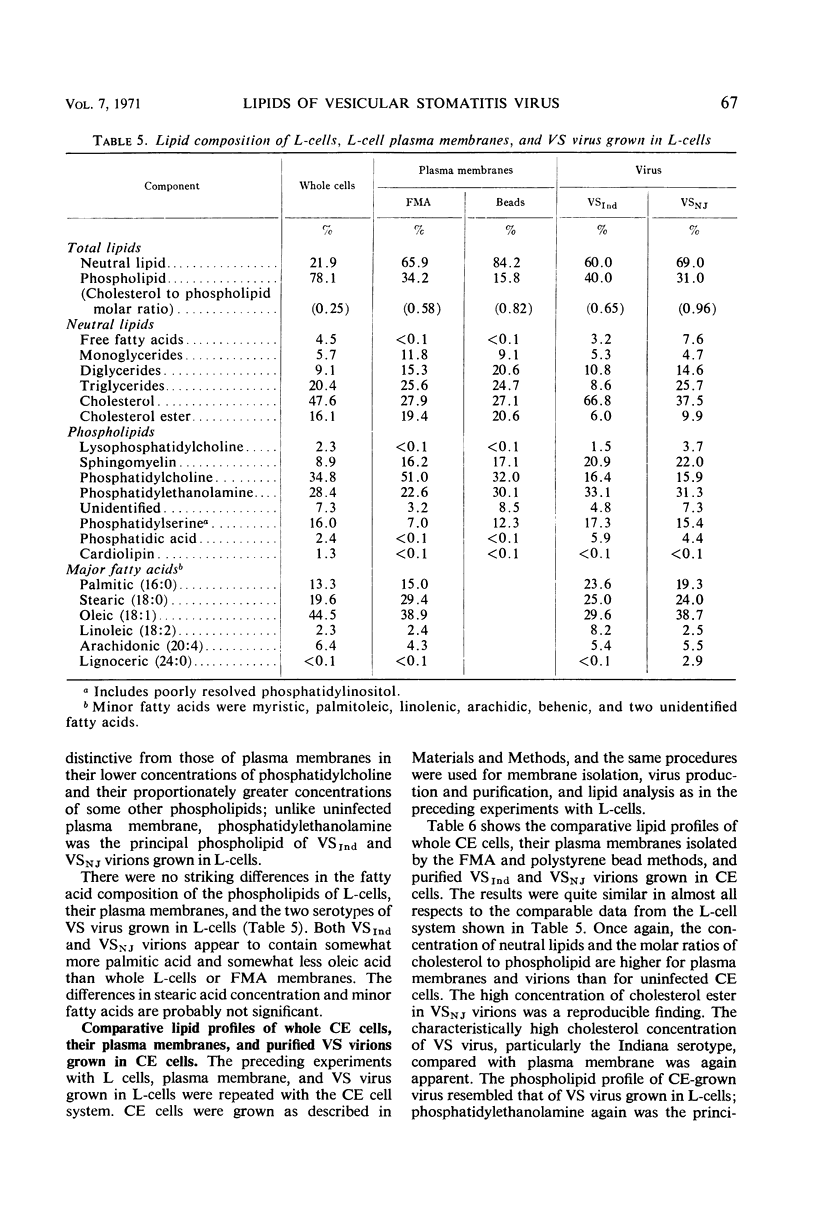
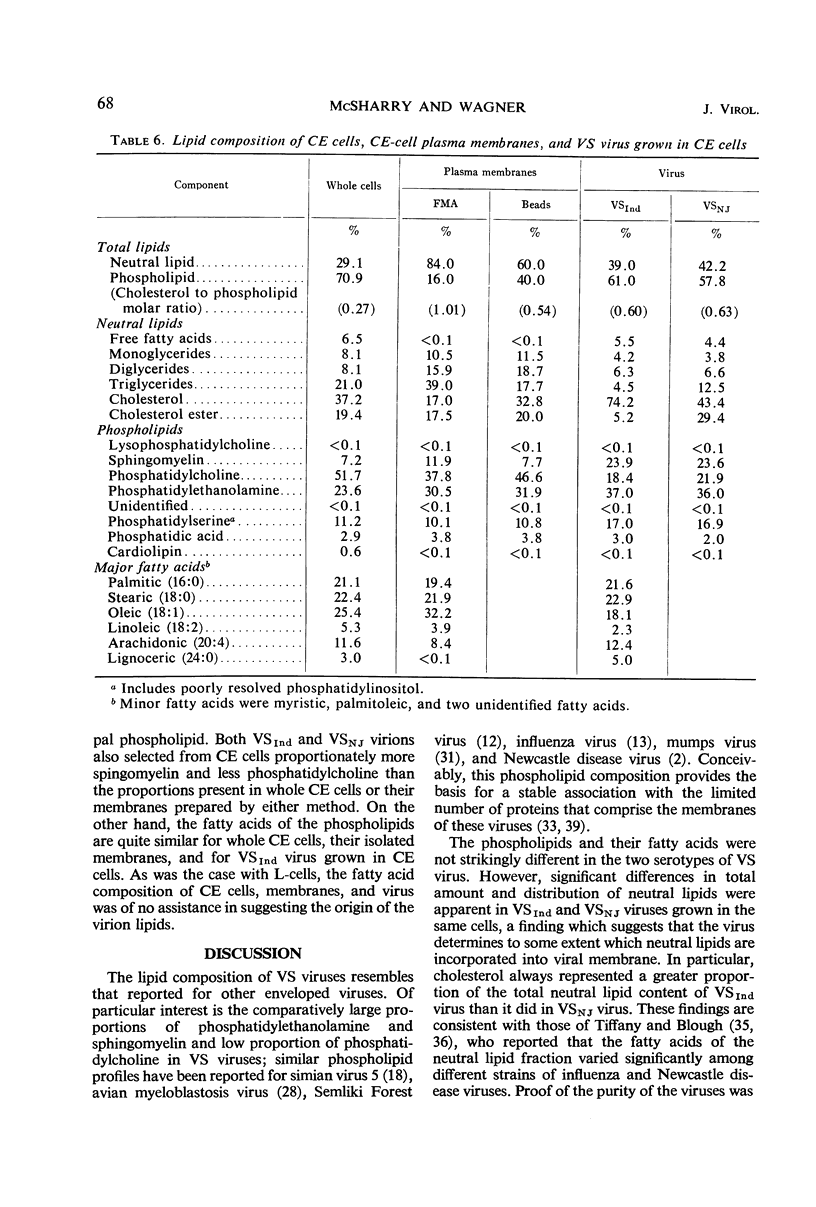
Abstract
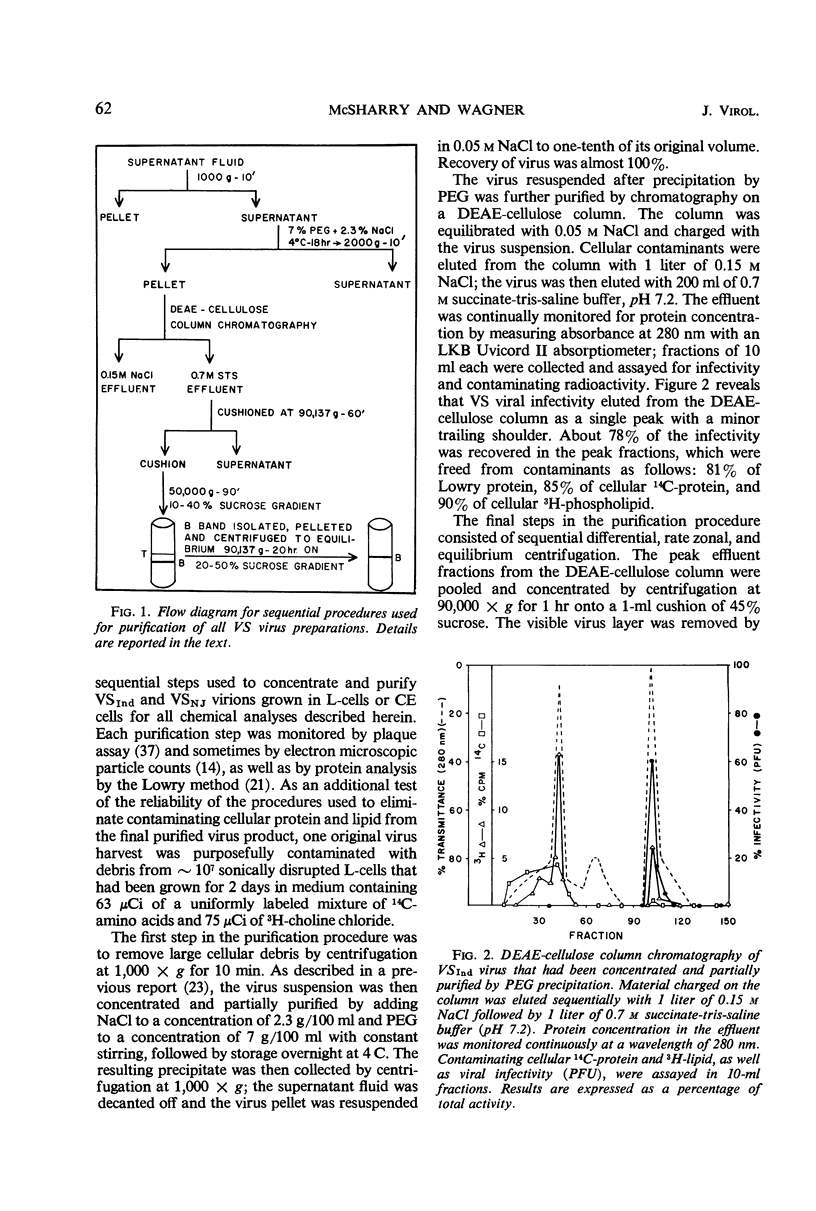
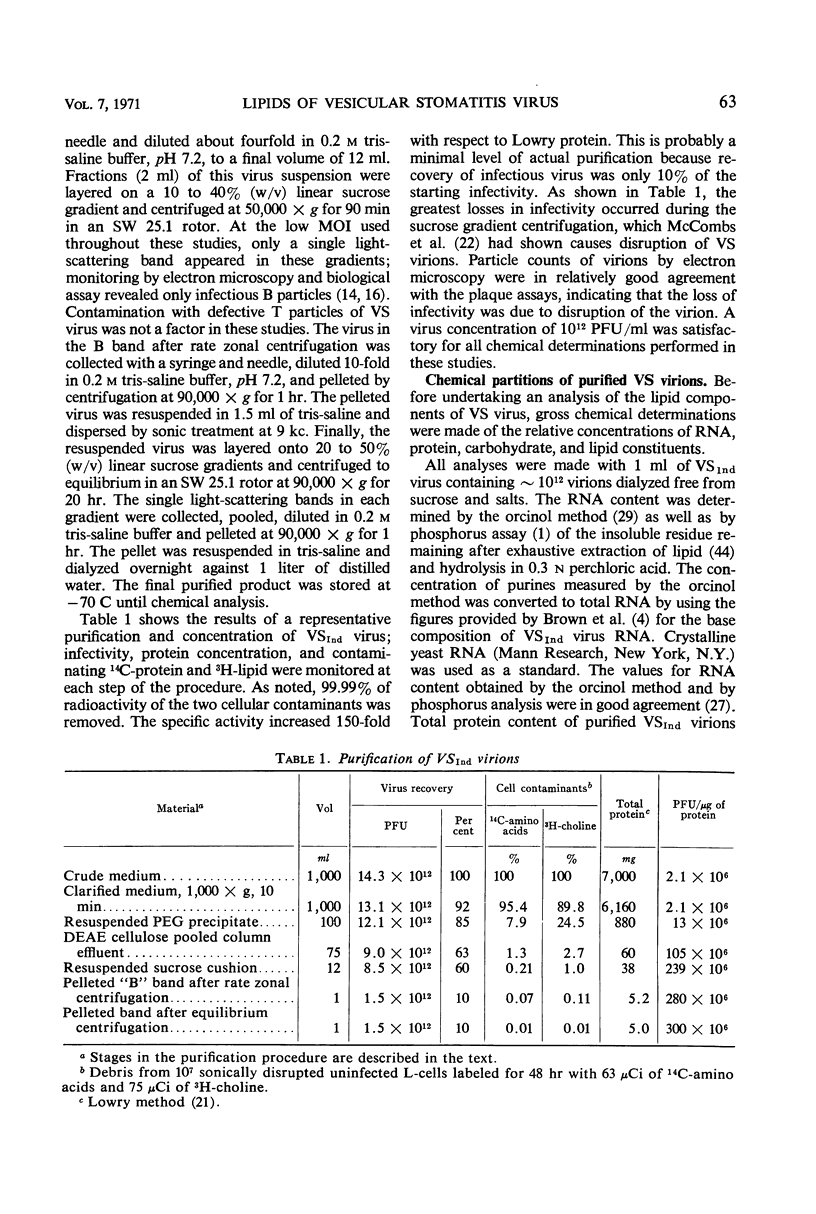
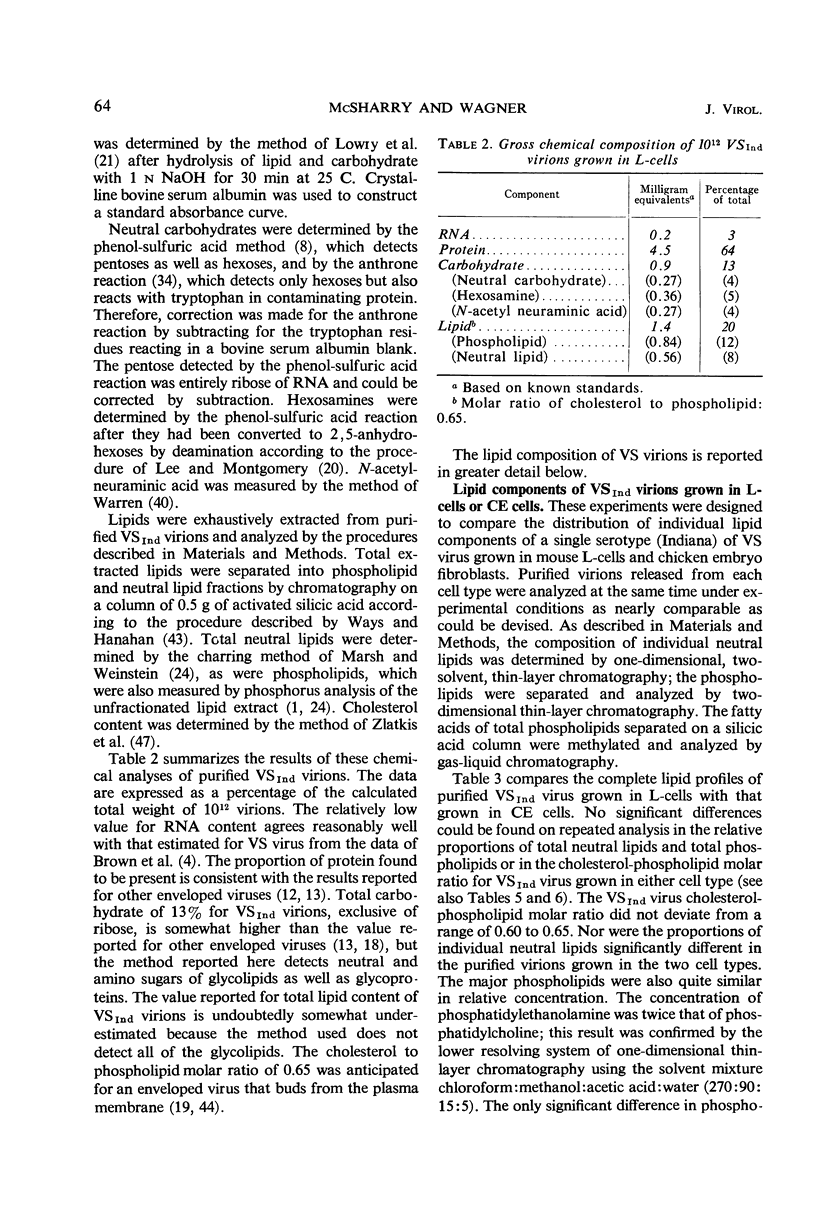
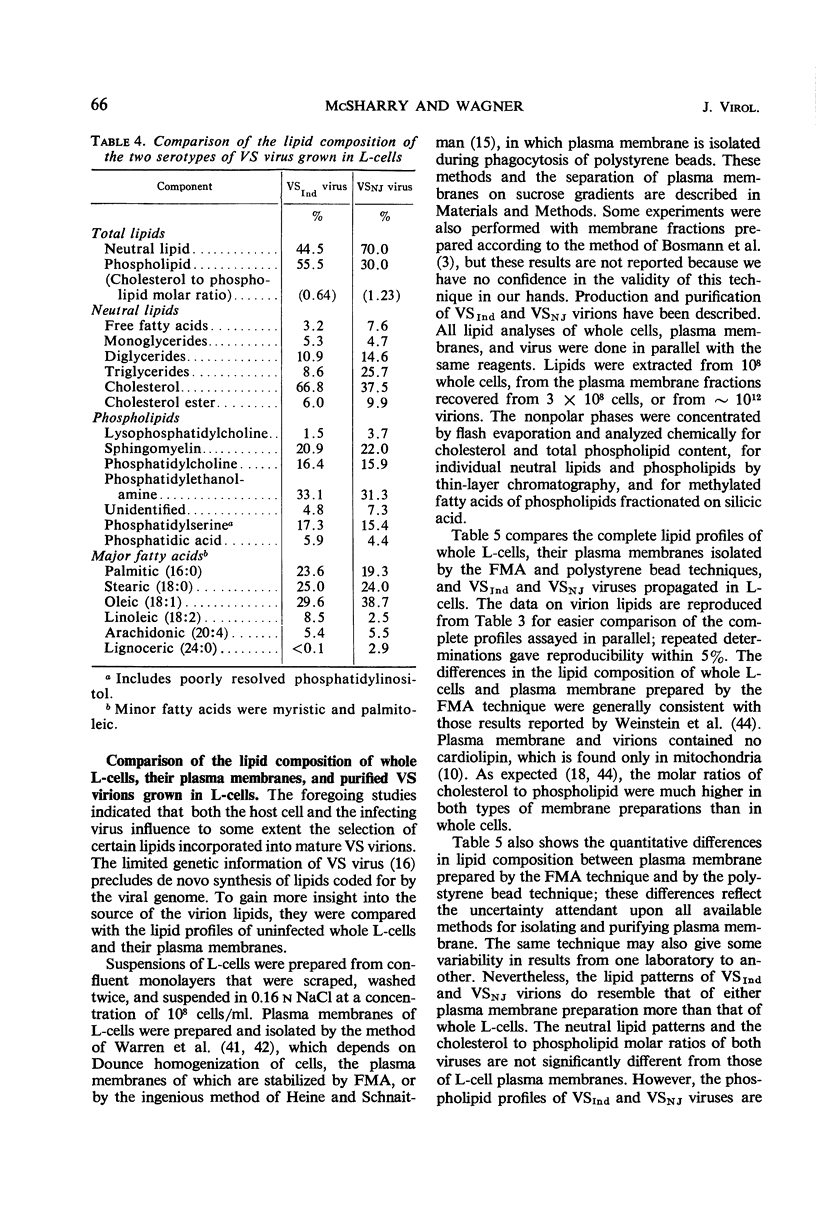
Methods are described for the production of vesicular stomatitis (VS) virus of sufficient purity for reliable chemical analysis. VS virions released from infected cells were concentrated and purified at least 150-fold by sequential steps of precipitation with polyethylene glycol, column chromatography, rate zonal centrifugation, and equilibrium centrifugation. The Indiana serotype (VSInd virus) propagated in L-cells was found to contain 3% ribonucleic acid, 64% protein, 13% carbohydrate, and 20% lipid; the molar ratio of cholesterol to phospholipid was 0.6 or greater. Thin-layer chromatography revealed no unusual neutral lipids or phospholipids and gas-liquid chromatography revealed no unusual fatty acids incorporated into VS virions. The antigenically distinct New Jersey serotype (VSNJ virus) grown in L-cells showed a similar lipid profile except that the proportion of neutral lipids was larger than in VSInd virus also grown in L-cells. This differences was less pronounced when the lipid composition of VSInd and VSNJ viruses grown in chick embryo cells was compared, but VSNJ virus grown in either cell type always contained larger amounts of neutral lipids other than cholesterol than did VSInd virus. The lipid composition of both VSInd and VSNJ viruses grown in L-cells or chick embryo cells more closely resembled that of plasma membrane than of whole cells. A consistent finding was the relatively large amounts of phosphatidylethanolamine and sphingomyelin and the relatively small amounts of phosphatidylcholine in both VS viruses compared with uninfected whole L-cells and chick embryo cells or their plasma membranes. The methods available for isolation of plasma membranes were inadequate for conclusive comparison of the lipids of VS virions with the lipids of the plasma membranes of their host cells. Nevertheless, the data obtained are consistent with two hypotheses: (i) the lipid composition of VS viruses primarily reflects their membrane site of maturation, and (ii) the newly synthesized viral proteins inserted into cell membranes influence the proportions of phospholipids and neutral lipids selected for incorporation into the viral membrane.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Blough H. A., Lawson D. E. The lipids of paramyxoviruses: a comparative study of Sendai and Newcastle disease viruses. Virology. 1968 Oct;36(2):286–292. doi: 10.1016/0042-6822(68)90146-3. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Hagopian A., Eylar E. H. Cellular membranes: the isolation and characterization of the plasma and smooth membranes of HeLa cells. Arch Biochem Biophys. 1968 Oct;128(1):51–69. doi: 10.1016/0003-9861(68)90008-8. [DOI] [PubMed] [Google Scholar]
- Brown F., Martin S. J., Cartwright B., Crick J. The ribonucleic acids of the infective and interfering components of vesicular stomatitis virus. J Gen Virol. 1967 Oct;1(4):479–486. doi: 10.1099/0022-1317-1-4-479. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Huang A. S. Comparison of membrane protein glycopeptides of Sindbis virus and vesicular stomatitis virus. J Virol. 1970 Aug;6(2):176–182. doi: 10.1128/jvi.6.2.176-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Pearce C. A. Evidence for a host cell component in vesicular stomatitis virus. J Gen Virol. 1968 Mar;2(2):207–214. doi: 10.1099/0022-1317-2-2-207. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Talbot P., Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. doi: 10.1099/0022-1317-7-3-267. [DOI] [PubMed] [Google Scholar]
- FROMMHAGEN L. H., FREEMAN N. K., KNIGHT C. A. The lipid constituents of influenza virus, chick allantoic membrane and sedimentable allantoic protein. Virology. 1958 Feb;5(1):173–175. doi: 10.1016/0042-6822(58)90015-1. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Rouser G., Fleischer B., Casu A., Kritchevsky G. Lipid composition of mitochondria from bovine heart, liver, and kidney. J Lipid Res. 1967 May;8(3):170–180. [PubMed] [Google Scholar]
- Freeman C. P., West D. Complete separation of lipid classes on a single thin-layer plate. J Lipid Res. 1966 Mar;7(2):324–327. [PubMed] [Google Scholar]
- Friedman R. M., Pastan I. Nature and function of the structural phospholipids of an arbovirus. J Mol Biol. 1969 Feb 28;40(1):107–115. doi: 10.1016/0022-2836(69)90299-x. [DOI] [PubMed] [Google Scholar]
- Galasso G. J. Enumeration of VSV particles and a demonstration of their growth kinetics by electron microscopy. Proc Soc Exp Biol Med. 1967 Jan;124(1):43–48. doi: 10.3181/00379727-124-31662. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Comparative sedimentation coefficients of RNA extracted from plaque-forming and defective particles of vesicular stomatitis virus. J Mol Biol. 1966 Dec 28;22(2):381–384. doi: 10.1016/0022-2836(66)90143-4. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. II. Immunological comparisons of viral antigens. J Virol. 1970 Jul;6(1):20–27. doi: 10.1128/jvi.6.1.20-27.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Lipids of plasma membranes of monkey and hamster kidney cells and of parainfluenza virions grown in these cells. Virology. 1969 Jun;38(2):255–268. doi: 10.1016/0042-6822(69)90367-5. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Plasma membrane lipids and parainfluenza virus assembly. Virology. 1970 Apr;40(4):939–947. doi: 10.1016/0042-6822(70)90140-6. [DOI] [PubMed] [Google Scholar]
- LEE Y. C., MONTGOMERY R. Determination of hexosamines. Arch Biochem Biophys. 1961 May;93:292–296. doi: 10.1016/0003-9861(61)90265-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Marsh J. B., Weinstein D. B. Simple charring method for determination of lipids. J Lipid Res. 1966 Jul;7(4):574–576. [PubMed] [Google Scholar]
- McCombs R. M., Melnick M. B., Brunschwig J. P. Biophysical studies of vesicular stomatitis virus. J Bacteriol. 1966 Feb;91(2):803–812. doi: 10.1128/jb.91.2.803-812.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J., Benzinger R. Concentration and purification of vesicular stomatitis virus by polyethylene glycol "precipitation". Virology. 1970 Mar;40(3):745–746. doi: 10.1016/0042-6822(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Nakai T., Howatson A. F. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. doi: 10.1016/0042-6822(68)90267-5. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. PURIFICATION AND PARTIAL CHEMICAL ANALYSIS OF SINDBIS VIRUS. Virology. 1963 Jul;20:433–445. doi: 10.1016/0042-6822(63)90092-8. [DOI] [PubMed] [Google Scholar]
- SOULE D. W., MARINETTI G. V., MORGAN H. R. Studies on the hemolysis of red blood cells by mumps virus. IV. Quantitative study of changes in red blood cell lipides and of virus lipides. J Exp Med. 1959 Jul 1;110(1):93–102. doi: 10.1084/jem.110.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siakotos A. N. Analytical separation of nonlipid water soluble substances and gangliosides from other lipids by dextran gel column chromatography. J Am Oil Chem Soc. 1965 Nov;42(11):913–919. doi: 10.1007/BF02632444. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Carbohydrate content of the membrane protein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):437–448. doi: 10.1016/0022-2836(70)90313-x. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany J. M., Blough H. A. Fatty acid composition of three strains of Newcastle disease virus. Virology. 1969 Mar;37(3):492–494. doi: 10.1016/0042-6822(69)90237-2. [DOI] [PubMed] [Google Scholar]
- Tiffany J. M., Blough H. A. Myxovirus envelope proteins: a directing influence on the fatty acids of membrane lipids. Science. 1969 Feb 7;163(3867):573–574. doi: 10.1126/science.163.3867.573. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R., LEVEY A. H., SNYDER R. M., RATCLIFF G. A., Jr, HYATT D. F. BIOLOGIC PROPERTIES OF TWO PLAQUE VARIANTS OF VESICULAR STOMATITIS VIRUS (INDIANA SEROTYPE). J Immunol. 1963 Jul;91:112–122. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. A., Snyder R. M. Structural proteins of vesicular stomatitis viruses. J Virol. 1969 Apr;3(4):395–403. doi: 10.1128/jvi.3.4.395-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ways P., Hanahan D. J. Characterization and quantification of red cell lipids in normal man. J Lipid Res. 1964 Jul;5(3):318–328. [PubMed] [Google Scholar]
- Weinstein D. B., Marsh J. B., Glick M. C., Warren L. Membranes of animal cells. IV. Lipids of the L cell and its surface membrane. J Biol Chem. 1969 Aug 10;244(15):4103–4111. [PubMed] [Google Scholar]
- Wetzel M. G., Korn E. D. Phagocytosis of latex beads by Acahamoeba castellanii (Neff). 3. Isolation of the phagocytic vesicles and their membranes. J Cell Biol. 1969 Oct;43(1):90–104. doi: 10.1083/jcb.43.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZLATKIS A., ZAK B., BOYLE A. J. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953 Mar;41(3):486–492. [PubMed] [Google Scholar]
- Zee Y. C., Hackett A. J., Talens L. Vesicular stomatitis virus maturation sites in six different host cells. J Gen Virol. 1970;7(2):95–102. doi: 10.1099/0022-1317-7-2-95. [DOI] [PubMed] [Google Scholar]



