared impaired subjects to a healthy control group, and the impaired group was found to have a greater degree of sway compared to the healthy group [34]. The SSE exercises might be beneficial because postural sway has been associated with low back symptoms in a working population [35]. The results from our investigation might be different due to additionally detected impairments in balance performance among subjects with pronounced functional limitations and severe LBP problems [34,35].
This study provides information regarding impaired body balance associated with trunk muscle imbalance, which can be used to improve treatment strategies. It has been reported that patients with LBP show increased postural sway along the A/P displacement [16]. Therefore, the displacement of A/P COP sway might be linked to both passive components of musculoskeletal function and active muscular tension, which decrease body sway capacity. It is possible that the postural compensation strategies found following SSE might be enhanced for patients with LBP. Limitations in this study indicated that there was no evidence to suggest that the exercise programs would have similar dose/response curves or that a 4-week period would represent a reasonable intervention time for either program. If motor control retraining is found to be an integral part of the treatment of LBP, further evaluation of the dominant side and differential effectiveness of treatment may be important for establishing a mechanism of action for exercise therapies.
Conclusions
The results of this study indicated significant improvements in pain and balance sway performance, especially regarding A/P COP sway, in patients with LBP. This A/P COP sway suggests that the use of a SSE program might enhance the coordination of postural adjustability. Although examining postural sway in a sitting position is necessary to investigate the association, this study suggests that SSE therapy might be associated with neuromuscular mechanisms to compensate for postural control. Follow-up studies are needed to investigate the characteristics of the back muscles and the factors mediating neuromuscular differences in patients with LBP. The improvement of A/P balance in the SSE group indicates that postural adjustability might help patients avoid further injuries following repeated unexpected perturbations.
Footnotes
Source of support: This work was supported by the National Agenda Project (NAP) funded by the Korea Research Council of Fundamental Science & Technology (P-09-JC-LU63-C01). This research was also partially supported by Korea University and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2010-0003015)
References
- 1.van Tulder MW, Ostelo R, Vlaeyen JW, et al. Behavioral treatment for chronic low back pain: a systematic review within the framework of the Cochrane Back Review Group. Spine. 2001;26:270–81. doi: 10.1097/00007632-200102010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Abenhaim L, Rossignol M, Valat JP, et al. The role of activity in the therapeutic management of back pain. Report of the International Paris Task Force on Back Pain. Spine (Phila Pa 1976) 2000;25:1S–33S. doi: 10.1097/00007632-200002151-00001. [DOI] [PubMed] [Google Scholar]
- 3.Sung PS. Multifidi muscles median frequency before and after spinal stabilization exercises. Arch Phys Med Rehabil. 2003;84:1313–18. doi: 10.1016/s0003-9993(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 4.Borkan JM, Cherkin DC. An agenda for primary care research on low back pain. Spine. 1996;21:2880–84. doi: 10.1097/00007632-199612150-00019. [DOI] [PubMed] [Google Scholar]
- 5.Lee TR, Kim YH, Sung PS. Spectral and entropy changes for back muscle fatigability following spinal stabilization exercises. J Rehabil Res Dev. 2010;47:133–42. doi: 10.1682/jrrd.2009.07.0088. [DOI] [PubMed] [Google Scholar]
- 6.Lee T, Kim YH, Sung PS. A comparison of pain level and entropy changes following core stability exercise intervention. Med Sci Monit. 2011;17(7):CR362–68. doi: 10.12659/MSM.881846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung PS, Zurcher U, Kaufman M. Nonlinear analysis of electromyography time series as a diagnostic tool for low back pain. Med Sci Monit. 2005;11(1):CS1–5. [PubMed] [Google Scholar]
- 8.Hodges PW, Richardson CA. Contraction of the abdominal muscles associated with movement of the lower limb. Phys Ther. 1997;77:132–42. doi: 10.1093/ptj/77.2.132. discussion 142–44. [DOI] [PubMed] [Google Scholar]
- 9.Sparto PJ, Parnianpour M, Reinsel TE, Simon S. The effect of fatigue on multijoint kinematics, coordination, and postural stability during a repetitive lifting test. J Orthop Sports Phys Ther. 1997;25:3–12. doi: 10.2519/jospt.1997.25.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Turner CH. Effects of tissue viscoelasticity on mechanical loading models using rats. Bone. 1999;25:742. doi: 10.1016/s8756-3282(99)00233-1. [DOI] [PubMed] [Google Scholar]
- 11.Richardson CA, Jull GA, Hodges P, Hides J. Therapeutic exercise for spinal segmental stabilization in low back pain. London: Harcourt Publishers; 1999. [Google Scholar]
- 12.Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine. 2000;25:3140–51. doi: 10.1097/00007632-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 13.Mientjes MI, Frank JS. Balance in chronic low back pain patients compared to healthy people under various conditions in upright standing. Clin Biomech (Bristol, Avon) 1999;14:710–16. doi: 10.1016/s0268-0033(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 14.Nault ML, Allard P, Hinse S, et al. Relations between standing stability and body posture parameters in adolescent idiopathic scoliosis. Spine. 2002;27:1911–17. doi: 10.1097/00007632-200209010-00018. [DOI] [PubMed] [Google Scholar]
- 15.Nies N, Sinnott PL. Variations in balance and body sway in middle-aged adults. Subjects with healthy backs compared with subjects with low-back dysfunction. Spine. 1991;16:325–30. doi: 10.1097/00007632-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Hamaoui A, Do MC, Bouisset S. Postural sway increase in low back pain subjects is not related to reduced spine range of motion. Neurosci Lett. 2004;357:135–38. doi: 10.1016/j.neulet.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 17.Henry SM, Hitt JR, Jones SL, Bunn JY. Decreased limits of stability in response to postural perturbations in subjects with low back pain. Clin Biomech (Bristol, Avon) 2006;21:881–92. doi: 10.1016/j.clinbiomech.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Cholewicki J, Juluru K, Radebold A, et al. Lumbar spine stability can be augmented with an abdominal belt and/or increased intra-abdominal pressure. Eur Spine J. 1999;8:388–95. doi: 10.1007/s005860050192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas JS, Lavender SA, Corcos DM, Andersson GB. Trunk kinematics and trunk muscle activity during a rapidly applied load. J Electromyogr Kinesiol. 1998;8:215–25. doi: 10.1016/s1050-6411(98)00008-x. [DOI] [PubMed] [Google Scholar]
- 20.Klenerman L, Slade PD, Stanley IM, et al. The prediction of chronicity in patients with an acute attack of low back pain in a general practice setting. Spine. 1995;20:478–84. doi: 10.1097/00007632-199502001-00012. [DOI] [PubMed] [Google Scholar]
- 21.Sung PS, Spratt KF, Wilder DG. A possible methodological flaw in comparing dominant and nondominant sided lumbar spine muscle responses without simultaneously considering hand dominance. Spine. 2004;29:1914–22. doi: 10.1097/01.brs.0000137071.47606.19. [DOI] [PubMed] [Google Scholar]
- 22.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 23.Alaranta H, Rytokoski U, Rissanen A, et al. Intensive physical and psychosocial training program for patients with chronic low back pain. A controlled clinical trial. Spine. 1994;19:1339–49. doi: 10.1097/00007632-199406000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Million R, Hall W, Nilsen KH, Baker RD, Jayson MI. Assessment of the progress of the back-pain patient 1981 Volvo Award in Clinical Science. Spine. 1982;7:204–12. doi: 10.1097/00007632-198205000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–73. [PubMed] [Google Scholar]
- 26.Hides JA, Jull GA, Richardson CA. Long-term effects of specific stabilizing exercises for first-episode low back pain. Spine. 2001;26:E243–48. doi: 10.1097/00007632-200106010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Jonasdottir G, Palmgren J, Humphreys K. Analysis of binary traits: testing association in the presence of linkage. BMC Genet. 2005;6(Suppl 1):S92. doi: 10.1186/1471-2156-6-S1-S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 29.Anagnostis C, Mayer TG, Gatchel RJ, Proctor TJ. The million visual analog scale: its utility for predicting tertiary rehabilitation outcomes. Spine. 2003;28:1051–60. doi: 10.1097/01.BRS.0000061989.94487.9B. [DOI] [PubMed] [Google Scholar]
- 30.Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Acad Emerg Med. 1998;5:1086–90. doi: 10.1111/j.1553-2712.1998.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 31.Unruh AM, Ritchie J, Merskey H. Does gender affect appraisal of pain and pain coping strategies? Clin J Pain. 1999;15:31–40. doi: 10.1097/00002508-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Ward MM, Leigh JP. The relative importance of pain and functional disability to patients with rheumatoid arthritis. J Rheumatol. 1993;20:1494–99. [PubMed] [Google Scholar]
- 33.Hodges PW, Moseley GL. Pain and motor control of the lumbopelvic region: effect and possible mechanisms. J Electromyogr Kinesiol. 2003;13:361–70. doi: 10.1016/s1050-6411(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 34.Kuukkanen TM, Malkia EA. An experimental controlled study on postural sway and therapeutic exercise in subjects with low back pain. Clin Rehabil. 2000;14:192–202. doi: 10.1191/026921500667300454. [DOI] [PubMed] [Google Scholar]
- 35.Takala EP, Korhonen I, Viikari-Juntura E. Postural sway and stepping response among working population: reproducibility, long-term stability, and associations with symptoms of the low back. Clin Biomech (Bristol, Avon) 1997;12:429–37. doi: 10.1016/s0268-0033(97)00033-8. [DOI] [PubMed] [Google Scholar]