Summary
Background
Abnormal regulation of Wnt/β-catenin signaling and subsequently increased β-catenin expression have been found to be involved in the proliferation and growth of colon cancer cells. Whether the down-regulation of β-catenin in colon cancer may result in compromised invasion and migration in vitro still remains to be determined.
Material/Methods
A human colon cancer cell line (LoVo cells) was transfected with small interfering RNA (siRNA) targeting β-catenin. RT-PCR, Western blot assay, flow cytometry, cell adhesion assay, scratch wound assay, and matrigel invasion assay were performed, and the correlation between cell invasion and migration and β-catenin expressions was analyzed.
Results
siRNA-mediated down-regulation of β-catenin elevated the E-cadherin expression but reduced the MMP-7 and CD44v6 expressions, which increased the adhesion between LoVo cells but decreased the adhesion of LoVo cells to fibronectin. Significant inhibition of cell invasion and migration was also observed following RNA interference with β-catenin siRNA.
Conclusions
siRNA-mediated downregulation of β-catenin could be valuable for defining gene expression and functional programs downstream of oncogenic β-catenin signals, which, in turn, may be helpful to isolate novel diagnostic markers, and for designing tumor-specific intervention at downstream targets of oncogenic β-catenin.
Keywords: β-catenin, RNA interference, invasion, migration, colon cancer
Background
β-catenin is a multi-functional protein that serves as a structural protein at the adherent junctions and acts as a transcriptional co-activator mediating the canonical Wnt signaling [1–4]. Its deregulation is important in the genesis of a number of human malignancies, particularly colorectal cancer [5]. Under physiological conditions, β-catenin mainly locates in cell membranes and functions as a component of the E-cadherin/catenin complex to control cell-cell adhesion and influence cell migration. The cytosolic β-catenin is maintained at a low level through degradation by the “destruction complex” [6–8]. In this complex, Axin acts as a scaffold protein, upon which adenomatous polyposis coli (APC) tumor suppressor protein, glycogen synthase kinase 3β (GSK-3β) and casein kinase 1α (CK1α) bind to facilitate the sequential phosphorylation of β-catenin by kinase CK1α and GSK-3β. Accordingly, phosphorylated β-catenin is recognized by β-transducin-repeat-containing protein and constantly degraded by the ubiquitin-proteasome pathway. Upon ligation of Wnts to their receptors, composed of frizzled proteins and low-density lipoprotein receptor-related protein 5/6, the cytoplasmic protein disheveled (Dvl) is recruited, phosphorylated and activated. Activation of Dvl induces the dissociation of GSK-3β from Axin and leads to the inhibition of GSK-3β. Next, the phosphorylation and degradation of β-catenin is inhibited as a result of the inactivation of the “destruction complex”. Subsequently, stabilized β-catenin translocates into the nucleus. Nuclear β-catenin is the ultimate effector, binding to Tcf/Lef (T-cell factor/lymphoid enhancing factor) to activate transcription of downstream genes, including c-myc[9],cyclin D1[10], MMP7[11], and CD44[12], which are involved in the control of cellular proliferation, differentiation, apoptosis, invasion and metastasis [13,14].
In the majority of colorectal cancers, abnormally high amounts and stabilization of β-catenin accompanied by mutations in APC or β-catenin gene have been detected, and it has been suggested that the transcriptional activation of β-catenin/TCF plays a critical role in colon carcinogenesis [15]. The APC protein, which acts as a tumor suppressor protein, can down-regulate the transcriptional activation mediated by Wnt/β-catenin, but the protein products of mutant APC genes present in colorectal tumors were defective in this activity. Furthermore, mutations of β-catenin are common phosphorylation motifs in its NH2-terminal domain.
Suppression of β-catenin gene expression with either antisense oligodeoxynucleotide or small interfering RNA (siRNA) has been attempted to further confirm its potential role in the neoplastic growth of colon cancer cells [15,16]. Systemic administration of β-catenin antisense oligodeoxynucleotide inhibited proliferation, anchorage-independent growth, and cellular invasiveness of APC-mutant human colon carcinoma cells, including SW480, Colo201, and DLD-1. After antisense-mediated suppression of β-catenin/Tcf transcriptional activity, there is a corresponding decrease in the expression of cyclin D1[15]. Furthermore, siRNA directed against β-catenin significantly downregulated c-myc and cyclin D1 gene expression, leading to reduced growth of SW480 and HCT116 colon cancer cells in soft agar and in nude mice [16]. These studies indicate that β-catenin plays a critical role in the neoplastic growth of colon cancers and genes inactivating targeting of β-catenin may have potential as a therapeutic agent to treat colon cancer.
In the present study, the effect of β-catenin on the invasion and migration, 2 important malignant phenotypes of cancer cells, was investigated by RNA interference (RNAi) in colon cancer cells (LoVo cells) in vitro, which is important for better defining its role in maintaining the malignant phenotype.
Material and Methods
Cell line and culture
A highly metastatic human colon adenocarcinoma cell line (LoVo cells) with APC mutation was purchased from the Basic Research Center of Shandong Tumor Hospital & Institute. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) containing 10% fetal bovine serum (FBS; Sijiqing Biological Engineering Materials Co., Ltd, Hangzhou, China) in a humidified atmosphere with 5% CO2 at 37°C.
RNA oligonucleotides and transfection
According to the sequence in the study of Verma et al. [16], small interfering RNA (siRNA) targeting β-catenin (CTNNB1, NM001904) was synthesized by Shanghai GenePharma Co., Ltd. The sense was 5′-AGCUGAUAUUGAUGGACAGdTdT-3′, which extends between amino acids 79 and 85 of β-catenin. A scrambled siRNA without homology to any mammalian gene sequence served as a negative control. The sense was: 5′-GUCAUUGACUUAUCGAUG GdTdT-3′.
LoVo cells in logarithmic phase were harvested by trypsinization and seeded at 1×106 cells/well into 6-well plates to yield 80–90% confluence. Transfections were performed by using lipofectamine 2000 (Invitrogen) and 100 nM β-catenin-siRNA according to the manufacturer’s instructions. Medium was refreshed 6 h after transfection, and assay done 48 h after transfection.
RNA isolation and reverse transcription-PCR (RT-PCR)
Two days after transfection, cells were collected and homogenized in Trizol reagent (Invitrogen), and total RNA was extracted according to the manufacturer’s instructions. RT-PCR was performed by the SuperScript one-step method according to the manufacturer’s instructions (Invitrogen). The primers used in PCR were as follows: β-catenin: forward: 5′-ATCATCGTGAGGGCTTACTGG-3′, reverse: 5′-CATCCCTTCCTGTTTAGTTGC-3′; β-actin: forward: 5′-AGCATCCTAGAACTCTGTGC-3′, reverse: 5′-ATTTCGGACCCCTGAACATA-3′.
Western blot assay
Two days after transfection, LoVo cells were lysed in RIPA Lysis Buffer (Beyotime Institute of Biotechnology, Nanjing, China) on ice. Centrifugation was performed and supernatant collected for the analysis of protein concentration by BCA method. The extracted proteins were separated by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes (Millipore, Billerica, USA) which were incubated overnight at 4°C with corresponding primary antibody and then with horseradish peroxidase (HRP) conjugated secondary antibody (Zhongshan Biological Technology, Beijing, China). Primary antibodies included mouse anti-β-catenin (sc-7963, Santa Cruz; 1: 500), mouse anti-E-cadherin (sc-21791, Santa Cruz; 1: 500), mouse anti-MMP-7 Ab-1 (Clone ID2, NeoMarkers; 1: 100), and mouse anti-β-actin.
Flow cytometry
Two days after transfection, cells were collected, washed twice with phosphate buffer saline (PBS) and re-suspended in 100 μl of PBS containing 1% bovine serum albumin (BSA). Then, FITC-labeled mouse anti-human CD44v6 monoclonal antibody (Jingmei Biotech Co., Ltd., Shanghai, China) was added to the suspension, followed by incubation for 30 min in dark at room temperature. FITC-labeled mouse IgG1 (Jingmei Biotech Co., Ltd., Shanghai, China) served as an isotype control. The CD44v6 expression was measured by flow cytometry (BD, Oxnard, USA). Gates were set to exclude dead cells and a total of 10,000 gated cells were analyzed.
Test of homogeneity adhesion (between tumor cells)
LoVo cells (1×104/well) were seeded into a 96-well plate to yield 100% confluence. Then, LoVo cells that were transfected with β-catenin siRNA or scrambled siRNA for 48 h were seeded into the same plate (1×104/well), followed by incubation for 2 h at 37°C. Then, the culture medium was removed and cells were gently washed 3 times in PBS to collect non-adherent cells followed by counting cell number. The adhesion rate was calculated as adhesion rate = [1 − (non-adherent cells/total cells)]×100%. The experiment was performed in sextuplicate wells.
Test of heterogeneity adhesion (between tumor cells and extracellular matrix)
A 96-well plate was treated with fibronectin (4 μg/well, R&D Systems) overnight at 37°C. Then, non-specific adhesion was excluded by incubating a plate with 100 μl of DMEM containing 2% BSA for 1 h at 37°C. Following removal of the above DMEM, LoVo cells (1×104) that were transfected with siRNA were seeded into this 96-well plate, followed by incubation for 2 h at 37°C. The adhesion rate was calculated as above.
Scratch wound assay
The scratch wound assay was done as described previously [17]. Briefly, 24 h post-transfection, the confluent monolayer LoVo cells were wounded with a plastic pipette tip, the dislodged cells were removed by rinsing in PBS, then the wound area of monolayer LoVo cells were photographed under a phase-contrast microscope and cells were incubated in DMEM at 37°C in a humidified incubator with 5% CO2. The ability of these cells to migrate into the wound area was assessed at 24 and 48 h later. Cells were gently washed with PBS and the wound area was photographed. Assay was performed in triplicate wells.
Matrigel invasion assay
The invasion activity of LoVo cells was measured as described previously [18] with modifications. A 24-well transwell chamber (Costar Co., USA) containing polycarbonate membrane with a pore size of 8 μm was used. The upper chambers were coated with 100 μl of Matrigel (1 mg/ml, BD). The membranes were rehydrated with warm serum-free medium containing 2% BSA for 1 h. siRNA transfected LoVo cells (2×104 cells) were seeded into the upper chamber, and the lower chambers were filled with 600 μl of serum-free DMEM containing 10 μg/ml fibronectin as a chemoattractant. Incubation was performed for 24 h at 37°C in a humidified incubator with 5% CO2. Then, cells on the top surface of the membranes were gently wiped off with cotton swabs, and those that remained on the membranes were then fixed in methanol and stained with crystal violet. The invasive cells that were attached to the bottom surface of the membranes were photographed and counted under a microscope at a magnification of 400×. Five fields were randomly selected and invasive cells were counted and averaged. The experiment was performed in triplicate.
Statistical analysis
Quantitative data were expressed as mean ± standard deviation (SD) and analyzed by 2-sample t-test. Two-tailed P values of <0.05 were accepted as statistically significant. Statistical analysis was performed with the SPSS version 13.0 statistical software package (SPSS Inc., Chicago, IL).
Results
β-catenin siRNA reduced mRNA and protein expression of β-catenin in LoVo cells
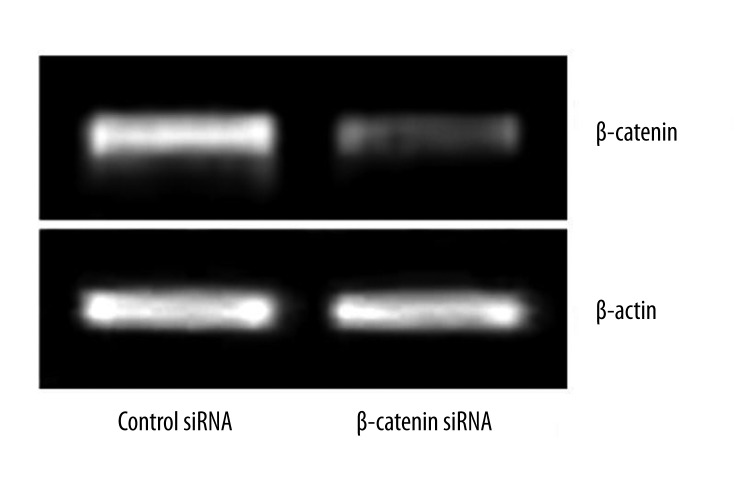
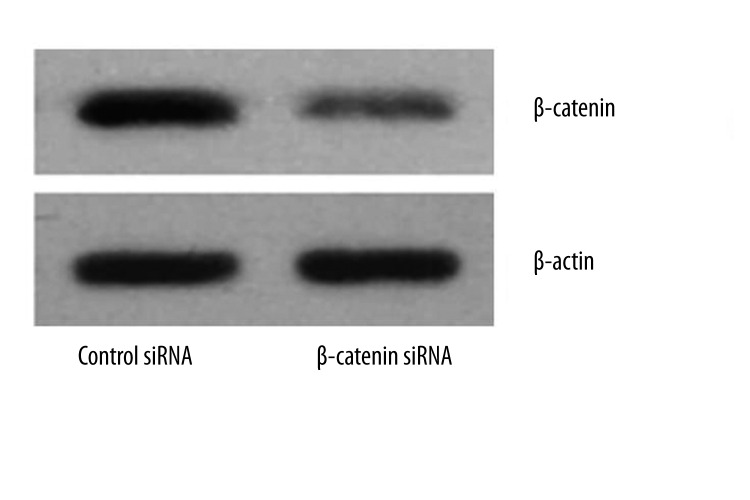
A human colon cell line, LoVo, with APC mutation, results in increased β-catenin levels. The increased levels of β-catenin lead to the enhanced expression of β-catenin/TCF-regulated genes. In this study, RNAi was used in an attempt to decrease β-catenin expression in the cell lines. To identify whether siRNA targeting β-catenin effectively inhibited β-catenin expression in LoVo cells, RT-PCR and Western blot assay were performed at 48 h after transfection. Results showed both the mRNA and protein expressions of β-catenin were significantly down-regulated in β-catenin siRNA transfected LoVo cells (Figures 1, 2). Collectively, these results indicated significant knockdown of β-catenin mRNA and protein expression by β-catenin siRNA.
Figure 1.
mRNA expression of β-catenin in LoVo cells following β-catenin siRNA transfection. LoVo cells were transfected with 100 nM siRNA targeting β-catenin or scrambled siRNA. At 48 h after transfection, mRNA expression of β-catenin was measured by RT-PCR.
Figure 2.
Protein expression of β-catenin in LoVo cells following β-catenin siRNA transfection. LoVo cells were transfected with β-catenin or scrambled siRNA (100 nM). At 48 h after transfection, the protein expression of β-catenin was measured by Western blot assay.
siRNA-mediated down-regulation of β-catenin regulated expressions of E-cadherin, MMP-7 and CD44v6
The E-cadherin-catenin complex plays a crucial role in epithelial cell-cell adhesion and in the maintenance of tissue architecture. Perturbation in the expression or function of this complex may result in loss of intercellular adhesion, with possible consequent cell transformation and tumor progression. Re-establishment of adherent junctions in cancer cells by restoration of cadherin expression [19] exerts tumor-suppressive effects, including decreased proliferation and motility. MMP-7 is another target gene of β-catenin/TCF; it is overexpressed in 80% of human colorectal cancers and is known to be an important factor for early tumor growth, with a potential function also for later progression steps such as invasion and metastasis. CD44 is a cell surface molecule that has diverse functions in cell–cell and cell–matrix interactions and may be a determinant of metastasis and invasive behavior in carcinomas. CD44v6 has been postulated to be involved in both carcinogenesis and tumor progression.
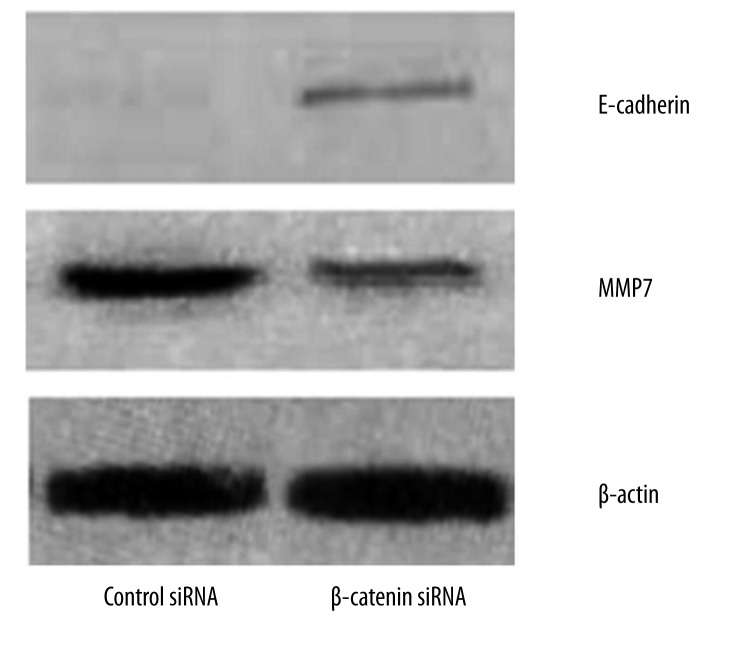
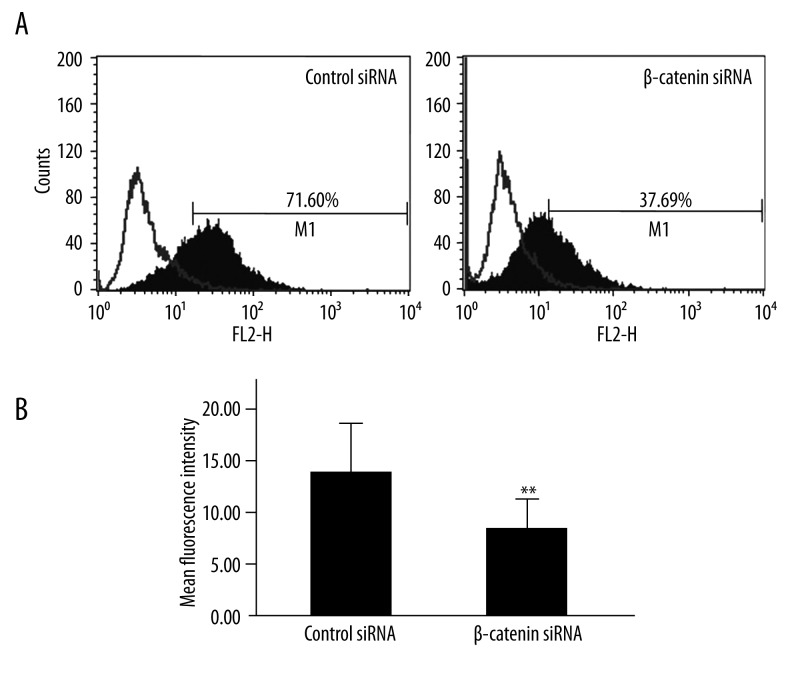
We next examined whether β-catenin inhibition resulted in alterations in expressions of E-cadherin, MMP-7 and CD44v6. Western blot assay was performed and results revealed E-cadherin expression was elevated but MMP-7 expression down-regulated in cells following β-catenin siRNA transfection (Figure 3). As shown in Figure 4, flow cytometry indicated LoVo cells transfected with β-catenin siRNA displayed significantly lower CD44v6 expression (7.73±3.08) when compared with scrambled siRNA treated cells (13.29±4.04) (P<0.001).
Figure 3.
Effects of β-catenin down-regulation on E-cadherin and MMP-7 expressions in LoVo cells. LoVo cells were transfected with β-catenin or scrambled siRNA (100 nM). At 48 h after transfection, the protein expressions of E-cadherin and MMP-7 were deterimined by Western blot assay.
Figure 4.
(A) CD44v6 expression in LoVo cells following β-catenin siRNA transfection. The white histogram represents isotype control, and black histogram represents CD44v6 at 48 h after transfection. Depicted is the fluorescence intensity vs. cell count. (B) The mean fluorescence intensity of positive staining is shown. (** P<0.001 vs. scrambled siRNA, n=6)
Effects of β-catenin siRNA on cell adhesion
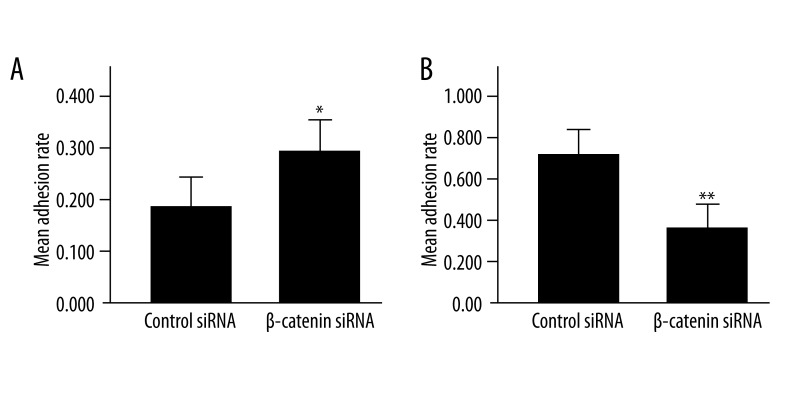
siRNA-mediated down-regulation of β-catenin regulated expressions of E-cadherin and CD44v6, which are closely related to intercellular and cell-matrix adhesion. To determine whether the β-catenin induced disruption of cell adhesion, cell adhesion assays were performed, and the results showed that the homogeneity and heterogeneity adhesion rates were 17.5±5.2% and 67.7±6.9%, respectively, in β-catenin siRNA-treated cells and 29.2±5.8% and 32.3±5.1%, respectively, in scrambled siRNA-treated cells, showing significant differences between the 2 groups. These findings suggest that siRNA-mediated down-regulation of β-catenin markedly reduced the homogeneity adhesion and increased the heterogeneity adhesion (P<0.01) (Figure 5).
Figure 5.
LoVo cell adhesion following β-catenin siRNA transfection. (A) The homogeneity adhesion between LoVo cells transfected with β-catenin siRNA increased significantly as compared to cells with scrambled siRNA transfection; (B) The heterogenicity adhesion of LoVo cells to fibronectin declined significantly after β-catenin siRNA transfection. (* P<0.01 vs. scrambled siRNA, ** P<0.001 vs. scrambled siRNA, n=6).
Inhibition of cell migration by β-catenin siRNA
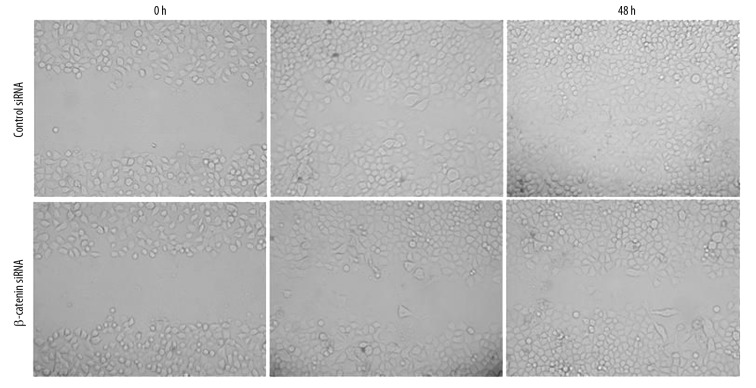
To address whether β-catenin overexpression plays a role in regulating the invasion and migration of LoVo cells, we investigated the influence of β-catenin down-regulation on the LoVo cell migration by scratch wound assay. Results showed scrambled siRNA-treated LoVo cells markedly increased in the wound 24 h later, and the wound was almost entirely occupied 48 h later. By contrast, in the β-catenin siRNA treated cells, only a few cells were found in the wound area 24 h later, and the wound area was also clear even 48 h later. These findings demonstrate that β-catenin down-regulation significantly inhibited the migration ability of LoVo cells (Figure 6).
Figure 6.
LoVo cell migration following β-catenin siRNA transfection. Following β-catenin or scrambled siRNA transfection, representative photomicrographs (100×) of LoVo cell migration were taken at 0, 24 and 48 h after wounding. The migration activity was different between LoVo cells with β-catenin siRNA transfection and those with scrambled siRNA transfection.
Down-regulation of β-Catenin inhibited invasion of LoVo cells
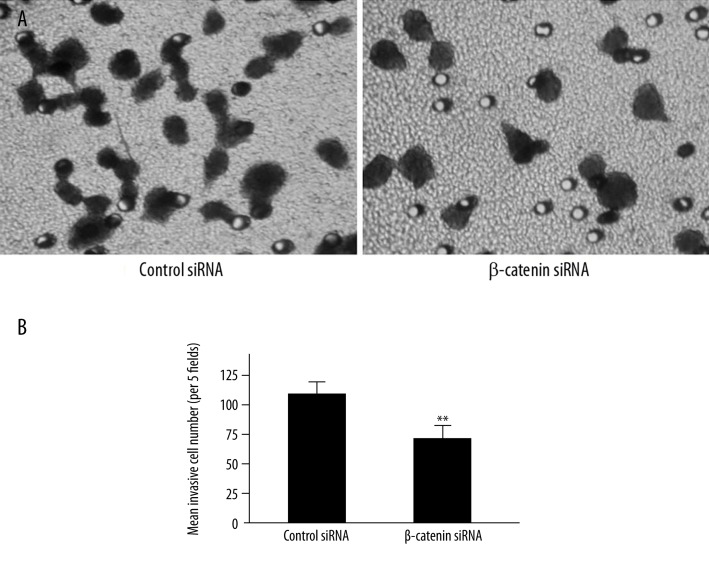
Previous work has demonstrated that the intranuclear localization of β-catenin increases cellular proliferation [20]. We further evaluated the invasion ability of β-catenin siRNA or scrambled siRNA-treated LoVo cells by matrigel invasion assay. Our results showed that after 24 h incubation, the number of β-catenin siRNA-treated cells across the matrigel-coated membrane was 68.8±8.7/5 fields, which was significantly lower than that of scrambled siRNA-treated LoVo cells (111.0±9.6/5 fields) (P<0.001) (Figure 7).
Figure 7.
LoVo cell invasion following β-catenin siRNA transfection. (A) After incubation for 24 h, invasive cells in scrambled siRNA group and β-catenin siRNA group were stained by crystal violet and photographed under a microscope (400×). (B) Invasion ability was quantitated and data were expressed as histograms. (** P<0.001 vs. scrambled siRNA, n=3).
Discussion
A characteristic feature of malignancies, including colon cancer, is the ability of cancer cells to invade surrounding tissues and migrate into distal tissues, which is a main cause of treatment failure and death in cancer patients. Thus, inhibition of invasion and metastasis of cancer cells has been a feasible strategy for the treatment of malignancies [21]. Abnormal regulation of Wnt/β-catenin signaling and subsequent increase of β-catenin expression has been found to promote cell proliferation and be related to carcinogenesis in colon cancer [22]. Whether β-catenin down-regulation in LoVo cells can compromise the invasion and migration potentials in vitro remains to be determined.
In the present study, our results showed that siRNA targeting β-catenin resulted in efficient and specific down-regulation of endogenous β-catenin at mRNA and protein levels in LoVo cells in vitro, and the siRNA-mediated β-catenin down-regulation dramatically inhibited the invasion and migration of LoVo cells, which may be related to the up-regulation of E-cadherin and reduction of CD44v6 and MMP-7 in LoVo cells.
The invasion and migration of cancer cells are complicated, multi-step processes involving the alteration of cell adhesion to extracellular matrix, as well as disruption of cell-cell junctions [23]. Liotta [24] proposed a 3-step hypothesis of tumor cell invasion of extracellular matrix: the first step is tumor cell attachment via cell surface receptors, which specifically bind to the components of matrix such as laminin and fibronectin; the second step is degradation of local matrix by tumor cell-associated proteases; and the third step is tumor cell locomotion into the region of matrix modified by proteolysis. Continued invasion of the matrix may take place by cyclic repetition of these steps.
In the present study, we assessed the invasion ability of LoVo cells in vitro by matrigel invasion assay, which is widely accepted as a biologically active basement membrane model to mimic the invasion process of tumor cells in vivo[25]. Our results showed siRNA-mediated down-regulation of β-catenin significantly inhibited the invasion of LoVo cells through the matrigel-coated transwell.
The decreased homogeneity adhesion and increased heterogeneity adhesion may contribute to the compromised migration of tumor cells from the primary site, and both homogeneity and heterogeneity adhesions are the basis for the invasion and metastasis of tumor cells [21]. Our results revealed siRNA-mediated down-regulation of β-catenin decreased the adhesion between LoVo cells and increased that of LoVo cells to fibronectin.
The invasion of tumor cells requires both migration and degradation of extracellular matrix [26]. Cell motility is a pivotal factor in metastasis and is necessary for migration through the matrix and entry into circulation, leading to distant metastasis [27]. In our study, results showed that β-catenin siRNA significantly inhibited the degradation of extracellular matrix and the chemotaxis of LoVo cells across the matrigel-coated membrane toward fibronectin, a chemoattractant. Scratch wound assay showed a slower wound-closure following transfection with β-catenin siRNA, indicating decreased motility of cancer cells.
A large number of genes relevant with tumor formation and progression have been found to be transcriptionally activated by the β-catenin/TCF complex [28], in which MMP-7 and CD44 are thought to play important roles in the invasion and metastasis of cancers [29,30]. In addition, E-cadherin/catenin complex controls cell-cell adhesion and influences cell migration [31,32]. To further elucidate the mechanism underlying the inhibition of adhesion, invasion and migration of LoVo cells followed β-catenin silencing, we detected the E-cadherin, CD44v6 and MMP-7 expression in LoVo cells after transfection. E-cadherin is a calcium-dependent adhesion molecule that mediates intercellular homogeneity adhesion, which widely exists in various types of epithelial cells. Our results showed the E-cadherin expression was significantly increased at 48 h after transfection when compared with scrambled siRNA-treated cells, suggesting that siRNA-mediated down-regulation of β-catenin promotes the homogeneity adhesion of LoVo cells. CD44v6, a splice variant of adhesion molecule CD44, is involved in cell-matrix interactions and takes part in cell motility, and tumor growth, invasion and metastasis [33,34]. In this study, the CD44v6 expression in LoVo cells was markedly reduced at 48 h after β-catenin siRNA transfection. Proteases involved in degradation of extracellular matrix are indispensable for tumor cell invasion, which facilitates the migration of cancer cells through the basal membrane [35]. Among these proteases, matrix metalloproteinases (MMPs) appear to be particularly important [36], and the overproduction of MMPs has been associated with tumor growth and metastasis [37,38]. Moreover, MMP-7 has been found to be over-expressed in colon cancer [39]. Our results also revealed β-catenin down-regulation in LoVo cells significantly inhibited MMP-7 expression.
Conclusions
In conclusion, β-catenin over-expression may contribute to the invasion and migration of colon cancer cells through regulating E-cadherin, CD44v6 and MMP-7. siRNA-mediated β-catenin down-regulation dramatically inhibited the invasion and migration of LoVo cells, which may be attributed to the up-regulation of E-cadherin expression and down-regulation of CD44v6 and MMP-7 expressions in LoVo cells. We believe experiments such as that presented here are valuable for defining gene expression and functional programs downstream of oncogenic b-catenin signals. This, in turn, may be helpful to isolate novel diagnostic markers, and for designing tumor-specific intervention at downstream targets of oncogenic b-catenin.
Abbreviations
- APC
adenomatous polyposis coli
- BSA
bovine serum albumin
- DMEM
Dulbecco’s modified Eagle’s medium
- MMP
matrix metalloproteinase
- RNAi
RNA interference
- siRNA
small interfering RNA
- TCF
T-cell factor
Footnotes
Competing interests
All authors declare that they have no conflict of interest to disclose.
Source of support: This work was funded by the Natural Science Fund of Shandong Province, China (No. ZR2009CM138)
References
- 1.Coyle-Rink J, Del Valle L, Sweet T, et al. Developmental expression of Wnt signaling factors in mouse brain. Cancer Biol Ther. 2002;1:640–45. doi: 10.4161/cbt.313. [DOI] [PubMed] [Google Scholar]
- 2.Streaker ED, Beckett D. The biotin regulatory system: kinetic control of a transcriptional switch. Biochemistry. 2006;45:6417–25. doi: 10.1021/bi052599r. [DOI] [PubMed] [Google Scholar]
- 3.Xing Y, Takemaru K, Liu J, et al. Crystal structure of a full-length β-catenin. Structure. 2008;16:478–87. doi: 10.1016/j.str.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixelius J, Cross MJ, Matsumoto T, et al. Endostatin action and intracellular signaling: β-catenin as a potential target? Cancer Lett. 2003;196:1–12. doi: 10.1016/s0304-3835(03)00267-2. [DOI] [PubMed] [Google Scholar]
- 5.López-Knowles E, Zardawi SJ, McNeil CM, et al. Cytoplasmic localization of beta-catenin is a marker of poor outcome in breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2010;19:301–9. doi: 10.1158/1055-9965.EPI-09-0741. [DOI] [PubMed] [Google Scholar]
- 6.Nelson WJ, Nusse R. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 2004;303:1483–87. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansson EA, Are A, Greicius G, et al. The Wnt/β-catenin signaling pathway targets PPARγ activity in colon cancer cells. Proc Natl Acad Sci USA. 2005;102:1460–65. doi: 10.1073/pnas.0405928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng XX, Wang ZC, Chen XY, et al. Correlation of Wnt-2 expression and β-catenin intracellular accumulation in Chinese gastric cancers: relevance with tumour dissemination. Cancer Lett. 2005;223:339–47. doi: 10.1016/j.canlet.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 9.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 10.Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–27. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brabletz T, Jung A, Dag S, et al. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155:1033–38. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wielenga VJ, Smits R, Korinek V, et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol. 1999;154:515–23. doi: 10.1016/S0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge X, Wang X. Role of Wnt canonical pathway in hematological malignancies. J Hematol Oncol. 2010;3:33. doi: 10.1186/1756-8722-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong NA, Pignatelli M. β-catenin – a linchpin in colorectal carcinogenesis? Am J Pathol. 2002;160:389–401. doi: 10.1016/s0002-9440(10)64856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roh H, Green DW, Boswell CB, et al. Suppression of β-catenin inhibits the neoplastic growth of APC-mutant colon cancer cells. Cancer Res. 2001;61:6563–68. [PubMed] [Google Scholar]
- 16.Verma UN, Surabhi RM, Schmaltieg A, et al. Small interfering RNAs directed against β-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clinical Cancer Res. 2003;9:1291–300. [PubMed] [Google Scholar]
- 17.Faux MC, Ross JL, Meeker C, et al. Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion. J Cell Sci. 2004;117:427–39. doi: 10.1242/jcs.00862. [DOI] [PubMed] [Google Scholar]
- 18.Albini A, Iwamoto Y, Kleinman HK, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–45. [PubMed] [Google Scholar]
- 19.Vleminckx K, Vakaet L, Mareel M, et al. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–19. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 20.Romiti A, Zullo A, Borrini F, et al. Relationship between beta-catenin expression and epithelial cell proliferation in gastric mucosa with intestinal metaplasia. World J Gastroenterol. 2005;11:4400–3. doi: 10.3748/wjg.v11.i28.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Yang H, Liu H, et al. Effect of staurosporine on the mobility and invasiveness of lung adenocarcinoma A549 cells: an in vitro study. BMC Cancer. 2009;9:174. doi: 10.1186/1471-2407-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi BR, Gwak J, Kwon HM, et al. Oligodeoxyribozymes that cleave β-catenin messenger RNA inhibit growth of colon cancer cells via reduction of β-catenin response transcription. Mol Cancer Ther. 2010;9:1894–902. doi: 10.1158/1535-7163.MCT-10-0056. [DOI] [PubMed] [Google Scholar]
- 23.Dasgupta P, Rizwani W, Pillai S, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liotta LA. Tumor invasion and metastasis – role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 25.Yuan K, Singh R, Rezonzew G, Siegal G. In vitro matrices for studying tumor cell invasion. In: Wells A, editor. Cell Motility in Cancer Invasion and Metastasis. Netherlands: Springer; 2006. pp. 25–54. [Google Scholar]
- 26.Su F, Li H, Yan C, et al. Depleting MEKK1 expression inhibits the ability of invasion and migration of human pancreatic cancer cells. J Cancer Res Clin Oncol. 2009;135:1655–63. doi: 10.1007/s00432-009-0612-6. [DOI] [PubMed] [Google Scholar]
- 27.Larkins TL, Nowell M, Singh S, Sanford GL. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer. 2006;6:181. doi: 10.1186/1471-2407-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo GQ, Li JH, Wen JF, et al. Effect and mechanism of the Twist gene on invasion and metastasis of gastric carcinoma cells. World J Gastroenterol. 2008;14:2487–93. doi: 10.3748/wjg.14.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiomi T, Okada Y. MT1-MMP and MMP-7 in invasion and metastasis of human cancers. Cancer Metastasis Rev. 2003;22:145–52. doi: 10.1023/a:1023039230052. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Yu Q. E-cadherin negatively regulates CD44-hyaluronan interaction and CD44-mediated tumor invasion and branching morphogenesis. J Biol Chem. 2003;278:8661–68. doi: 10.1074/jbc.M208181200. [DOI] [PubMed] [Google Scholar]
- 31.Jawhari A, Farthing M, Pignatelli M. The importance of the E-cadherin-catenin complex in the maintenance of intestinal epithelial homoeostasis: more than intercellular glue? Gut. 1997;41:581–84. doi: 10.1136/gut.41.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verras M, Sun Z. Roles and regulation of Wnt signaling and β-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 34.Zhang JC, Wang ZR, Cheng YJ, et al. Expression of proliferating cell nuclear antigen and CD44 variant exon 6 in primary tumors and corresponding lymph node metastases of colorectal carcinoma with Dukes’ stage C or D. World J Gastroenterol. 2003;9:1482–86. doi: 10.3748/wjg.v9.i7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellyei S, Schally AV, Zarandi M, et al. GHRH antagonists reduce the invasive and metastatic potential of human cancer cell lines in vitro. Cancer Lett. 2010;293:31–40. doi: 10.1016/j.canlet.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Abécassis I, Olofsson B, Schmid M, et al. RhoA induces MMP-9 expression at CD44 lamellipodial focal complexes and promotes HMEC-1 cell invasion. Exp Cell Res. 2003;291:363–76. doi: 10.1016/j.yexcr.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 38.Duffy MJ, Maguire TM, Hill A, et al. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–57. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kioi M, Yamamoto K, Higashi S, et al. Matrilysin (MMP-7) induces homotypic adhesion of human colon cancer cells and enhances their metastatic potential in nude mouse model. Oncogene. 2003;22:8662–70. doi: 10.1038/sj.onc.1207181. [DOI] [PubMed] [Google Scholar]