Summary
Background
Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS) is a rare and severe adverse drug reaction with an associated mortality of 10–20%. Clinical worsening despite discontinuation of the culprit drug is considered a characteristic feature of DIHS/DRESS. Besides the early recognition of the syndrome and discontinuation of its causative drug, the mainstay of treatment is systemic corticosteroids. Nevertheless, treatment of severe DIHS/DRESS is not well defined, as corticosteroids may sometimes not be effective, and decreasing the dose may be associated with flaring of the disease.
Case Report
A 38-year-old woman with high fever, malaise, abdominal pain, rash, and elevated liver enzymes received immediate high-dose N-acetylcysteine, because acetaminophen hepatotoxicity was suspected. N-acetylcysteine administration was associated with a significant clinical improvement. However, within the next week DIHS/DRESS syndrome was diagnosed, which explained all the symptoms, and which was subsequently treated with prednisone and valganciclovir.
Conclusions
New options necessary to improve treatment of severe DIHD/DRESS have to consider its sequential pathogenetic mechanisms. N-acetylcysteine might neutralize the drug-derived reactive metabolites, which are responsible for protein adduct formation and specific T cell stimulation, and replete the glutathione stores that counterbalance oxidative stress. Prednisone might inhibit lymphoproliferation and valganciclovir might prevent complications related to HHV-6 reactivation. We therefore propose the unprecedented combination of N-acetylcysteine, prednisone and valganciclovir as a treatment option for DIHS/DRESS.
Keywords: drug-induced hypersensitivity syndrome, drug reaction with eosinophilia and systemic symptoms, HHV-6, N-acetylcysteine, valganciclovir
Background
Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS) is a severe drug-induced adverse reaction that in most cases occurs 3 to 6 weeks after drug administration [1–4]. The diagnosis can be confirmed if 5 out of the following 6 criteria are fulfilled: 1. maculopapular rash developing >3 weeks after starting the triggering drug; 2. fever >38°C; 3. lymphadenopathy; 4. leucocytosis (>10×109/L), atypical lymphocytosis, and eosinophilia; 5. hepatitis (ALT >100 U/L); and 6. HHV-6 reactivation [1].
Because of the complexity and variability of DIHS/DRESS, another diagnostic scoring system has been proposed by the RegiSCAR study group [3,5]. Numerous systemic complications may occur, including pneumonitis, pancreatitis, renal failure, encephalitis, thyroid disease, spleen rupture, eosinophilic colitis or esophagitis, coronary artery thrombosis, and myocarditis [1,2,4]. A mortality rate of 10–20% has been reported [1,2,4]. The most common drugs associated with DIHS/DRESS are: antiepileptic drugs (carbamazepine, phenytoin, phenobarbital, lamotrigine, levetiracetam), sulfonamide or its derivates (sulfamethoxazole, sulfadiazine, sulfasalazine, dapsone), allopurinol, minocycline, mexiletine, nevirapine, abacavir, and others [1–4].
The DIHS/DRESS has several important clinical features that cannot be explained only by drug antigen-driven oligoclonal T cell expansion. These include paradoxical worsening of clinical symptoms, frequent flare-ups and a stepwise development of multiorgan failures when the causative drugs are withdrawn. The pathophysiology of DIHS/DRESS resembles that of graft versus host diseases (GvHD) and immune reconstitution syndrome (IRS) [1]. Herpesviruses, including herpesvirus-6 (HHV-6), herpesvirus-7 (HHV-7), Epstein Barr virus (EBV) and cytomegalovirus (CMV), can be reactivated during DIHS/DRESS in the same sequential manner as in GvHD [6–11]. Autoimmune diseases may occur months/years after clinical resolution of DIHS/DRESS. For example, diabetes type I, autoimmune thyroiditis, systemic sclerosis, and systemic lupus erythematosus (SLE) have been described[4,12].
The standard treatment of DIHS/DRESS is with moderate- or high-dose corticosteroids [1–3]. Nevertheless, treatment of severe DIHS/DRESS is not well defined. Indeed, sometimes corticosteroid treatment is not effective, and tapering the dose may be associated with disease flaring. Adjunctive treatment with acetylcysteine [13,14], intravenous immunoglobulins [1,2], plasmapheresis [1], and cyclosporine [3] have been tried with varying results. We report on a woman with sulfasalazine-induced DIHS/DRESS treated using an unprecedented combination of N-acetylcysteine, prednisone, and valganciclovir.
Case Report
A 38-year-old woman was admitted to hospital on September 2010 because of high fever and a generalized erythematous rash. She complained of fever with myalgias over the previous week. She had taken a course of acetaminophen 500 mg 3 times daily (a total of 19 tablets). She was of North African origin (Morocco) and living in Italy. She had 4 children, of whom 1 suffered from diabetes type I since the age of 2 years. Ten years earlier she was diagnosed with vitiligo. Six years earlier she underwent a thyroidectomy for unknown reason and since then had been on L-thyroxine 125 μg daily. Four weeks prior to this admission she was prescribed sulfasalazine 500 mg TDS because of arthralgias. On the morning of the hospital admission she had discontinued sulfasalazine on her own. The month prior to hospital admission the patient was fasting for cultural reasons (Muslim Ramadan). On physical examination she had a fever 40°C, a non-itchy generalized erythematous rash and mild facial edema. She was agitated and she complained of diffuse malaise, asthenia and abdominal pain. There were signs of dehydration, and body weight loss of 3 kg over 1 month. Her blood pressure and cardiovascular examination were normal. Bilateral cervical lymphadenopathy was found.
Chest x-ray examination showed a left base consolidation. Laboratory analysis showed: white blood cells 4×109/L, neutrophils 75%, hemoglobin 10.8 g/L, MCV 63, AST 541 IU/L, ALT 389 IU/L, LDH 1,703 IU/L, PT INR 1.21, PTT RATIO 1.41, gamma-globulin 16.5% (8.73 g/L), CRP 80 mg/L, urinary ketones 300 mg/L, albumin 26 g/L. Acetaminophen blood level 10 hours after the last drug assumption was 3 μg/mL. She was admitted in the Infectious Diseases Department because viral hepatitis was suspected.
The prolonged fasting for Ramadan was considered a risk factor for acetaminophen hepatotoxicity at therapeutic doses [15]. Therefore intravenous N-acetylcysteine was immediately administered (10 g the first 2 hours, followed by 2.5 g every 6 hours for the next 3 days), followed by temporary hypotension (from 130/75 to 80/50 mmHg). Amoxi-clavulanate 2 g TDS was given for treatment of the lung consolidation (as above). On day +2 of admission, significant improvement was observed, with resolution of malaise, abdominal pain and erythematous rash. Liver function test results were markedly reduced (Figure 1).
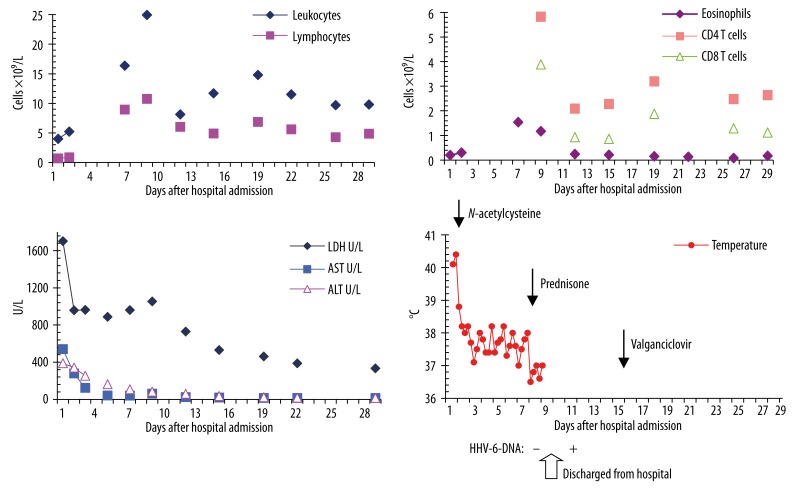
Figure 1.
Course of the following laboratory parameters: leukocytes and lymphocytes (A), eosinophils, CD4, and CD8 T cells (B), AST (aspartate aminotransferase), ALT (alanine aminotransferase), LDH (lactate dehydrogenase) (C), course of fever, sequence of the combination treatment, and timing of HHV-6-DNA detection: − = not detected, + = detected (D).
Nevertheless, she continued to have persistent fever, elevated LDH (Figure 1), enlarging lymphadenopathies, liver and spleen, lymphocytosis (from 0.7 to 8.95×109/L with 1.62×109/L atypical lymphocytes), high CD4+ cell count (5.832×109/L), leucocytosis (from 4 to 16.37×109/L), eosinophilia (from 0.2 to 1.54×109/L) for 1 week (Figure 1), which was strongly suggestive of sulfasalazine-related DIHS/DRESS syndrome. She has started taking this drug 4 weeks previously. On day +7, treatment with 75 mg prednisone daily was commenced and amoxi-clavulanate was discontinued. Fever disappeared and 2 days later the patient was discharged. She was advised to avoid taking sulfasalazine and other sulphonamides, including sulfamethoxazole.
Microbiological analysis for HAV-IgM, HBs-Ag, HBV-DNA, HCV-Ab, Legionella-Ag and Streptococcus pneumoniae-Ag in urine were all negative. HAV-IgG, HBs-Ab, and HBc-Ab were detected. HLA study showed HLA-B*18*53. Herpesvirus screening was performed and tested positive for CMV- EBV-, and HHV-6-IgG (1:40); however, she tested negative for CMV-, EBV-, and HHV-6-DNA (day +8). HHV-6-DNA was detected in blood and serum samples on day +12 from admission time. After 3 weeks the HHV-6-IgG titer was increased from 1:40 to 1:320. Further search for CMV or EBV reactivation remained negative. On day +15 valganciclovir 900 mg twice daily was also introduced. Four days later, because of general malaise, the dose was halved to 450 mg twice daily. After 3 months of treatment, first prednisone and then valganciclovir were discontinued. The patient’s clinical status was unremarkable and all laboratory values were within normal range. Eighteen months following her illness she continues to do well.
Discussion
Sulfasalazine metabolism
Usually sulfasalazine is split in the colon by bacterial azo-reduction into 5-ASA and sulfapyridine [16]. Sulfapyridine is almost completely absorbed in the colon and metabolized by hydroxylation, glucuronidation, and acetylation [16]. Various oxidizing cytochrome P450 (CYP) enzymes, including CYP2C9, CYP2E1, and CYP3A4, can oxidize aryl amines to reactive metabolites [17] and sulfapyridine will be converted into the unstable sulfapyridine hydroxylamine intermediate, which auto-oxidizes to nitroso-sulfapyridine [18].
Induction of a T cell response to the drug protein adducts
The results of the patch tests and lymphocyte transformation tests indicate that drug-specific T cells are the driving force behind DIHS/DRESS [1]. Sulfamethoxazole (SMX) is commonly used to investigate the chemical and cellular basis of drug hypersensitivity [18]. The SMX metabolite nitroso-SMX binds covalently to cysteine residues on cellular protein; this binding above a certain threshold may cause direct toxicity and provides an antigenic signal to nitroso-SMX specific T cells [18].
Dendritic cells, as antigen-presenting cells, play an important role in immunity, differentiating between tolerogenic and immunogenic responses [19]. Dendritic cell maturation and costimulatory signals are required for T cell immunogenicity together with the antigen. There are a series of exogenous and endogenous signals that mature dendritic cells, leading to expression of costimulatory molecules. Exogenous signals include conserved microbial products that interact with pathogen-recognition receptors, such as toll-like receptors (TLRs). This may be of relevance to the increased risk of drug hypersensitivity reactions associated with viral infections such as EBV, human herpes 6, and HIV. Endogenous signals derive from necrotic cell death, apoptotic cell death or oxidative stress [19].
In an experiment, antigen-presenting cells were incubated with SMX in the presence of bacterial endotoxins, flu viral proteins, cytokines, inflammatory molecules, oxidants, and hyperthermia [19]. Such conditions, also referred to as “danger” conditions, significantly increased the formation of SMX-protein adducts. Soluble antioxidants were shown to prevent SMX-hydroxyamine auto-oxidation and to reduce nitroso-SMX back to SMX-hydroxylamine, limiting nitroso-SMX protein binding and T cell stimulatory capacity of nitroso-SMX protein adducts. Therefore it was suggested that acute and chronic infections treated with SMX require antioxidant supplementation [19].
Another experiment demonstrated that nitroso-SMX increases dendritic cell costimulatory signalling via CD40 [18]. Activation of antigen-presenting cells through CD40 signalling is known to precipitate animal models of autoimmune diseases [18]. Several autoimmune diseases have been reported to occur at intervals of months to years after clinical resolution of DIHS/DRESS (see below). It was also demonstrated that CD4+CD25+Foxp3+ regulatory T (Treg) cells are expanded at the acute stage of DIHS/DRESS [12]. This expansion may reflect an attempt to limit collateral tissue damage induced by activation of effector T cells while allowing latent herpesviruses to reactivate [12]. Otherwise human herpesvirus binding TLR2 on regulatory T cells could induce their proliferation [20]. Peripheral Treg cells are also induced by reactive oxygen species [21].
Crossreactivity within drug classes
Nitroso-SMX responsive T cell clones from patients with SMX hypersensitivity reaction displayed in vitro reactivity toward nitroso metabolites of sulfadiazine and sulfapyridine. T cell receptor cross-reactivity with nitroso sulfonamides displaying different side chains was thus demonstrated, and shows the clear potential for hypersensitivity reaction to develop different drug structures within the same chemical class through metabolite formation and targeting of identical binding sites on protein [22]. The above described patient was therefore advised to avoid the entire class of sulfonamides and its derivates.
Reactivation of human herpesvirus 6 (HHV-6) and other herpesviruses
In 1997 a rise of HHV-6 antibody titre was described for the first time in a patient with DIHS/DRESS and a fulminant hemophagocytic syndrome [6]. In addition, increased HHV-6 DNA in the serum was detected by quantitative PCR and by in situ hybridization in the skin of patients with DIHS/DRESS [7,8]. It was also shown that other herpesviruses, HHV-7, EBV, and CMV, could reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in GvHD [9,10]. HHV-6 reactivation was found in 62 of 100 patients with DIHS/DRESS and was associated with flaring and severity of the syndrome. HHV-6 DNA was detected in patients’ serum from day 10 to day 27 after clinical onset, but not earlier [11].
The pathophysiological role of herpesvirus reactivation in DIHS/DRESS is not clear. First, we do not know if during asymptomatic HHV-6 infection the virus does not replicate, if it replicates at a low level, or reactivates temporarily in localized compartments like the salivary gland or lymphatic tissue. In 1 patient with GvHD, HHV-6 reactivation in saliva was observed 10 days before onset of rash, and salivary HHV-6 DNA became negative during the rash, followed by detection of HHV-6 DNA in the blood [23]. An ongoing virus replication or virus reactivation could add to the “danger” conditions that lead to dendritic cell maturation and expression of costimulatory molecules, thus favoring the immunogenicity of the drug protein adducts (see above). This would increase the rate of allergic drug reactions, as seen in infectious mononucleosis and HIV infection. Second, hypo-immunoglobulinemia, and low B cell and CD56 cell counts observed in the initial phase of some patients with DIHS/DREES may reflect immune depression caused by drug administration [24] and/or may be a consequence of excessive regulatory T cell expansion (see below). Drug-discontinuation may reconstitute immunity, and the paradoxical worsening of symptoms after drug discontinuation could be interpreted as immune reconstitution syndrome (IRS) [25]. Third, T cell activation and proliferation (induced by the drug metabolite protein adducts) may reactivate HHV-6 and other herpesviruses, which may be responsible for late organ complications such as nephritis, encephalitis, pneumonitis, myocarditis. Corticosteroid treatment may favor herpesvirus reactivation, and the clinical flare-ups observed when the corticosteroid dose is tapered too fast may reflect an IRS. Currently, anti-cytomegalovirus drugs such as valganciclovir, cidofovir, and foscarnet are used to treat HHV-6 infections because in vitro studies show that they also have activity against HHV-6 [26].
DIHS/DRESS, T regulatory cell expansion, hypogammaglobulinemia, reactivation of latent herpesviruses, and autoimmune disease
Several autoimmune diseases (type I diabetes mellitus, thyroiditis, systemic sclerosis-like manifestations, and SLE) have been reported to occur at intervals of several months to years after clinical resolution of DIHS/DRESS [4,12]. A dramatic expansion of T regulatory (Treg) cells has recently been found in the acute stage of DIHS/DRESS [4,12]. Treg cells are CD4+CD25+FoxP3+ T cells specialized in suppressing the activation of the immune system and thereby maintaining immune system homeostasis and tolerance to self antigens. The suppressive capacity of Treg cells became defective after clinical resolution of DIHS/DRESS [12]. One possible explanation for Treg dysfunction is that Treg cells might be exhausted owing to repeated and excessive activation of effector T cells by drug protein adducts and/or reactivated herpesviruses. The functional defect of Treg cells could be responsible for the development of autoimmune disease that can occur months to years after the resolution of DIHS/DRESS [4,12]. Also human herpesvirus binding TLR2 present on Treg cells has been shown to temporarily abrogate their suppressive phenotype [20]. Foxp3 expression, a key regulator of regulatory T cell function, was found to decrease following TLR2 stimulation of Treg cells [20].
Unfortunately we missed the opportunity to measure the quote of the CD4+CD25+FoxP3+ T cells among the excessively increased CD4 T cells of the described patient (Figure 1). The patient’s increased number of CD4 compared to CD8 T cells and her mild skin lesions might reflect the excessive expansion of Treg cells, since Treg cells are thought to alleviate the skin lesion severity of DIHS/DRESS [12]. However, excessive Treg cells are thought to allow latent herpesviruses to reactivate in an uncontrolled fashion [4,12]. Hypogammaglobulinemia observed at the onset of DIHS/DRESS may be related to the expansion of Treg cells, because Treg cells have been shown to be capable of inducing B cell death [12].
Why is repeated administration of acetaminophen and food restriction considered to be risk factors for DRESS syndrome?
Acetaminophen-induced toxicity is a clinically important model of drug-induced liver injury. Increased formation of the reactive intermediate metabolite N-acetyl-p-benzo-quinone-imine (NAPQI) and depletion of cellular glutathione have been proposed as the major reasons for the hepatotoxicity of acetaminophen overdose [27]. In rare circumstances, even “therapeutic” doses of acetaminophen can lead to hepatotoxicity [15]. CYP2E1, among other CYP enzymes, was found to be the main activator of acetaminophen to NAPQI [27]. In rats the repeated administration of subtoxic doses of acetaminophen has been shown to induce hepatic CYP enzymes (CYP2E1, CYP3A, CYP1A) [28].
CYP2E1 is capable of carrying out the oxidation of acetone, a product of fatty acid oxidation, in a pathway that leads to the production of glucose, termed the propane diol pathway of gluconeogenesis [27]. This explains the marked induction of CYP2E1 seen in fasted rats, because fasting results in an increase in acetone. Acetone has been shown to be an inducer of CYP2E1 by substrate stabilization of the enzyme [27].
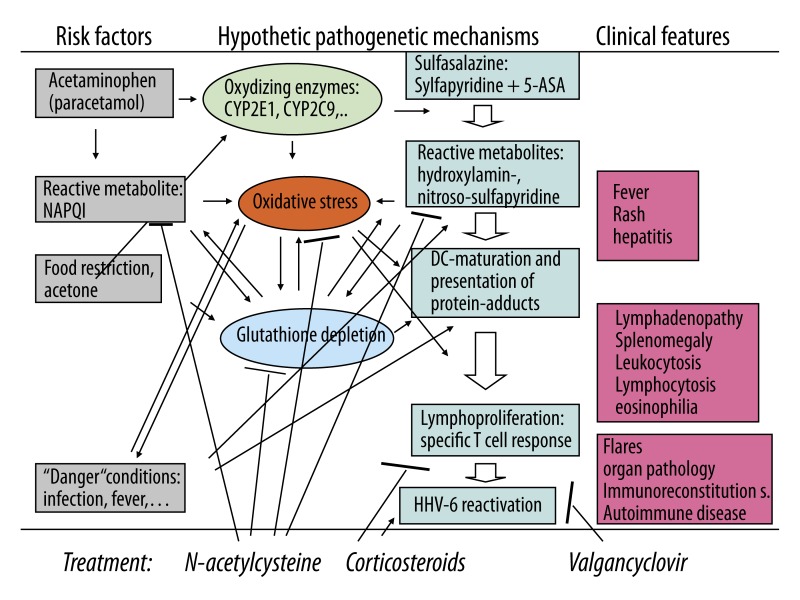
CYP2E1 induction by repeated administration of acetaminophen and by acetone resulting from food restriction may have: 1. increased oxidative stress due to increased NAPQI formation; 2. increased oxidation of sulfapyridine to its hydroxylamine metabolite, and 3. depleted cellular glutathione stores and thus decreased the capacity to neutralize the reactive metabolites and thus favoring their covalent binding to cellular proteins (Figure 2).
Figure 2.
Hypothetical pathogenetic mechanisms and treatment effects of combined treatment of HSS/DRESS in the described patient. ↑ – increase or activation; T– decrease or inhibition.
N-acetylcysteine
Acetaminophen poisoning, either intentional or unintentional, has become the most common cause of acute liver failure. When given within the first 24 hours after ingestion, N-acetylcysteine can effectively prevent or minimize liver damage caused by acetaminophen, even after massive overdoses [29]. Additionally, it has been shown that intravenous N-acetylcysteine improves transplant-free survival in early stage of non-acetaminophen acute liver failure [29–31]. N-acetylcysteine, a synthetic precursor of glutathione, is used to treat patients with acetaminophen overdose for up to 48 hours after ingestion. It is well established that early treatment with N-acetylcysteine can improve the scavenging of the reactive metabolite N-acetyl-p-benzoquinone imine. Recent studies in mice showed that delayed treatment with high N-acetylcysteine doses can protect against acetaminophen overdose by 2 further mechanisms – by scavenging of reactive oxygen and peroxynitrite due to cysteine supply for glutathione synthesis, and by providing excess amino acids (amino acids not used for glutathione synthesis) as substrates for the Krebs cycle, supporting the mitochondrial energy metabolism [32]. Furthermore, in animal studies N-acetylcysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure [33], and has nephroprotective properties in cyclosporine-, cisplatin-, and isophosphamide-induced nephrotoxicity, and in ischemia/reperfusion kidney injury [34].
The defective detoxification of reactive intermediates of the drugs metabolized by CYP450 has been considered the triggering factor of the DRESS syndrome since its first description [35]. It had been shown that SMX-hydroxylamine and not SMX caused dose-dependent toxicity to lymphocytes of normal volunteers in vitro, and that toxicity was decreased by co-incubating the lymphocytes with N-acetylcysteine [36]. In another experiment, SMX-hydroxylamine produced concentration-dependent toxicity in HIV-infected T-lymphoblasts – toxicity that was significantly greater than the toxicity seen among non-infected T-lymphoblasts. HIV-infected cells had significantly lower glutathione concentration than non-infected cells. Incubation with SMX-hydroxylamine produced a concentration-dependent decline in glutathione content in both infected and non-infected T-lymphoblasts. Co-incubation with glutathione or N-acetylcysteine reduced the toxicity of SMX-hydroxylamine in HIV-infected cells [37]. In anecdotal case reports of sulphasalazine-related life-threatening adverse effects, N-acetylcysteine had been used and was suggested to be beneficial [38]. In other cases of DIHS/DRESS its beneficial effect was not confirmed [39]. It seems obvious that the potential beneficial effect of N-acetylcysteine administration is eliminated if it is given too late or if its use is not combined with other treatments. However, N-acetylcysteine as treatment of DIHS/DRESS has not gained wide acceptance, and N-acetylcysteine is not mentioned in reviews on treatment of this syndrome [1–3]. Its use has recently been suggested in combination with intravenous immunoglobulin administration [40].
Conclusions
To the best of our knowledge no treatment of DIHS/DRESS with the combination of N-acetylcysteine, corticosteroid and valganciclovir has been previously reported. We do not know if the favorable course of the DIHS/DRESS in the reported patient was a consequence of treatment. Nevertheless, the knowledge of the sequential pathomechanisms describes above provides a rational basis for such a combined treatment. The immediate administration of N-acetylcysteine, which was associated with significant clinical improvement, might have neutralized the drug-derived reactive metabolites, which are responsible for protein adduct formation and specific T cell stimulation, and repleted the glutathione stores that counterbalance oxidative stress. The corticosteroid might have inhibited lymphoproliferation. Valganciclovir might have prevented HHV-6-associated pathology, including immune reconstitution syndrome (Figure 2).
Footnotes
Source of support: Departmental sources
References
- 1.Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2006;55:1–8. doi: 10.2332/allergolint.55.1. [DOI] [PubMed] [Google Scholar]
- 2.Eshki M, Allanore L, Musette P, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol. 2009;145:67–72. doi: 10.1001/archderm.145.1.67. [DOI] [PubMed] [Google Scholar]
- 3.Walsh SA, Creamer D. Drug reaction with eosinophilia and systemic symptoms (DRESS): a clinical update and review of current thinking. Clin Exp Dermatol. 2011;36:6–11. doi: 10.1111/j.1365-2230.2010.03967.x. [DOI] [PubMed] [Google Scholar]
- 4.Aota N, Shiohara T. Viral connection between drug rashes and autoimmune diseases: how autoimmune responses are generated after resolution of drug rashes. Autoimmun Rev. 2009;8:488–94. doi: 10.1016/j.autrev.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609–11. doi: 10.1111/j.1365-2133.2006.07704.x. [DOI] [PubMed] [Google Scholar]
- 6.Descamps V, Bouscarat F, Laglenne S, et al. Human herpesvirus 6 infection associated with anticonvulsant hypersensitivity syndrome and reactive haemophagocytic syndrome. Br J Dermatol. 1997;137:605–8. doi: 10.1111/j.1365-2133.1997.tb03795.x. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, Inagi R, Aono T, et al. Human herpesvirus 6 infection as a risk factor for the development of severe drug-induced hypersensitivity syndrome. Arch Dermatol. 1998;134:1108–12. doi: 10.1001/archderm.134.9.1108. [DOI] [PubMed] [Google Scholar]
- 8.Tohyama M, Yahata Y, Yasukawa M, et al. Severe hypersensitivity syndrome due to sulfasalazine associated with reactivation of human herpesvirus 6. Arch Dermatol. 1998;134:1113–17. doi: 10.1001/archderm.134.9.1113. [DOI] [PubMed] [Google Scholar]
- 9.Seishima M, Yamanaka S, Fujisawa T, et al. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br J Dermatol. 2006;155:344–49. doi: 10.1111/j.1365-2133.2006.07332.x. [DOI] [PubMed] [Google Scholar]
- 10.Kano Y, Hiraharas K, Sakuma K, Shiohara T. Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br J Dermatol. 2006;155:301–6. doi: 10.1111/j.1365-2133.2006.07238.x. [DOI] [PubMed] [Google Scholar]
- 11.Tohyama M, Hashimoto K, Yasukawa M, et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. 2007;157:934–40. doi: 10.1111/j.1365-2133.2007.08167.x. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi R, Kano Y, Yamazaki Y, et al. Defective regulatory T cells in patients with severe drug eruptions: timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol. 2009;182:8071–79. doi: 10.4049/jimmunol.0804002. [DOI] [PubMed] [Google Scholar]
- 13.Gabay C, De Bandt M, Palazzo E. Sulphasalazine-related life-threatening side effects: is N-acetylcysteine of therapeutic value? Clin Exp Rheumatol. 1993;11:417–20. [PubMed] [Google Scholar]
- 14.Lau G, Kwan C, Chong SM. The 3-week sulphasalazine syndrome strikes again. Forensic Sci Int. 2001;122:79–84. doi: 10.1016/s0379-0738(01)00476-5. [DOI] [PubMed] [Google Scholar]
- 15.Moling O, Cairon E, Rimenti G, et al. Severe hepatotoxicity after therapeutic doses of acetaminophen. Clin Ther. 2006;28:755–60. doi: 10.1016/j.clinthera.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Klotz U. Clinical pharmacokinetics of sulphasalazine, its metabolites and other prodrugs of 5-aminosalicylic acid. Clin Pharmacokinet. 1985;10:285–302. doi: 10.2165/00003088-198510040-00001. [DOI] [PubMed] [Google Scholar]
- 17.Vyas PM, Roychowdhury S, Svensson CK. Role of human cyclooxygenase-2 in the bioactivation of dapsone and sulfamethoxazole. Drug Metab Dispos. 2006;34:16–18. doi: 10.1124/dmd.105.006890. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson JP, Naisbitt DJ, Farrell J, et al. Sulfamethoxazole and its metabolite nitroso sulfamethoxazole stimulate dendritic cell costimulatory signaling. J Immunol. 2007;178:5533–42. doi: 10.4049/jimmunol.178.9.5533. [DOI] [PubMed] [Google Scholar]
- 19.Lavergne SN, Wang H, Callan HE, et al. “Danger” conditions increase sulfamethoxazole-protein adduct formation in human antigen-presenting cells. J Pharmacol Exp Ther. 2009;331:372–81. doi: 10.1124/jpet.109.155374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutmuller RP, Morgan ME, Netea MG, et al. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006;27:387–93. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Kraaij MD, Savage ND, van der Kooij SW, et al. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc Natl Acad Sci USA. 2010;107:17686–91. doi: 10.1073/pnas.1012016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castrejon JL, Berry N, El-Ghaiesh S, et al. Stimulation of human T cells with sulfonamides and sulfonamide metabolites. J Allergy Clin Immunol. 2010;125:411–18. doi: 10.1016/j.jaci.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Shiohara T, Kano Y. A complex interaction between drug allergy and viral infection. Clin Rev Allergy Immunol. 2007;33:124–33. doi: 10.1007/s12016-007-8010-9. [DOI] [PubMed] [Google Scholar]
- 24.Kano Y, Inaoka M, Shiohara T. Association between anticonvulsant hypersensitivity syndrome and human herpesvirus 6 reactivation and hypogammaglobulinemia. Arch Dermatol. 2004;140:183–88. doi: 10.1001/archderm.140.2.183. [DOI] [PubMed] [Google Scholar]
- 25.Shiohara T, Kurata M, Mizukawa Y, Kano Y. Recognition of Immune Reconstitution Syndrome Necessary for Better Management of Patients with Severe Drug Eruptions and Those under Immunosuppressive Therapy. Allergol Int. 2010;59:333–43. doi: 10.2332/allergolint.10-RAI-0260. [DOI] [PubMed] [Google Scholar]
- 26.De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217–45. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez FJ. The 2006 Bernard B. Brodie Award Lecture. Cyp2e1. Drug Metab Dispos. 2007;35:1–8. doi: 10.1124/dmd.106.012492. [DOI] [PubMed] [Google Scholar]
- 28.Kim SJ, Lee MY, Kwon do Y, et al. Alteration in metabolism and toxicity of acetaminophen upon repeated administration in rats. J Pharmacol Sci. 2009;111:175–81. doi: 10.1254/jphs.09151fp. [DOI] [PubMed] [Google Scholar]
- 29.Lee WM, Hynan LS, Rossaro L, et al. Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–64. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mumtaz K, Azam Z, Hamid S, et al. Role of N-acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation. Hepatol Int. 2009;3:563–70. doi: 10.1007/s12072-009-9151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kortsalioudaki C, Taylor RM, Cheeseman P, et al. Safety and efficacy of N-acetylcysteine in children with non-acetaminophen-induced acute liver failure. Liver Transpl. 2008;14:25–30. doi: 10.1002/lt.21246. [DOI] [PubMed] [Google Scholar]
- 32.Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–54. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bémeur C, Vaquero J, Desjardins P, Butterworth RF. N-acetylcysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure in mice: antioxidant and anti-inflammatory mechanisms. Metab Brain Dis. 2010;25:241–49. doi: 10.1007/s11011-010-9201-2. [DOI] [PubMed] [Google Scholar]
- 34.Chen N, Aleksa K, Woodland C, et al. N-Acetylcysteine prevents ifosfamide-induced nephrotoxicity in rats. Br J Pharmacol. 2008;153:1364–72. doi: 10.1038/bjp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS) Semin Cutan Med Surg. 1996;15:250–57. doi: 10.1016/s1085-5629(96)80038-1. [DOI] [PubMed] [Google Scholar]
- 36.Rieder MJ, Uetrecht J, Shear NH, Spielberg SP. Synthesis and in vitro toxicity of hydroxylamine metabolites of sulfonamides. J Pharmacol Exp Ther. 1988;244:724–28. [PubMed] [Google Scholar]
- 37.Rieder MJ, Krause R, Bird IA, Dekaban GA. Toxicity of sulfonamide-reactive metabolites in HIV-infected, HTLV-infected, and noninfected cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:134–40. [PubMed] [Google Scholar]
- 38.Gabay C, De Bandt M, Palazzo E. Sulphasalazine-related life-threatening side effects: is N-acetylcysteine of therapeutic value? Clin Exp Rheumatol. 1993;11:417–20. [PubMed] [Google Scholar]
- 39.Lau G, Kwan C, Chong SM. The 3-week sulphasalazine syndrome strikes again. Forensic Sci Int. 2001;122:79–84. doi: 10.1016/s0379-0738(01)00476-5. [DOI] [PubMed] [Google Scholar]
- 40.Cumbo-Nacheli G, Weinberger J, Alkhalil M, et al. Anticonvulsant hypersensitivity syndrome: is there a role for immunomodulation? Epilepsia. 2008;49:2108–12. doi: 10.1111/j.1528-1167.2008.01720.x. [DOI] [PubMed] [Google Scholar]