Abstract
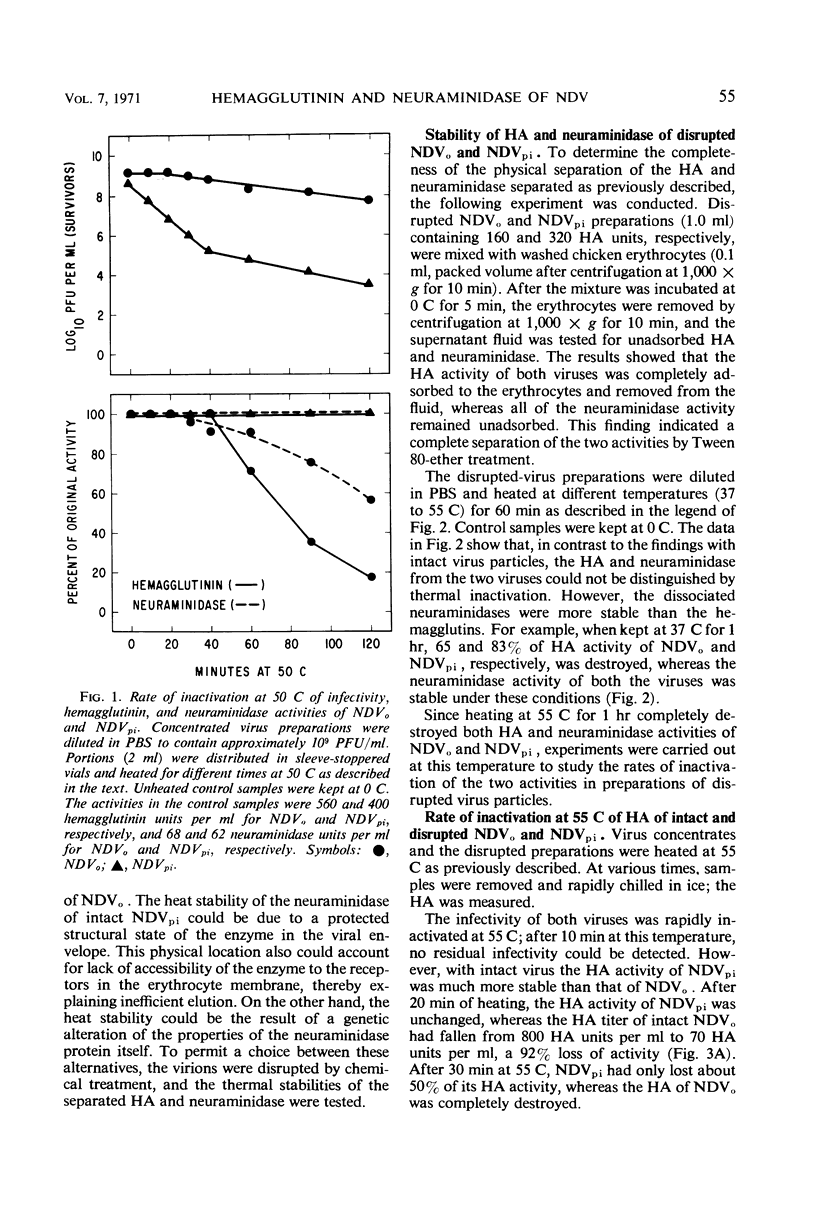
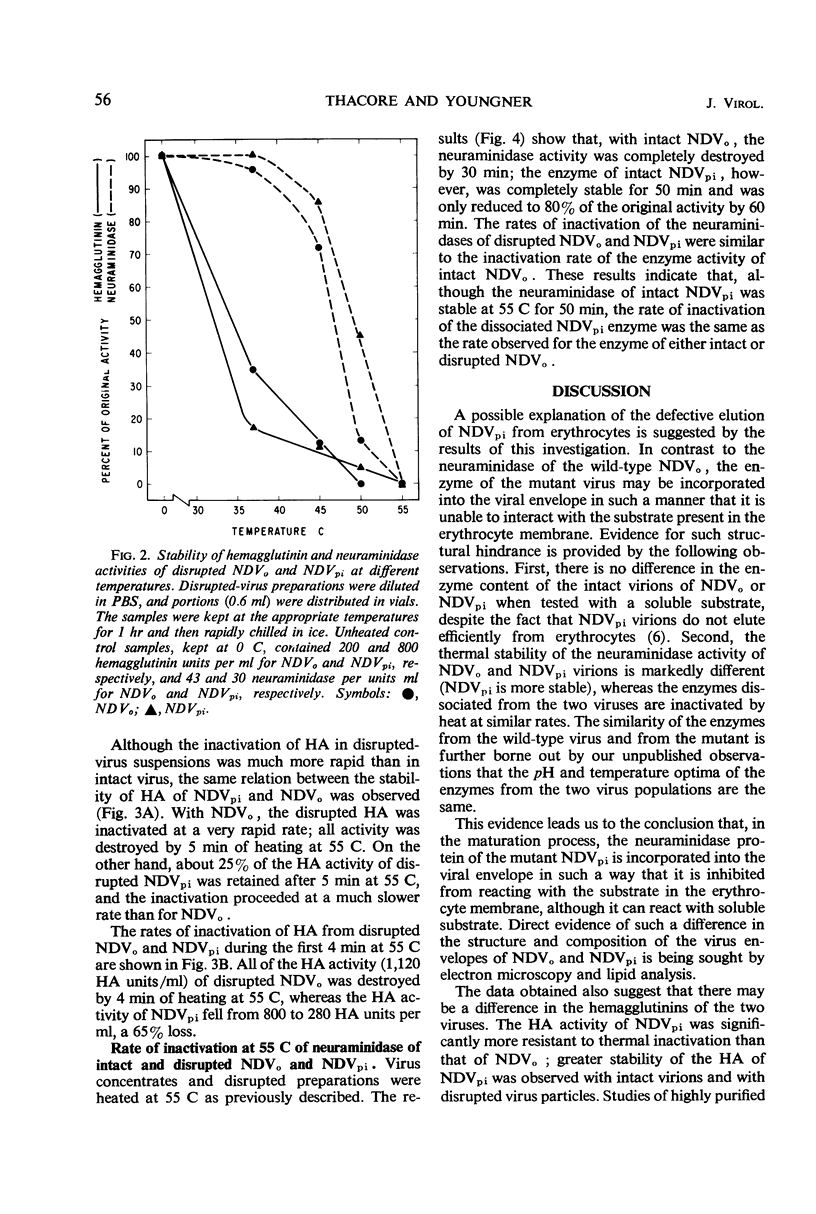
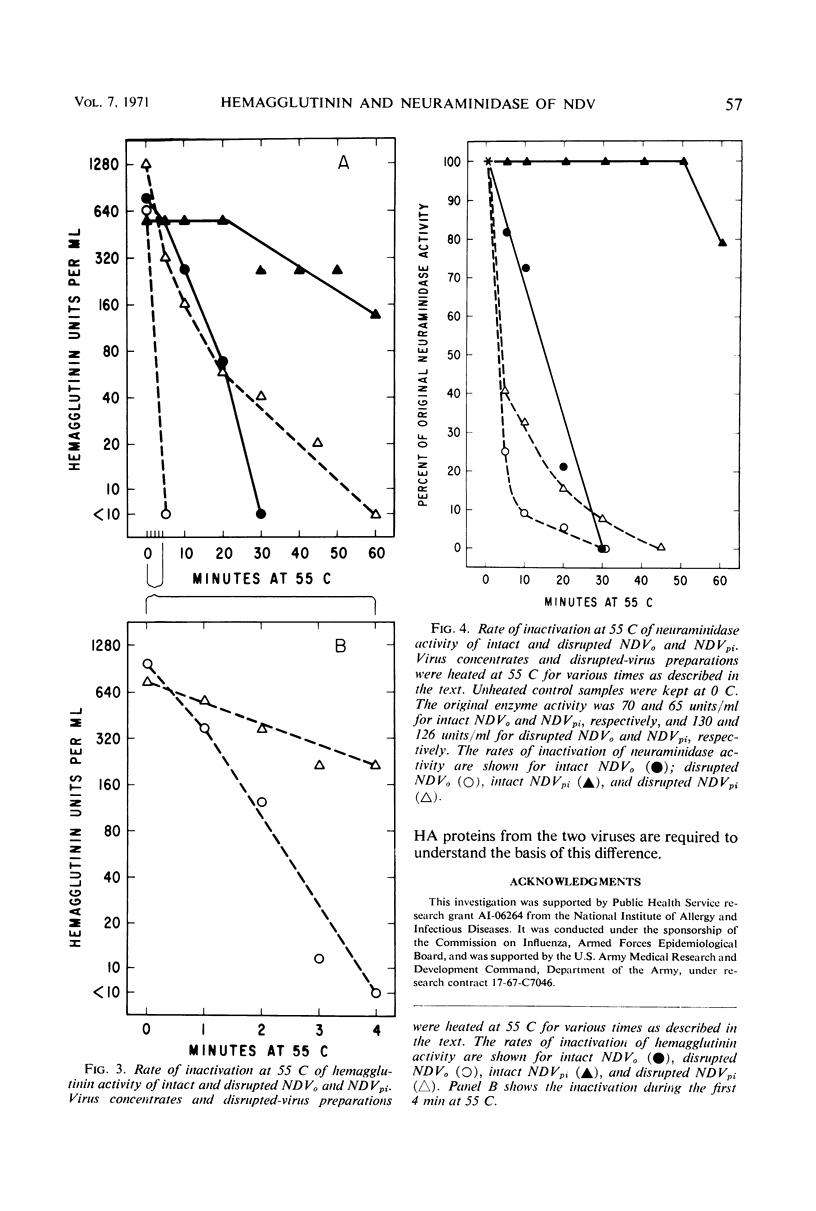
Data were obtained which indicated the possible cause of the defective elution from erythrocytes of the mutant virus (NDVpi) isolated from L cells persistently infected with the Herts strain of Newcastle disease virus (NDVo). The chicken erythrocyte receptors for the mutant and wild-type viruses were equally sensitive to the action of Vibrio cholera filtrate neuraminidase; this suggests that the failure of NDVpi to elute from chicken erythrocytes is not due to a specific neuraminidase-resistant receptor for this virus on the erythrocyte membrane. There was no difference in the enzyme content of the intact virions of NDVo and NDVpi when tested with a soluble substrate, indicating that the inefficient elution of NDVpi was not due to a reduced enzyme content. The neuraminidase activity of intact NDVpi virions was significantly more stable at 55 C than the enzyme of NDVo virions, whereas the dissociated enzymes of the two viruses were inactivated at the same rate. On the basis of these findings, it seems likely there is a structural difference between the two viruses. The neuraminidase protein of the mutant NDVpi may be incorporated into the viral envelope in such a manner that it is prevented from reacting with the substrate in the erythrocyte membrane, although it can react with a soluble substrate. The hemagglutinin activity of both intact and disrupted NDVpi was significantly more resistant to thermal inactivation than that of the wild-type NDVo. This finding suggests a genetic difference in the hemagglutinin protein of the two viruses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DiGioia G. A., Licciardello J. J., Nickerson J. T., Goldblith S. A. Thermal inactivation of Newcastle disease virus. Appl Microbiol. 1970 Mar;19(3):451–454. doi: 10.1128/am.19.3.451-454.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam E. A., Cheyne I. M., White D. O. The structural proteins of Newcastle disease virus. Virology. 1969 Sep;39(1):118–129. doi: 10.1016/0042-6822(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Kilbourne E. D. Identification in a recombinant influenza virus of structural proteins derived from both parents. Virology. 1966 Nov;30(3):493–501. doi: 10.1016/0042-6822(66)90125-5. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with Newcastle disease virus. II. Ribonucleic acid and protein synthesis in cells infected with mutants isolated from persistently infected L cells. J Virol. 1970 Jul;6(1):42–48. doi: 10.1128/jvi.6.1.42-48.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with newcastle disease virus: I. Properties of mutants isolated from persistently infected L cells. J Virol. 1969 Sep;4(3):244–251. doi: 10.1128/jvi.4.3.244-251.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Scott A. W., Hallum J. V., Stinebring W. R. Interferon production by inactivated Newcastle disease virus in cell cultures and in mice. J Bacteriol. 1966 Oct;92(4):862–868. doi: 10.1128/jb.92.4.862-868.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



