Summary
Background
Surface chemistry of dental implant plays an important role in osseointegration. Heat treatment might alter surface chemistry and result in different biological response. The aim of this study was to investigate the roles of heat treatment of H2O2/HCl-treated Ti implants in cell attachment, proliferation and osteoblastic differentiation.
Material/Methods
Sandblasted, dual acid-etched and H2O2/HCl heat-treated discs were set as the control group and sandblasted, dual acid-etched H2O2/HCl-treated discs were the test group. Both groups’ discs were sent for surface characterization. MC3T3-E1 cells were seeded on these 2 groups’ discs for 3 hours to 14 days, and then cell attachment, cell proliferation and cell differentiation were evaluated.
Results
Scanning electron microscope analysis revealed that the titanium discs in the 2 groups shared the same surface topography, while x-ray diffraction examination showed an anatase layer in the control group and titanium hydride diffractions in the test group. The cell attachment of the test group was equivalent to that of the control group. Cell proliferation was slightly stimulated at all time points in the control group, but the alkaline phosphatase (ALP) activity and osteocalcin (OC) production increased significantly in the test group compared with those in the control group at every time point investigated (p<0.05 or p<0.01). Moreover, the osteoblastic differentiation-related genes AKP-2, osteopontin (OPN) and OC were greatly up-regulated in the test group (p<0.05 or p<0.01).
Conclusions
The results implied that surface chemistry played an important role in cell response, and H2O2/HCl etched titanium surface without subsequent heat treatment might improve osseointegration response.
Keywords: titanium implant, heat treatment, anatase, titanium hydride
Background
Pure titanium (Ti) and titanium alloys have been widely used as dental implant materials because of their compatible mechanical properties, chemical stability and good biocompatibility. However, Ti is intrinsically bioinert, and long-term maintenance of osseointegration and stability of Ti-based implants is still a problem, especially in compromised conditions. Osseointegration is closely related to the interactions that occur between the implant surface and its surrounding bone tissue, and they are mainly determined by the nature of the surface properties, such as surface chemistry, energy, roughness, and topography [1–6].
In order to optimize the surface properties of dental implants, various physical and chemical methods have been applied, such as machining [7,8], sand-blasting [9,10] and acid etching [11–13]. A direct surface treatment that will increase the bioactivity of dental implants is to modify the outermost atomic layers of Ti; H2O2 treatment is a simple and effective technique. Wang et al reported that an anatase gel layer could form on Ti substrates due to the chemical treatment with aqueous H2O2/HCl solution and subsequent heating at a moderate temperature [14,15]. The anatase gel layer was highly bioactive and deposited apatite in the Kokubo’s simulated body fluid (SBF) within a day or so. This means that approaches using H2O2/HCl solution and heat treatment can give the implant the ability to deposit apatite by itself to confer osteoconductivity. Recently, a new surface which combined the advantages of roughness and bioactivity to produce a synergetic biological effect was developed by sand-blasting, dual acid etching and H2O2/HCl heat treatment [16]. In vitro and in vivo studies all demonstrated that this dental surface has high bioactivity and promotes cell growth and peri-implant bone formation [16–18].
However, we encountered an interesting phenomenon during the preparation of that new implant surface – the new dental implant without the final heat treatment presented a much higher bioactivity both in cell and in animal experiments (unpublished data). These results seem contradict those of previous studies [14,15]. According to Wang’s investigation, H2O2/HCl solution-treated Ti without the subsequent heat treatment could not deposit apatite within 7 days of staking in SBF solution. Thus, it appears that dental implants should not have such high bioactivity. The purpose of this study is to elucidate this confusing phenomenon.
Material and methods
Substrates preparation and characterization
Sample preparation
Pure Ti flat discs with a diameter of 10 mm or 30 mm were used for substrate materials in this study (Xihu Biomaterials Research Institute, Hangzhou, China). All the specimens were first mechanically polished by SiC abrasive sandpapers (from grit 320, to 500, to 800, and to 1200) with a grinding and polishing machine (Saphir 360, ATM, Germany). Then, all the substrates were sandblasted with course grits (green silicon carbide) at 4 MPa pressure and ultrasonically cleaned in acetone, alcohol and distilled water for 20 min each. Thereafter, the discs were treated in a solution of 0.11 mol/L HF and 0.09 mol/L HNO3 for 10 min at room temperature. Subsequently, the substrates were treated with a mixed solution composed of 5.80 mol/L HCl and 8.96 mol/L H2SO4 at 80°C for 30 min. Finally, the specimens were etched in an aqueous mixture of HCl (0.1 mol/L) and H2O2 (8.8 mol/L) at 80°C for 20 min. Between every 2 steps all the discs were thoroughly rinsed with distilled water and dried by nitrogen gas. After that, half of the substrates were randomly selected and set as the test group. The remaining discs were further heated in air at 400°C for 1 h and allowed to cool naturally, and these discs were set as the control group.
Before cell culture, all the specimens (including the control group and test group) were ultrasonically cleaned in acetone, alcohol, and distilled water for 20 min each, dried by nitrogen gas, then both sides of the Ti discs were sterilized with ultraviolet light for 1 h.
Surface characterization of the Ti discs
Surface morphology of the Ti discs was observed by field-emission scanning electron microscopy (FSEM, FEI, SIRION100, Eindhoven, Netherlands). The crystalline structure was evaluated by thin-film x-ray diffractometry (XRD). Low-angle x-ray diffractometry patterns were recorded with a Rigaku RAD-II diffractometer (Rigaku, Tokyo, Japan) using CuKa radiation operating under 40 kV and 25mA acceleration at an angle of incidence of 1°.
Cell culture
Cell seeding
Mouse pre-osteoblast cells MC3T3-E1 (Cell Bank, Chinese Academy of Sciences, Shanghai, China) were cultured in alpha-Minimum Essential Medium (Gibco, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco, USA), and maintained at 37 °C in a 5% CO2 humidified atmosphere. Cells were seeded at a density of 1×105 cells per well into 6-well culture plates (Corning, NY), to which the Ti discs had been added. After 1 day of culture, the discs were transferred to new 6-well plates to minimize the cells adhering to cell culture plates instead of Ti discs. In order to evaluate cell differentiation, basic medium was supplemented with 10 mM β-Glycerolphosphate (Sigma-Aldrich, USA) and 50 mg/L ascorbin-2-phosphate (Sigma-Aldrich, USA). Medium was changed every 3 days. Each experiment was performed in triplicate for each group and repeated 3 times; however, examiners were blinded to the group assignments.
Cell attachment
Cell attachment was first observed by SEM examination. The discs with cells cultured on them for 24 h, were fixed in 2% glutaraldehyde at 4° for 2 h, followed by washing with deionized water and slow sequential dehydration steps in 10% increments of ethanol for 5 min each. Samples were then placed in pressurized liquid CO2/siphon for 1 h using a CO2 critical point dryer. The discs were next mounted, sputter coated with gold and examined by FSEM.
Cell attachment was also quantitatively determined by measurement of total DNA content in cell layers with a PicoGreen dsDNA Quantitation Kit (Invitrogen, USA). Briefly, cells were seeded onto Ti discs at a cell density of 1×105 cell per disc in 2 ml standard medium and cultured for 3 h, and after that non-adherent cells were gently washed off with PBS. Then cells were trypsinized and resuspended in 500 μl PBS, and stored at −80°C until assay. After thawing at room temperature, 100 μl of the cell lysate was mixed with 100 μl DNA-binding fluorescent dye solution following the protocol provided by the kit. The samples were detected by a fluorescence spectrometer (Spectra M2, Molecular, USA) at an excitation wavelength of 480 nm and emission wavelength of 520 nm against a standard curve.
Cellular proliferation
Proliferation of MC3T3-E1 on Ti discs was also assayed using the dsDNA Quantitation Kit. Cells were seeded onto Ti discs at a cell density of 1×105 cells per disc in 2 ml standard medium and cultured for 1 day, 3 days and 7 days. At these time points, each Ti disc was assessed as described above.
ALP activity assay
ALP activity was determined using a commercial phosphatase substrate kit (Wako, Japan), which is a colorimetric endpoint assay measuring the enzymatic conversion of p-nitrophenyl phosphate (pNPP) to the yellowish product p-nitrophenol (pNP) in the presence of ALP. Following gentle removal of culture medium and washing with PBS, the cells were lysed by CelLytic Buffer (Sigma, USA). A 20 μL volume of cell lysate mixed with 100 μL working assay solution was shaken for 1 min with a plate mixer, and then incubated at 37°C for 15 min. Then the addition of 80 μL stop solution to each well terminated the reactions. After that, the 96-well plate was shaken for another 1 min and read at 405 nm with a spectrophotometer (Spectra M2, Molecular, USA). ALP activity was calibrated by per unit total cellular protein. Total protein was determined with a BCA protein assay kit (Pierce, USA). ALP activity was expressed as nanomole of p-nitrophenol liberated per microgram of total cellular protein per hour.
Osteocalcin release
The production of osteocalcin was measured as the release of extracellular matrix protein into the culture medium by Mouse Osteocalcin EIA Kit (Biomedical Technologies, USA). Briefly, a 25 μL cell culture medium sample and 100 μL osteocalcin antiserum were placed in a 96-well EIA plate and incubated at 4°C for 24 h. The well was washed with wash buffer and 100 μL streptavidin-horseradish reagent was added, and then incubated at room temperature for 30 min. Then 50 μL TMB conjugated hydrogen peroxide solution were added to each well and allowed to incubate at room temperature for 15 min. When adding 100 μL stop solution, absorbance was measured at 450 nm on a spectrophotometer. Data were expressed as total nanograms of mouse OC in the medium per milligram of cell layer protein.
Gene expression analysis
Cells were cultured on Ti discs over a time course of 7 days or 14 days. Upon harvest, cells were trypsinized and total RNA was extracted using the RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The RNA samples were quantified and their purity was determined by standard spectrophotometric method. RNA was reverse transcribed with an RT-PCR kit (Takara Bio, Otsu, Japan) and real-time PCR was performed in a CFX96 Thermal Cycler (Bio-Rad, USA) with an SYBR PrimeScript RT -PCR kit (Takara Bio). The sequences of primers for AKP-2, OPN, OC and β-actin genes are given in Table 1. The PCR conditions were: 95.0°C for 10 sec, followed by 95.0°C 5 sec, 60°C, and 34 sec for 40 cycles. All samples were analyzed in triplicate. The comparative Ct-value method was used to calculate the relative quantity of AKP-2, OPN, OC and β-actin. Expression of the housekeeping gene β-actin was used as an internal control to calibrate results.
Table 1.
Forward (F) and reverse (R) primers for target genes.
| Gene name | Amplicon length(bp) | 5′-3′primer sequence |
|---|---|---|
| AKP-2 | 164 | F 5′-TGCCTACTTGTGTGGCGTGAA-3′ |
| R 5′-TCACCCGAGTGGTAGTCACAATG-3′ | ||
| OPN | 124 | F 5′-TACGACCATGAGATTGGCAGTGA-3′ |
| R 5′-TATAGGATCTGGGTGCAGGCTGTAA-3 | ||
| OC | 178 | F 5′-AGCAGCTTGGCCCAGACCTA-3′ |
| R 5′-TAGCGCCGGAGTCTGTTCACTAC-3′ | ||
| β-actin | 131 | F 5′-TGACAGGATGCAGAAGGAGA-3′ |
| R 5′-GCTGGAAGGTGGACAGTGAG-3′ |
Statistical analysis
The results are expressed as means ± standard deviations. Statistical significance was assessed using Student’s 2-tailed t test in SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). P values less than 0.05 or 0.01 were judged to be statistically significant.
Results
Surface characteristics of the discs
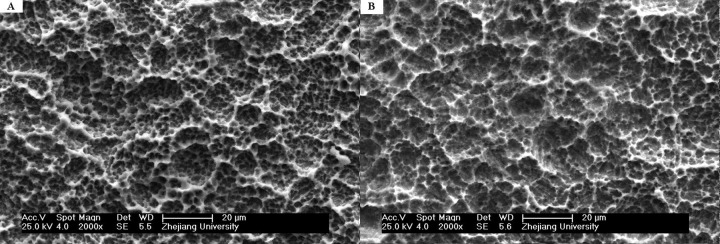
SEM images show similar surface topography and roughness of these 2 substrates. The surfaces are quite irregular for test and control group discs, and they both show a porous network structure and 1 to 2 μm microtextured property – macro-pits embraced smaller, round-shaped micro-pits (Figure 1). The SEM evaluations also demonstrate no residual sandblasting particles on either surface.
Figure 1.
SEM images of 2 different Ti surfaces. (A) Sandblasted, dual acid-etched and H2O2/HCl etched Ti disc (test group) has an irregular surface with numerous irregular micropits and indentations; (B) Sandblasted, dual acid-etched and H2O2/HCl heat treated Ti disc (control group) has a similar porous network structure surface.
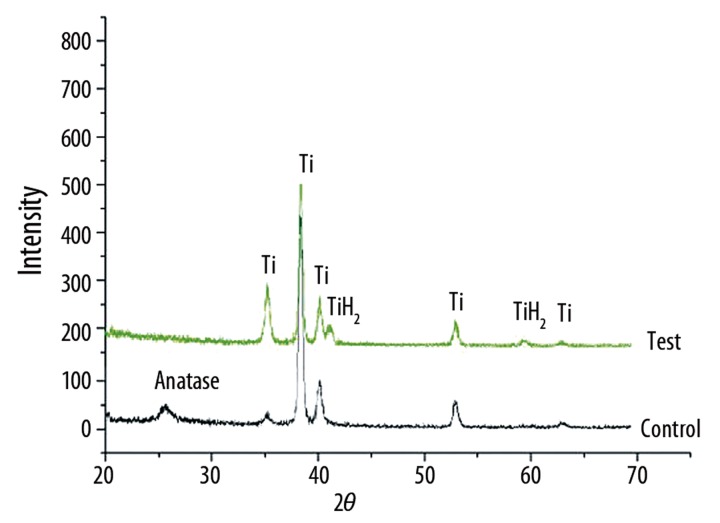
XRD analysis demonstrates a titanium dioxide layer on the control surface and the crystal structure of this titanium dioxide is proved to be anatase (Figure 2, control).
Figure 2.
XRD patterns of the 2 different specimens. It shows anatase exists only in heat treated surface disc (control), whereas TiH2 exists in the surface of test group disc (test).
Although there is no anatase on the surface of test discs, titanium hydride (TiH2) diffraction appears on the x-ray diffractometry pattern of the test surface (Figure 2, test).
Cell attachment
As shown in Figure 3, the cells were fully spread on both discs by 24 h, with elongated cell shape and numerous cellular processes. However, it seems that there is no significant difference between the cell attachment and cell spreading on the test disc (Figure 3A) and control disc (Figure 3B).
Figure 3.
SEM images of mouse MC3T3-E1 cells attached and spread with many cellular processes on the surfaces of test disc (A) and control disc (B).
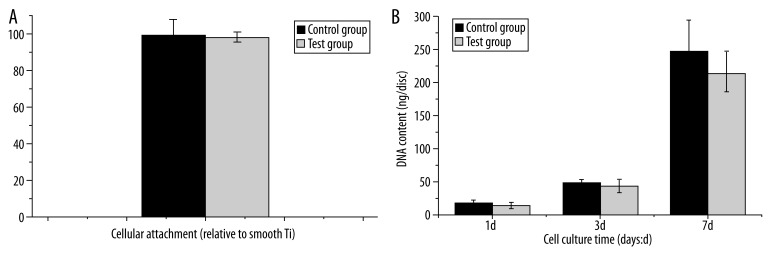
Cellular attachment was also measured 3 h after seeding by measurement of total DNA content, with optical density used as a relative measure of cell number. No differences in initial attachment were evident for the 2 surfaces assessed (P>0.05, Figure 4A).
Figure 4.
(A) Cellular attachment of MC3T3-E1 to Ti surfaces was determined 3 hours after cell seeding. The data was expressed by the ratio relative to cells on machined Ti discs. No significant difference in cellular attachment was observed between different surfaces(n=6, p>0.05). (B) Cell proliferation was determined by fluorescence assay. The cellular DNA content was higher in control group than test group, but there was no significant difference(values = mean ±SD; p>0.05; n=6).
Cell proliferation
Cell number was determined spectrophotometrically at day 1, day 3 and day 7 after seeding onto Ti surface. Figure 4B shows the DNA content of the 2 groups. As demonstrated, despite the lack of statistical significance, the general trend suggests that cell proliferation was slightly stimulated at all time points in the control group (P>0.05).
Osteogenic differentiation response
The alkaline phosphatase activity (ALP), normalized by total cellular protein, is plotted in Figure 5. At day 7, ALP was 0.314±0.025 (nmol pNPP/h)/(μg protein) for MC3T3-E1 cells on the test surface, almost 1.5-fold higher than that on the control surface (P<0.01). ALP level increased with culture time in both groups. At day 14, ALP was 0.424±0.021 (nmol pNPP/h)/(μg protein) on the test surface, also significant higher than that on the control surface (P<0.01).
Figure 5.
The activity of intracellular ALP was much higher in test group than control group at every time point. ALP activity was normalized by total cellular protein. (value = mean ±SD; n=3, * p<0.05; ** p<0.01).
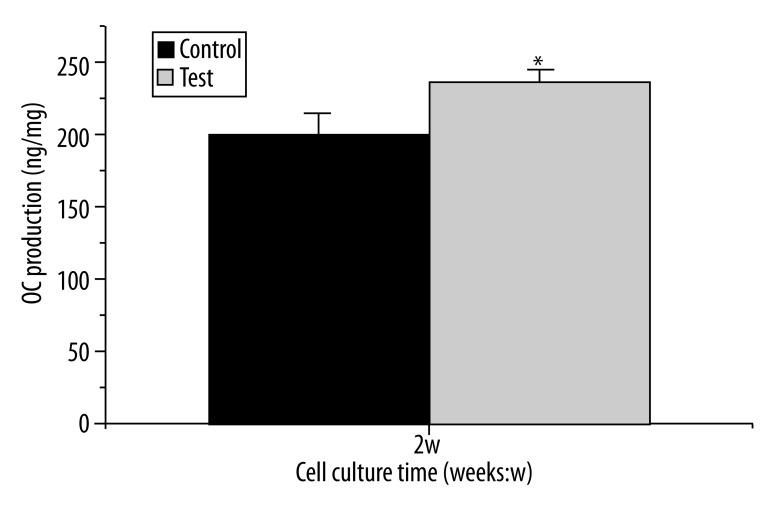
The OC production released into cell culture medium at day 14 is shown in Figure 6, and that also has been normalized by cell protein content. The test surface enhanced osteocalcin production compared with the control surface (248.4±22.3 vs. 196.3±15.8) (ng/μg protein), and the difference was statistically significant (p<0.05).
Figure 6.
Effect of different Ti surfaces on osteocalcin production: osteocalcin content in culture medium was measured by ELISA method. Data were expressed as total ng mouse osteocalcin/μg cell protein. Cells in test group produced more osteocalcin than control group at day 14 with statistically significant (values = mean ±SD; n=3, p<0.05).
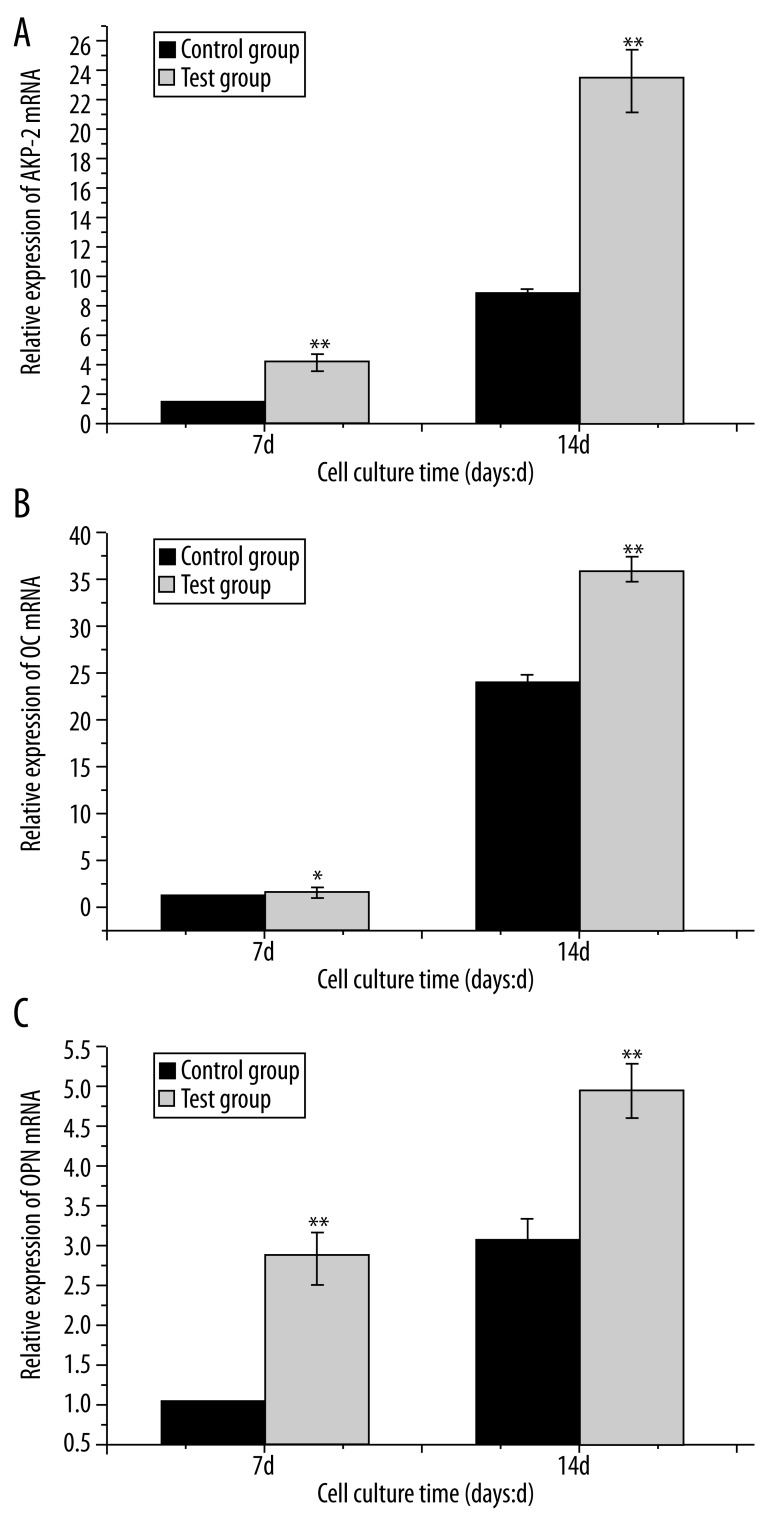
Gene expression analysis was conducted at day 7 and day 14 after seeding onto Ti surfaces. Degree of up-regulation/down-regulation at day 7 or day 14 was determined relative to day 7 in the control group (baseline). Figure 7 shows comparisons of mRNA transcript levels of bone-related genes between the 2 groups in the presence of osteogenic medium. Transcript levels of AKP-2, OPN and OC gene were significantly up-regulated in a time-dependent manner on both surfaces. The AKP-2, OPN and OC levels were higher in the test group than in the control group at all time points investigated, and there were significant differences between the 2 groups (p<0.05 or p<0.01).
Figure 7.
QPCR results of three osteogenic genes (A) AKP-2; (B) OPN; (C) OC on different Ti surfaces at day 7 and 14. The transcriptional levels of the above 3 genes were significantly up-regulated in test group compared with control group. (value= mean ±SD; n=3, * p<0.05; ** p<0.01). Gene expressions of day 7 in control group were set as 1.0.
Discussion
From the above results, we found that H2O2/HCl etched Ti surface without subsequent heat treatment showed superior biocompatibility than the control group, which was followed by heat treatment. Cell attachment was always used to determine whether the material was biocompatible. In the present study, cell attachment did not significantly differ between the 2 Ti surfaces over the first 3 h. This phenomenon might be attributed to the similar topography profile because topography has a leading effect on cell adhesion[19]. Cell proliferation rate was slightly lower over a time course of 7 days in the test group versus the control group, which was associated with higher cell differentiation. This result is in accordance with some previous reports [20,21]. In order to evaluate osteogenic differentiation response in different Ti surfaces, we examined the expression of several osteogenic differentiation-related makers by chemical method or real-time RT-PCR up to 2 weeks after confluence. The osteogenic differentiation proceeds sequentially with the appearance of specific osteogenic markers. Usually, ALP is expressed first, followed by OPN, while OC emerges last [22]. The present study revealed that ALP activity was higher in the test group at any given culture period, especially at 7 days, indicating significantly increased osteogenic differentiation response. Regarding the OC production, the value in the test group was also much higher than that in the control group, with a statistically significant difference. To evaluate cellular response at the molecular level, expression analysis of bone-related genes was performed. Markedly increased expression levels of Akp-2, OPN and OC mRNA confirmed a higher osteogenic differentiation in the test group at day 7 or day 14. All these results suggest that this bioactive surface (without heat treatment) allowed faster osteoblastic cell differentiation than did the control surface.
According to Wang’s reports [14], Ti substrate treated with the H2O2/HCl solution had an amorphous titania gel layer, and a subsequent heating at 400° for 1 h transformed the titania gel layer from an amorphous to an anatase crystal structure.
Furthermore, this anatase layer could deposit apatite within 1 day and shows high bioactivity. Therefore, this method is applicable in Ti dental implants and has been used in our serial studies. A new dental implant surface with high bioactivity has been developed in our lab [16–18]. Sandblasting and dual acid-etching were first used to clean out sandblasted particles and to form a rough surface, and then treated with H2O2/HCl heat-treatment, which slightly altered the surface topography and resulted in sub-micron pits on the surface. After those treatments, we achieved a new surface with moderate roughness (Sa 1.0–2.0 μm), which displays a stronger bone response than do smoother or rougher surfaces [23,24]. Moreover, this surface also has an anatase titania gel layer, which can induce apatite formation when soaked in SBF solution, and is expected to favor bone osseointegration. In fact, this new surface promoted bone growth around dental implant [16,17]. Nevertheless, this new surface with high bioactivity shows inferior biocompatibility when compared with the test surface, and that surprised us, because H2O2/HCl treated Ti without heating treatment could not form an anatase structure and could not induce apatite formation.
Cellular behaviors such as adhesion, morphological change, functional alteration, proliferation, and differentiation are greatly affected by surface properties, including topography, composition, roughness and hydrophilicity [25]. The 2 surfaces share almost the same topography and roughness, so distinct cell response between the control group and the test group in the present study cannot be explained by different surface topography and surface roughness, but the different surface chemistry appears to be a leading factor.
When Ti substrates are chemically treated with hydrogen peroxide solution, dissolution-deposition equilibrium of TiO2 should take part in the TiO2 growth, and such deposition from the solution allow many Ti-OH groups to become involved in the titania layer[26]. However, it has been suggested that the formation of apatite on titania gels is induced by the abundant Ti-OH groups on its surface, implying that a large number of Ti-OH groups are essential for induction of apatite nucleation [27]. Ti-OH groups on the Ti surface were negatively charged when soaking in the SBF, and the positively charged Ca2+ ions first adsorbed on the Ti surface and amorphous calcium titanate was formed. The calcium titanate became positively charged with the accumulation of Ca2+ ions, and hence it combined with the negatively charged phosphate ions in the SBF to form amorphous calcium phosphate. Lastly, the amorphous calcium phosphate transformed into crystalline apatite [28,29]. Nevertheless, the formation of apatite on surface is a prerequisite for the bioactivity of materials, not only because apatite is a main component of bone crystal, but also because the apatite layer preferentially adsorbs acellular proteins that serve as growth factors. Though no Ti-OH groups were detected with XRD examination, a large number of titanium hydroxyl groups were believed to form on test surface when treated with hydrogen peroxide solution. However, the bioactivity reduction in the control group might be partly due to the number of hydroxyl groups on the surface of the titania layers, which is greatly decreased by subsequent heat treatment through the processes of dehydration and polycondensation [15,30].
When Ti is treated with dual acid-etching, the acid must first dissolve the protective titanium oxide layer. During the process of Ti corrosion, native hydrogen ions are released and diffuse rapidly into the metal. As saturation in hydrogen is reached, titanium hydride is formed [31]. On the other hand, previous studies have confirmed that heat treatment can remove the titanium hydride from acid-etched surfaces [32,33], which is why titanium hydride diffractions only appear in the test surface. Regarding the biocompatibility of titanium hydride, there exist some controversial opinions. Some believed that the bioactivity of titanium hydride did not need to be addressed because the acid-etched surface, which was covered with a dense stoichiometric titanium oxide layer, could prevent any effusion and interaction [31]. Nevertheless, others suggested that the hydride concentration in Ti surfaces was positive correlated with bone formation [34]. In addition, our previous studies strongly implied that titanium hydride in implant surfaces could greatly enhance cell growth and bone formation [35,36]. The possible mechanism is that the hydride ion released from Ti surface can exchange with Ca2+ in the outer environment; the released H+ would attack or break the Ti-O bond, finally forming Ti-OH groups [37]. In short, both of them are important for apatite formation.
The anatase titania gel layer in the control group can promote cell growth. Conversely, Ti-OH groups and titanium hydride in the test group can greatly favor cell function – Ti-OH and titanium hydride might be the more important contributing factors for the enhanced cell response than anatase structure. That is the possible reason for superior biocompatibility in the test group. Of note, it seems hard to understand why the test surface that could not deposit apatite shows a higher level of bioactivity. In fact, the osteogenic cells adhere to the surfaces of materials earlier than apatite formation, so the higher ability of apatite deposition does not mean better cell response. The present study indicates that heat treatment of this titanium surface is unsuitable for implant osseointegration and clinical application. However, further investigations should be done to explore the differences of these 2 implant surfaces in animal experiments.
Conclusions
We have demonstrated that sandblasted, dual acid-etched and H2O2/HCl titanium discs with or without the following heat treatment shared similar surface topography. Heat treatment altered the surface chemistry and consequently resulted in a different cell response. H2O2/HCl etched titanium surface without subsequent heat treatment showed superior bone anabolic ability than the control group.
Footnotes
Source of support: This work was sponsored by Grant of Health Bureau of Zhejiang Province(2012KYA189)
References
- 1.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667–81. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 2.Huang Z, Daniels RH, Enzerink RJ, et al. Effect of nanofiber-coated surfaces on the proliferation and differentiation of osteoprogenitors in vitro. Tissue Eng Part A. 2008;14:1853–59. doi: 10.1089/ten.tea.2007.0399. [DOI] [PubMed] [Google Scholar]
- 3.Xue W, Liu X, Zheng X, Ding C. In vivo evaluation of plasma-sprayed wollastonite coating. Biomaterials. 2005;26:3455–60. doi: 10.1016/j.biomaterials.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Le Guehennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23:844–54. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Zeifang F, Grunze M, Delling G, et al. Improved osseointegration of PTFEP-coated titanium implants. Med Sci Monit. 2008;14(2):BR35–40. [PubMed] [Google Scholar]
- 6.Hilbig H, Wiener T, Armbruster FP, et al. Effects of dental implant surfaces on the expression of bone sialoprotein in cells derived from human mandibular bone. Med Sci Monit. 2005;11(4):BR111–15. [PubMed] [Google Scholar]
- 7.Luthen F, Lange R, Becker P, et al. The influence of surface roughness of titanium on beta1- and beta3-integrin adhesion and the organization of fibronectin in human osteoblastic cells. Biomaterials. 2005;26:2423–40. doi: 10.1016/j.biomaterials.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 8.Cassinelli C, Morra M, Bruzzone G, et al. Surface chemistry effects of topographic modification of titanium dental implant surfaces: 2. In vitro experiments. Int J Oral Maxillofac Implants. 2003;18:46–52. [PubMed] [Google Scholar]
- 9.Refai AK, Textor M, Brunette DM, Waterfield JD. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J Biomed Mater Res A. 2004;70:194–205. doi: 10.1002/jbm.a.30075. [DOI] [PubMed] [Google Scholar]
- 10.Guizzardi S, Galli C, Martini D, et al. Different titanium surface treatment influences human mandibular osteoblast response. J Periodontol. 2004;75:273–82. doi: 10.1902/jop.2004.75.2.273. [DOI] [PubMed] [Google Scholar]
- 11.Anselme K, Bigerelle M. Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomater. 2005;1:211–22. doi: 10.1016/j.actbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Giordano C, Sandrini E, Busini V, et al. A new chemical etching process to improve endosseous implant osseointegration: in vitro evaluation on human osteoblast-like cells. Int J Artif Organs. 2006;29:772–80. doi: 10.1177/039139880602900807. [DOI] [PubMed] [Google Scholar]
- 13.Sandrini E, Giordano C, Busini V, et al. Apatite formation and cellular response of a novel bioactive titanium. J Mater Sci Mater Med. 2007;18:1225–37. doi: 10.1007/s10856-007-0122-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang XX, Hayakawa S, Tsuru K, Osaka A. A comparative study of in vitro apatite deposition on heat-, H(2)O(2)-, and NaOH-treated titanium surfaces. J Biomed Mater Res. 2001;54:172–78. doi: 10.1002/1097-4636(200102)54:2<172::aid-jbm3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Wang XX, Hayakawa S, Tsuru K, Osaka A. Improvement of bioactivity of H(2)O(2)/TaCl(5)-treated titanium after subsequent heat treatments. J Biomed Mater Res. 2000;52:171–76. doi: 10.1002/1097-4636(200010)52:1<171::aid-jbm22>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Yang GL, He FM, Zhao SS, et al. Effect of H2O2/HCl heat treatment of implants on in vivo peri-implant bone formation. Int J Oral Maxillofac Implants. 2008;23:1020–28. [PubMed] [Google Scholar]
- 17.He FM, Yang GL, Li YN, et al. Early bone response to sandblasted, dual acid-etched and H2O2/HCl treated titanium implants: an experimental study in the rabbit. Int J Oral Maxillofac Surg. 2009;38:677–81. doi: 10.1016/j.ijom.2009.03.716. [DOI] [PubMed] [Google Scholar]
- 18.Yang XF, Chen Y, Yang F, et al. Enhanced initial adhesion of osteoblast-like cells on an anatase-structured titania surface formed by H2O2/HCl solution and heat treatment. Dent Mater. 2009;25:473–80. doi: 10.1016/j.dental.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Jayaraman M, Meyer U, Buhner M, et al. Influence of titanium surfaces on attachment of osteoblast-like cells in vitro. Biomaterials. 2004;25:625–31. doi: 10.1016/s0142-9612(03)00571-4. [DOI] [PubMed] [Google Scholar]
- 20.Boyan BD, Bonewald LF, Paschalis EP, et al. Osteoblast-mediated mineral deposition in culture is dependent on surface microtopography. Calcif Tissue Int. 2002;71:519–29. doi: 10.1007/s00223-001-1114-y. [DOI] [PubMed] [Google Scholar]
- 21.Zhao G, Schwartz Z, Wieland M, et al. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005;74:49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- 22.Beck GR, Jr, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci USA. 2000;97:8352–57. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1 – review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004;17:536–43. [PubMed] [Google Scholar]
- 24.Ivanoff CJ, Widmark G, Johansson C, Wennerberg A. Histologic evaluation of bone response to oxidized and turned titanium micro-implants in human jawbone. Int J Oral Maxillofac Implants. 2003;18:341–48. [PubMed] [Google Scholar]
- 25.Xie Y, Liu X, Huang A, et al. Improvement of surface bioactivity on titanium by water and hydrogen plasma immersion ion implantation. Biomaterials. 2005;26:6129–35. doi: 10.1016/j.biomaterials.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Osaka A, Tsuru K, Hayakawa S. Titania derived from combined chemical and thermal treatments of titanium: In vitro apatite forming ability. Phosphorus Res Bull. 2004;17:130–41. [Google Scholar]
- 27.Li P, Ohtsuki C, Kokubo T, et al. The role of hydrated silica, titania, and alumina in inducing apatite on implants. J Biomed Mater Res. 1994;28:7–15. doi: 10.1002/jbm.820280103. [DOI] [PubMed] [Google Scholar]
- 28.Takadama H, Kim HM, Kokubo T, Nakamura T. TEM-EDX study of mechanism of bonelike apatite formation on bioactive titanium metal in simulated body fluid. J Biomed Mater Res. 2001;57:441–48. doi: 10.1002/1097-4636(20011205)57:3<441::aid-jbm1187>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Takadama H, Kim HM, Kokubo T, Nakamura T. An X-ray photoelectron spectroscopy study of the process of apatite formation on bioactive titanium metal. J Biomed Mater Res. 2001;55:185–93. doi: 10.1002/1097-4636(200105)55:2<185::aid-jbm1005>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 30.Uchida M, Kim HM, Kokubo T, et al. Structural dependence of apatite formation on titania gels in a simulated body fluid. J Biomed Mater Res A. 2003;64:164–70. doi: 10.1002/jbm.a.10414. [DOI] [PubMed] [Google Scholar]
- 31.Szmukler-Moncler S, Bischof M, Nedir R, Ermrich M. Titanium hydride and hydrogen concentration in acid-etched commercially pure titanium and titanium alloy implants: a comparative analysis of five implant systems. Clin Oral Implants Res. 2010;21:944–50. doi: 10.1111/j.1600-0501.2010.01938.x. [DOI] [PubMed] [Google Scholar]
- 32.Perrin D, Szmukler-Moncler S, Echikou C, et al. Bone response to alteration of surface topography and surface composition of sandblasted and acid etched (SLA) implants. Clin Oral Implants Res. 2002;13:465–69. doi: 10.1034/j.1600-0501.2002.130504.x. [DOI] [PubMed] [Google Scholar]
- 33.Aronsson BO, Hjorvarsson B, Frauchiger L, et al. Hydrogen desorption from sand-blasted and acid-etched titanium surfaces after glow-discharge treatment. J Biomed Mater Res. 2001;54:20–29. doi: 10.1002/1097-4636(200101)54:1<20::aid-jbm3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 34.Lamolle SF, Monjo M, Rubert M, et al. The effect of hydrofluoric acid treatment of titanium surface on nanostructural and chemical changes and the growth of MC3T3-E1 cells. Biomaterials. 2009;30:736–42. doi: 10.1016/j.biomaterials.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F, Yang GL, He FM, et al. Cell response of titanium implant with a roughened surface containing titanium hydride: an in vitro study. J Oral Maxillofac Surg. 2010;68(5):1131–39. doi: 10.1016/j.joms.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 36.Cheng Z, Zhang F, He F, et al. Osseointegration of titanium implants with a roughened surface containing hydride ion in a rabbit model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:e5–12. doi: 10.1016/j.tripleo.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Cui X, Kim HM, Kawashita M, et al. Effect of hot water and heat treatment on the apatite-forming ability of titania films formed on titanium metal via anodic oxidation in acetic acid solutions. J Mater Sci Mater Med. 2008;19:1767–73. doi: 10.1007/s10856-007-3314-0. [DOI] [PubMed] [Google Scholar]