Summary
Background
Late-onset and solitary recurrence of gastric signet ring cell (SRC) carcinoma is rare. We report a successful surgical resection of late solitary locoregional recurrence after curative gastrectomy for gastric SRC carcinoma.
Case Report
The patient underwent total gastrectomy for advanced gastric carcinoma at age 52. Seven years after the primary operation, he visited us again with sudden onset of abdominal pain and vomiting. We finally decided to perform an operation, based on a diagnosis of colon obstruction due to the recurrence of gastric cancer by clinical findings and instrumental examinations. The laparotomic intra-abdominal findings showed that the recurrent tumor existed in the region surrounded by the left diaphragm, colon of splenic flexure, and pancreas tail. There was no evidence of peritoneal dissemination, and peritoneal lavage fluid cytology was negative. We performed complete resection of the recurrent tumor with partial colectomy, distal pancreatectomy, and partial diaphragmectomy. Histological examination of the resected specimen revealed SRC carcinoma, identical in appearance to the previously resected gastric cancer. We confirmed that the intra-abdominal tumor was a locoregional gastric cancer recurrence in the stomach bed. The patient showed a long-term survival of 27 months after the second operation.
Conclusions
In the absence of effective alternative treatment for recurrent gastric carcinoma, surgical options should be pursued, especially for late and solitary recurrence.
Keywords: gastric cancer, late recurrence, locoregional recurrence and surgical treatment
Background
Recurrences after curative resection for gastric cancer have been categorized as locoregional recurrence, peritoneal recurrence, and distant (including hematogenous) metastasis [1–6]. Peritoneal recurrence of gastric cancer, referred to as “carcinomatosis” because multiple metastases develop in most cases, is associated with a poor prognosis [1,2]. According to reports on the role and outcomes of surgical treatment of non-hepatic intra-abdominal recurrences of gastric cancer, surgical resection is the treatment of choice for selected patients in whom the recurrent tumors are completely resectable [7,8]. Recently, we encountered a patient with late-onset solitary intra-abdominal recurrence of gastric cancer 7 years after curative gastrectomy, which was diagnosed as a locoregional recurrence in the stomach bed. We report this case because it is quite rare to achieve curative resection of a late solitary non-hepatic intra-abdominal recurrence of gastric signet ring cell (SRC) carcinoma, as in this patient.
Case Report
In October 2001 a 52-year-old man underwent total gastrectomy (with D2 lymphadenectomy) plus splenectomy for type 3 advanced gastric cancer in the lesser curvature of the stomach body. The tumor (6.5×8.0 cm) was diagnosed histopathologically as a SRC carcinoma, and the depth of tumor invasion was confirmed as exposed-serosal (se). The tumor showed infiltrative growth, with severe fibrosis. There was a slight lymphatic invasion (ly1), no venous invasion (v0), and a lymph node metastasis (n1). The tumor was classified as stage IIIA (T3N1M0) according to the Japanese classification of gastric carcinoma (JCGC) [9], and also as stage IIIA (T4aN1M0) according to the recent JCGC revised 2010 [10]. Adjuvant chemotherapy with intravenous low-dose 5-fluorouracil and cisplatin was given for 5 days. Thereafter, oral adjuvant chemotherapy was administered with uracil-tegafur. However, the adjuvant chemotherapy was abandoned because of the development of adverse effects after 8 weeks. Three years later, the patient stopped visiting the hospital on his own will.
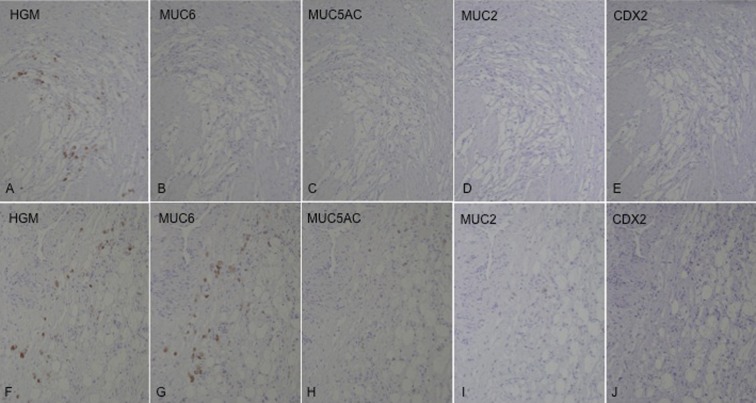
At the age of 58, 6 years and 5 months later, he visited us again with abdominal pain and vomiting of sudden onset and was diagnosed as having bowel obstruction. The patient was hospitalized for conservative treatment, which proved to be ineffective. Contrast-enema (Figure 1A) and colonoscopic examinations revealed obstruction of the large intestine at the splenic flexure. Abdominal CT (Figure 1B, C) showed a tumor in the same region. Serum tumor markers, including carcinoembryonic antigen and carbohydrate antigen 19-9, were within normal limits. We finally decided to perform an operation, based on a diagnosis of colon obstruction due to the recurrence of gastric cancer by clinical findings and instrumental examinations. The laparotomic intra-abdominal findings indicated that the recurrent tumor existed in the region surrounded by the left diaphragm, colon of splenic flexure, and pancreas tail. Although the tumor invasion to the surrounding organs was recognized, the tumor was not exposed to the intra-abdominal cavity. There was no evidence of peritoneal dissemination, and peritoneal lavage fluid cytology was negative. We performed complete resection of the recurrent tumor with partial colectomy of the splenic flexure, distal pancreatectomy, and partial diaphragmectomy (Figure 2). Histological examination of the resected specimen revealed SRC carcinoma (Figure 3A), identical in appearance to the previously resected gastric SRC carcinoma. The tumor was located in the peripancreatic tissue, surrounded by the transverse colon, diaphragm and pancreas, and SRC carcinoma had invaded the submucosal layer through the colonic proper muscle (Figure 3B), as well as the smooth muscle layer of the diaphragm (Figure 3C). However, the SRC carcinoma had not become exposed on the colonic mucosa or on the pleural surface of the diaphragm. We confirmed that the intra-abdominal tumor was a locoregional gastric cancer recurrence in the stomach bed. Immunohistochemical analysis was performed for both the primary and recurrent tumors by using HGM, MUC6 and MUC5AC antibodies as gastric phenotype markers and MUC2 and CDX2 antibodies as intestinal phenotype markers. Primary gastric SRC carcinoma showed HGM (+), and recurrent SRC carcinoma showed HGM (+) and MUC6 (+) (Figure 4). The clinical course after the second operation was favorable, and oral adjuvant chemotherapy with S-1 (TS-1, Taiho Pharmaceutical) was administered. The patient showed a long-term survival of 27 months after the second operation, but finally died of peritoneal carcinomatosis of gastric cancer.
Figure 1.
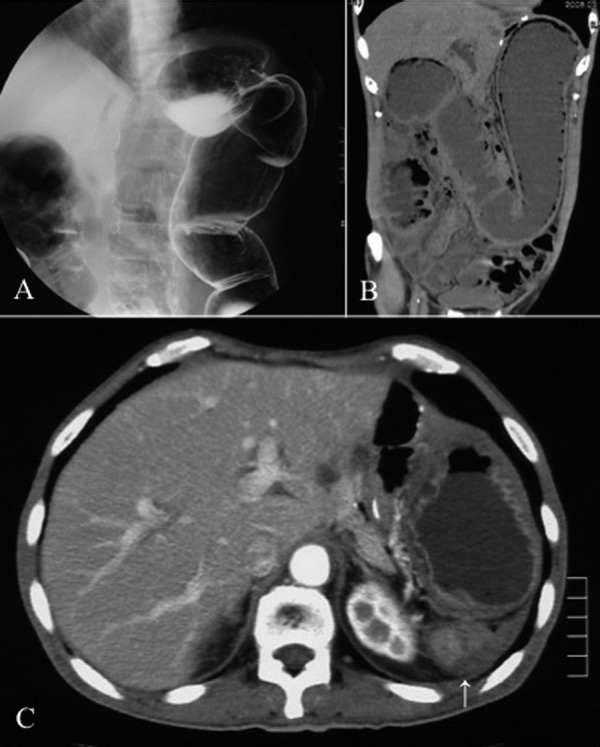
(A) Contrast enema examination indicated obstruction of the large intestine in the region of the splenic flexure. (B) Coronal CT showed dilatation of the intestinal loop from the ascending colon to the transverse colon. (C) Axial CT revealed a tumor (arrow) in the dorsal region of the intra-abdominal cavity where the spleen had originally existed.
Figure 2.
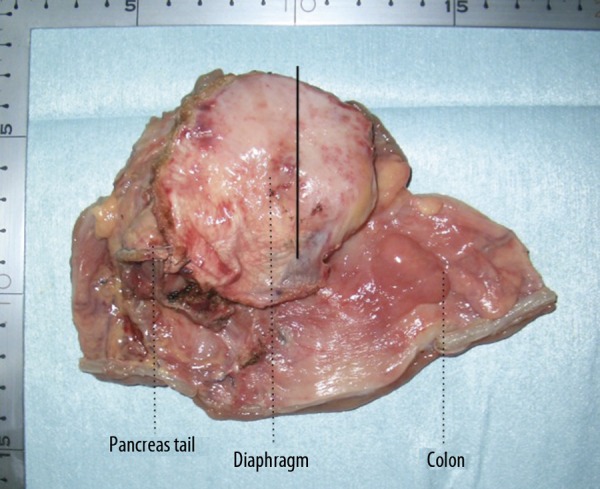
Macroscopic appearance of the resected tumor; the solid line in the resected specimen indicates the cutting surface.
Figure 3.
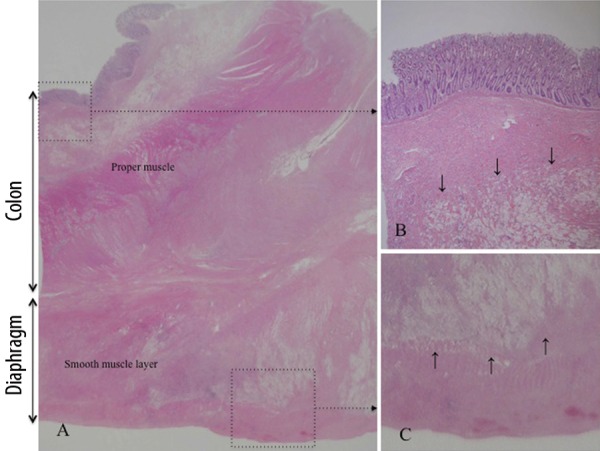
(A) Histological examination showed no mucosal cancerous lesion; however signet ring cells were identified in the submucosal tissue ((B), arrows), the proper muscle layer, the subserosal tissue of the colon, and the smooth muscle layer ((C), arrows) of the diaphragm. H&E, (A) Loupe image; (B, C) ×40.
Figure 4.
Immunohistochemical staining of the mucin phenotype markers showed HGM (+) in the primary tumor (A–E) and HGM (+) and MUC6 (+) in the recurrent tumor (F–J) ×100.
Discussion
The recurrence patterns after curative surgery for gastric cancer have been classified by pertinent studies as follows: locoregional recurrence, peritoneal recurrence, or distant (including hematogenous) metastasis [1–6]. Locoregional recurrence is defined as a recurrence in the stomach bed, anastomotic site, regional lymph nodes including the para-aortic lymph nodes, or in an adjacent structure by direct extension [4,6,11]. Moreover, in 1952, Thomson defined the stomach bed as immediately adjacent perigastric tissues, including the entire pancreas [12]. Peritoneal recurrence is confirmed by positive ascitic fluid cytology or visualization of the peritoneal nodules, including Krukenberg’s tumor [4]. In addition, peritoneal recurrence in cases of gastric cancer is generally multiple, referred to as peritoneal carcinomatosis [1,2]. Distant metastasis is defined as specific organ involvement via systemic metastasis, including periumbilical nodules and extra-abdominal lymph node metastases [4]. According to the definition of recurrence, we needed to confirm if the recurrence in this patient was actually a peritoneal or locoregional recurrence. At first, we assumed that the present tumor in this patient was a solitary peritoneal recurrence. Our careful histopathological investigation of the resected specimen revealed invasion close to the pancreatic tissue by SRC carcinoma. Based on these histopathological findings and the definition of recurrence, we determined that the recurrence in this patient was a locoregional recurrence in the stomach bed.
Many risk factors for locoregional recurrence have been reported, including the degree of gastric wall penetration [2,3,11]. In particular, extraluminal recurrence, which was classified into locoregional recurrence by Lehnert et al., was reported as being due to primary implantation of tumor cells into the resection area during gastrectomy [13]. Additionally, Iwanaga et al. reported the following 4 factors as possibly responsible for late recurrences after gastrectomy for gastric cancer: 1) a small volume of cancer left at the time of the surgery, 2) the cancer left during surgery was at a site unfavorable for the subsequent spread of cancer, 3) slow cancer cell proliferation, and 4) the high resistance of the host to cancer [14]. Histological evidence of serosal invasion was also observed in our present patient. We conjecture that a few tumor cells, which had been released at the initial operation, became implanted in the resection area of the left epigastric part, and grew for many years in the present case. Furthermore, the postoperative peritoneal adhesions might have blocked the spread of the tumor cells.
Tian et al. have reported that gastric SRC carcinoma can be classified into 4 phenotypes – gastric (G), intestinal, mixed gastric and intestinal and unclassified, according to the expression pattern of gastric and intestinal phenotype markers [15]. As the SRC carcinoma tissues stained positive for the gastric phenotype markers but not for the intestinal phenotype markers, both the primary and recurrent tumors were classified as having a G-phenotype. It is notable that although the expression of MUC6 disappeared in the primary tumor, it was restored in the recurrent tumor. MUC6 is a secretory mucin, which is present in normal gastric mucous neck cells and deep glands of the antrum. It has been reported that downregulation of MUC6 may contribute to malignant transformation of gastric epithelial cells and correlate with gastric carcinoma progression and a poor prognosis [16]. This finding leads to our presumption that MUC6 has some effect on the normal differentiation of gastric mucous cells. It has also been reported that a mucin phenotypic shift might be a comitant phenomenon of tumor progression due to increasing heterogeneity [15]. In contrast, in the present gastric SRC carcinoma, we observed a mucin phenotypic shift that restored MUC6 expression, which may contribute to differentiation. These biological features may have contributed to the rare phenomenon of late-onset peritoneal recurrence of SRC 9 years after the first operation. However, we cannot rule out the possibility of reduced stainability caused by decreased antigenicity associated with deterioration of the primary tumor specimen.
Although there have been many reports on the recurrence patterns and the clinical course of recurrent gastric cancer [1–5], only a few studies are available regarding surgical treatment of recurrence [8,13,17]. According to reports on the role and outcomes of surgical treatment of non-hepatic intra-abdominal recurrences of gastric cancer, surgical resection is a treatment of choice for selected patients in whom the recurrent tumors are completely resectable [7,8]. Surgical resection of gastric cancer recurrence is only rarely possible; however, when surgical resection is performed, a 20% 5-year survival can be expected [8,13]. In addition, some authors reported that the survival of patients who had complete resection was significantly longer than that of the other incomplete resection groups, including patients with unresectable tumors [17,18]. Altogether, the resectability of the recurrent tumor might be the most important prognostic factor for survival after resection [2,17,18]. According to a review of the pertinent literature to determine the current surgical options for recurrent or metastatic gastric cancer, solitary and late-appearing metachronous tumors are associated with an improved prognosis [13]. In the present case, the favorable factors for a second curative surgery were: the recurrence was late-appearing and isolated, and the intra-abdominal recurrent tumor could be completely resected en bloc without any need to touch. Therefore, our present patient showed a satisfactory long-term survival after resection of the gastric recurrent tumor.
Conclusions
In the absence of effective alternative treatment for recurrent gastric carcinoma, surgical options should be pursued, especially for solitary and late recurrence.
Footnotes
Source of support: Departmental sources
References
- 1.Roviello F, Marrelli D, de Manzoni G, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg. 2003;90:1113–19. doi: 10.1002/bjs.4164. [DOI] [PubMed] [Google Scholar]
- 2.Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–42. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 3.Maehara Y, Hasuda S, Koga T, et al. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87:353–57. doi: 10.1046/j.1365-2168.2000.01358.x. [DOI] [PubMed] [Google Scholar]
- 4.Moon YW, Jeung HC, Rha SY, et al. Changing patterns of prognosticators during 15-year follow-up of advanced gastric cancer after radical gastrectomy and adjuvant chemotherapy: A 15-year follow-up study at a single Korean institute. Ann Surg Oncol. 2007;14:2730–37. doi: 10.1245/s10434-007-9479-4. [DOI] [PubMed] [Google Scholar]
- 5.D’Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–16. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CW, Lo SS, Shen KH, et al. Incidence and factors associated with recurrence patterns after intended curative surgery for gastric cancer. World J Surg. 2003;27:153–58. doi: 10.1007/s00268-002-6279-7. [DOI] [PubMed] [Google Scholar]
- 7.Takeyoshi I, Ohwada S, Ogawa T, et al. The resection of non-hepatic intraabdominal recurrence of gastric cancer. Hepatogastroenterology. 2000;47:1479–81. [PubMed] [Google Scholar]
- 8.de Liaño AD, Yarnoz C, Aguilar R, et al. Surgical treatment of recurrent gastric cancer. Gastric Cancer. 2008;11:10–14. doi: 10.1007/s10120-007-0444-5. [DOI] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 2nd English edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma (in Japanese) The 14th Edition. Tokyo: Kanehara; 2010. [DOI] [PubMed] [Google Scholar]
- 11.Landry J, Tepper JE, Wood WC, et al. Patterns of failure following curative resection of gastric carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:1357–62. doi: 10.1016/0360-3016(90)90344-j. [DOI] [PubMed] [Google Scholar]
- 12.Thomson FB, Robins RE. Local recurrence following subtotal resection for gastric carcinoma. Surg Gynecol Obstet. 1952;95:341–44. [PubMed] [Google Scholar]
- 13.Lehnert T, Rudek B, Buhl K, Golling M. Surgical therapy for loco-regional recurrence and distant metastasis of gastric cancer. Eur J Surg Oncol. 2002;28:455–61. doi: 10.1053/ejso.2002.1260. [DOI] [PubMed] [Google Scholar]
- 14.Iwanaga T, Koyama H, Furukawa H, et al. Mechanisms of late recurrence after radical surgery for gastric carcinoma. Am J Surg. 1978;135:637–40. doi: 10.1016/0002-9610(78)90126-5. [DOI] [PubMed] [Google Scholar]
- 15.Tian MM, Zhao AL, Li ZW, Li JY. Phenotypic classification of gastric signet ring cell carcinoma and its relationship with clinicopathologic parameters and prognosis. World J Gastroenterol. 2007;13:3189–98. doi: 10.3748/wjg.v13.i23.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng H, Takahashi H, Nakajima T, et al. MUC6 down-regulation correlates with gastric carcinoma progression and a poor prognosis: an immunohistochemical study with tissue microarrays. J Cancer Res Clin Oncol. 2006;132:817–23. doi: 10.1007/s00432-006-0135-3. [DOI] [PubMed] [Google Scholar]
- 17.Song KY, Park SM, Kim SN, Park CH. The role of surgery in the treatment of recurrent gastric cancer. Am J Surg. 2008;196:19–22. doi: 10.1016/j.amjsurg.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 18.Nunobe S, Hiki N, Ohyama S, et al. Outcome of surgical treatment for patients with locoregional recurrence of gastric cancer. Langenbecks Arch Surg. 2011;396:161–66. doi: 10.1007/s00423-010-0730-2. [DOI] [PubMed] [Google Scholar]