Summary
Background
It is controversial whether an early reduction and internal fixation can reduce the occurrence of femoral neck fracture-induced osteonecrosis of the femoral head (ONFH). This prospective study was designed to reflect the relationship between injury-to-surgery interval (ISI) and traumatic ONFH based on a canine model of femoral neck fractures.
Material/Methods
Twenty-four dogs were equally divided randomly into 3 groups. A lateral L-shape approach centered left great trochanter was used for exposure of the femoral neck. A low-speed drill was used for making displaced fractures in the narrow femoral neck, with the femoral head kept in situ with ligamentum teres intact. In Group A, the fracture was immediately reduced and fixed with 3 parallel pins; while the operation was done 3 days later in Group B, and 3 weeks later in Group C. Another 2 dogs had their fractures untreated. Postoperatively, all dogs were fed separately and received regular x-ray examination. Left femoral heads were harvested for histological examination with a postoperative follow-up of 3.5 months.
Results
The canine model of femoral neck fractures could be achieved successfully. Radiological signs of post-fracture ONFH could not be detected at intervals of 2 weeks, 4 weeks, 1 month and 2 months. Histologically, there were 2 cases with ONFH in Group A, 1 case in Group B, and 2 cases in Group C. The difference had no statistical significance. For untreated fractures, obvious ONFH could be found radiologically.
Conclusions
A shorter ISI may not reduce the incidence of fracture-induced ONFH, which suggests that intrinsic factors play an important role in the occurrence of ONFH.
Keywords: osteonecrosis of the femoral head, femoral neck fractures, canine model, injury-to-surgery interval
Background
Osteonecrosis of the femoral head (ONFH) is a frequent and severe complication following femoral neck fractures. Although fragile blood supply to the femoral head is understood, and great advances in surgical technique and operational assessment criteria have been made, unfortunately, it seems that all these achievements cannot significantly reduce the incidence of post-fracture ONFH [1,2]. Conventionally, it is believed that shortening of injury-to-surgery interval (ISI) can reduce fracture-induced ONFH [3–5]. However, direct proof of the relationship between ISI and post-fracture ONFH could not be achieved for the absence of prospective or randomized controlled trials clinically. The relationship between ISI and ONFH following acute femoral neck fractures remains controversial [6].
Therefore, the best way to resolve the problem is to design a prospective or randomized controlled trail to reflect the relationship between ISI and fracture-induced ONFH [7]. Ethical considerations prevent this from being carried out clinically; we therefore designed a canine model of femoral neck fractures, based on which, reduction and fixation was done within different ISIs. The occurrence of ONFH was detected prospectively via radiological and histological examinations. We hypothesized that immediate and early operations could not reduce fracture-induced ONFH in the canine model of femoral neck fractures. Our experimental protocol was approved by the local animal welfare and ethics committee.
Material and Methods
Animals
We used beagle dogs obtained from the Laboratory Animal Center of Shanghai Sixth People’s Hospital, with an average age of 2.5 years and an average body weight of 15 kilograms (range, 14–17 kg). All animals had no special history of diseases and were housed separately. Before the day of each operation, they were fasted and intramuscularly injected with penicillin sodium. Twenty-four dogs were divided randomly and equally into 3 groups for the following operational management, and another 2 animals were used as controls.
Operational methods
Under aseptic conditions, a 6-cm-long L-shaped skin incision was made, centered left great trochanter. To expose the hip joint, the tensor fascia lata was split along the length of its bundles, and the gluteus medius muscle was partially detached from the anterior great trochanter. The hip joint capsule was opened, and electrocoagulation of soft tissue attachments at the base of femoral neck was performed circumferentially to interrupt the extraosseous blood supply to the femoral head; however, the medial and lateral circumflex arteries were not revealed. The ligamentum teres was kept intact. Low-speed drilling was employed to fracture and displace the femoral neck at the narrow base [8]. All fractures were categorized as type IV according to Garden criteria.
In Group A, 8 animals had their fracture immediately reduced and stabilized by 3 parallel Kirschner pins in the pattern of an inverted triangle; however in Group B, early surgery was defined to be performed on 3 days later, and 3 weeks later in Group C, which was considered as late. Another 2 dogs had their femoral neck fractures untreated and served as controls. The maneuvers were under direct vision for assurance of anatomical reduction of fractures and reliable stabilization. All operations were performed by the same team of surgeons. Postoperatively, all animals were housed separately and injected with penicillin sodium for prophylactic infection.
Radiological and histological examinations
All animals had radiological examinations 2 weeks postoperatively, then monthly for 3 times. The dogs were maintained in prone position with their hip joints in rear protraction and abduction under general anesthesia. Moreover, for animals in Group B and C, magnetic resonance imaging (MRI) was employed to detect the signal of the head after femoral neck fractures, but before the treatment of internal fixation. The timing for MRI was 2 days and 2 weeks postoperatively in Group B and Group C, respectively.
After last radiological examination, all animals were euthanized and bilateral femoral heads were harvested for histological examination. The general contour of the femoral head and articular cartilage was observed. For microscopic examination, tissue samples were obtained from the zone of weight bearing and the center of the femoral head. The samples were fixed with 10% formalin for 1 week and decalcified with 5 μM EDTA solution for 4 weeks. The specimens were embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin (HE).
Definition of ONFH
Radiologically, the sclerotic rim is a reactive bone remodeling at the necrotic-viable osseous junction. This pattern characterizes the stage II according to the modified Ficat-Arlet, Steinberg’s and ARCO systems. The presence of the crescent sign in the absence of segmental flattening classifies the lesion as stage III in all major staging systems. For more advanced cases, the femoral head would collapse and deform, with joint space narrowing [9].
Histologically, osteonecrosis showed an accumulation of bone marrow cell debris, bone trabeculae demonstrating empty lacunae and/or ghost nuclei in the lacunae, and an increase in the fat cells of the bone marrow.
Results
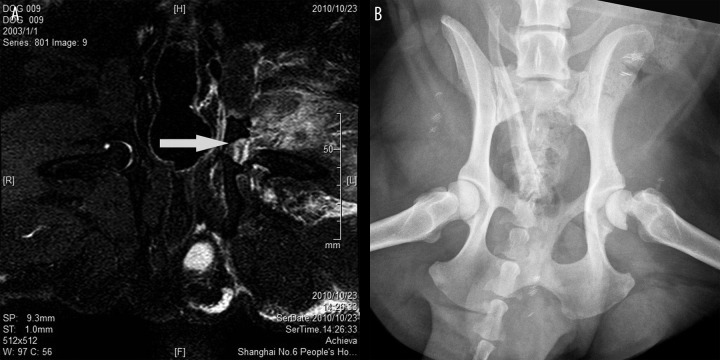
Femoral neck fractures can be successfully constructed via a lateral approach with known vessels preserved. One animal each died due to deep infection in Group A, B and C. For animals with fractures stabilized by paralleled pins, radiographic osteonecrosis of the femoral head did not appear, regardless of when the operation was performed (Figure 1). However, 2 dogs with untreated fractures had typical osteonecrosis 2 months postoperatively (Figure 2). In Group B and C, before fracture fixation surgery, a wide range of edema was detected and the signal in the femoral head was homogeneous on plain radiographs and MRI (Figure 3A,B).
Figure 1.
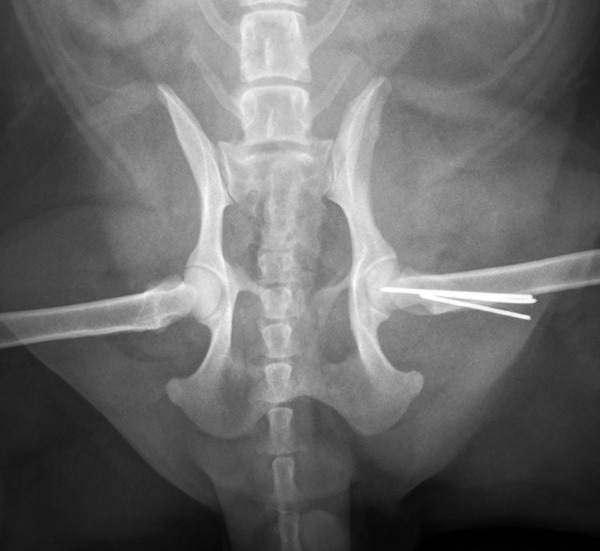
Animals with femoral neck fractures stabilized by parallel pins. Animals had their femoral neck fractures stabilized by parallel pins, radiographic osteonecrosis of the femoral head did not appear when fractures came to union.
Figure 2.
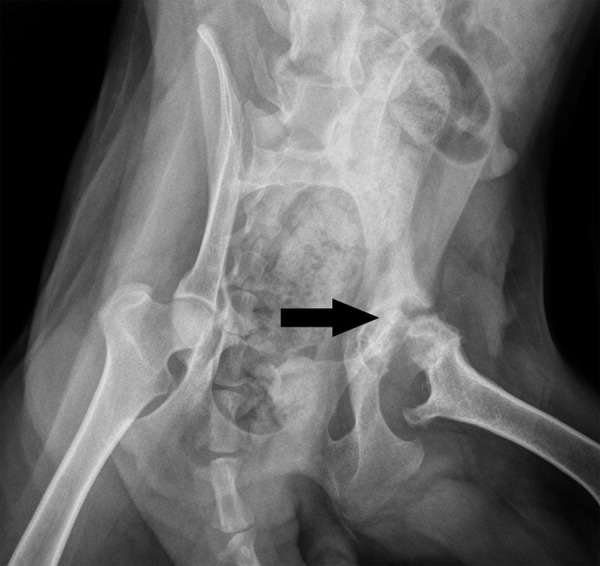
Radiograph of animals having fractures untreated. Apparently, osteonecrosis of the femoral head developed when dogs had their fractures left untreated. The femoral head lost its contour as the arrow indicated.
Figure 3.
Absence of osteonecrosis in animals having treatment delayed for three weeks. Before surgery of fracture fixation, wide range of edema was detected (A) and signal in the femoral head was homogeneous on MRI and plain radiograph (B).
When the last radiological examination was completed, all animals were sacrificed and femoral heads were harvested and prepared for histological examination. All these fractures came to union uneventfully. Morphologically, 21 animals in all fixation groups maintained their shape of femoral head, which had similar smoothness and luster. However, 2 animals without fracture fixation lost their contour of the head. Histologically, 2 femoral heads in Group A displayed accumulation of bone marrow cell debris, empty lacunae and an increase of fat cells, which were considered as ONFH, while there was 1 animal in Group B and 2 in Group C that had apparent osteonecrosis. For untreated femoral neck fractures, histological osteonecrosis was detected uneventfully (Figure 4). There were no statistical differences in incidence of fracture-induced ONFH in Group A, B and C, indicating that ISI did not affect the occurrence of osteonecrosis of the femoral head (Table 1).
Figure 4.
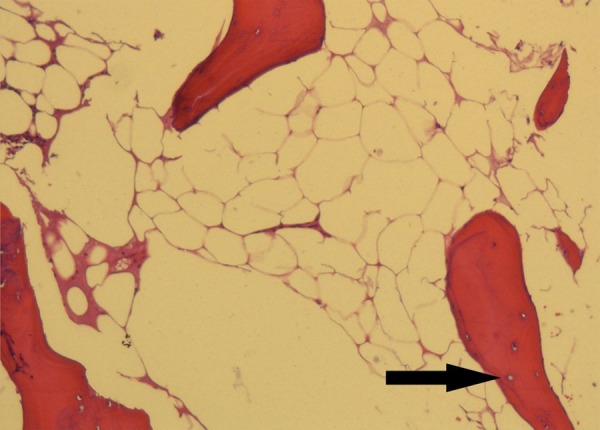
Characteristic osteonecrosis in animals with untreated fractures. An accumulation of bone marrow cell debris, empty lacunae in the lacunae as indicated, and an increase in the fat cells of the bone marrow was revealed.
Table 1.
Results of osteonecrosis occurrence in different treatment groups.
| Osteonecrosis of the femoral head | p value | |||
|---|---|---|---|---|
| Radiographically | Histologically | Total | ||
| Group A | 0/7 | 2/7 | 2/7 | >0.05 |
| Group B | 0/7 | 1/7 | 1/7 | |
| Group C | 0/7 | 2/7 | 2/7 | |
| Total | 0/21 | 5/21 | 5/21 | <0.001 |
| Untreated | 2/2 | 2/2 | 2/2 | |
Discussion
Nonunion and ONFH are frequent complications following femoral neck fractures [10]. Fortunately, with major advances in internal fixation and radiological evaluation, the occurrence of nonunion has been reduced for anatomical reduction and logical stabilization. However, post-fracture ONFH remains approximately 20% in all reported femoral neck fractures [7,11,12]. Post-fracture ONFH is notorious for induced pain and dysfunction, which might need hip replacement when head-salvage treatment fails [13].
Traditionally, it has been believed that the occurrence of ONFH can be reduced when ISI is shortened. Several adjectives have been introduced to describe the timing of ISI, including early, late, old and neglected. The definition of “early” is confused with a brief review of previous literature and “early” operation includes within 8 hours, 12 hours, 1 day and 3 days [14–16]. Due to ethics limitations, prospective and randomized controlled trials cannot be performed; therefore, the relationship between ISI and traumatic ONFH cannot be directly evaluated in humans.
Previous studies have reported debatable results of ONFH incidence when femoral neck fractures were performed within different ISIs. Asnis et al. [17] reported a series of femoral neck fractures fixed by cannulated screws in 141 patients, and found 13 cases resulted in ONFH radiologically or histologically at years, and another 13 patients had ONFH in a longer follow-up. The overall incidence of ONFH was 22%. Their patients had an average injury-to-surgery time of 2 days. Butt et al. [18] used closed reduction and internal fixation with parallel cannulated screws in 52 patients, with an average delay time of 6 days; 7 of them (13.5%) had ONFH at an average 40-month follow-up. The ONFH incidence at a mean follow-up of about 3 years was comparable to other studies. Interestingly, we also noticed that increasing neglected fractures of the femoral neck were reported in developing countries. The neglected facture, which is defined as more than 3 months since injuries, may be due to non-treatment, misdiagnosis, failed conventional management and inability to transport patients to a trauma center due to poverty and distance. Huang treated 16 cases of neglected femoral neck fractures; only 4 of them (25%) ended in ONFH at 2 to 8 year follow-up [19]. Although a meta-analysis has revealed that the difference of ONFH incidence following early (<12 h) and late (>12 h) surgery was not significant [7], it should be noted that enrolled articles for meta-analysis were neither randomized controlled nor prospective, which might lead to deviations. Another meta-analysis by us (unpublished data) showed that the general incidence of fracture-induced ONFH was 17.5% in young adults. When ISI was less than 3 weeks, the incidence of ONFH was 16.1%. Although it rose to 21.7% when ISI was beyond 3 weeks, there was no statistical difference. ISI did not significantly affect the incidence of postoperative ONFH based on nonrandomized case series.
Due to multiple ethics limitations, we designed the canine model of femoral neck fractures, and explored prospectively the relationship between ISI and the incidence of fracture-induced ONFH. To our knowledge, this is the first prospective study to reflect the intrinsic relationship between ISI and ONFH occurrence. The L-shaped lateral approach could facilitate preservation of known vessels around the hip. Although lateral muscles have to be detached, they could be reconstructed after the operation. Low-speed drilling could minimize adverse effects to neighboring bone tissue, and cellular features could not be altered for avoiding high temperature. All these details have been noted intraoperatively to eliminate individual bias of fractures.
In terms of intrinsic mechanism, severe hypoxia and ischemia is the common pathological phenomenon following skeletal fractures, which could alter cellular functions of osteogenesis and vasculogenesis [20–23]. Interestingly, the effects of a hypoxic microenvironment on osteogenic and vasculogenic cells are also debatable. Previous studies have indicated that hypoxia could enhance or reduce multiplication and osteogenic differentiation of mesenchymal stem cells (MSCs), which act as the main pre-osteogenitors [24–27]. Therefore, correspondingly, we speculate that short-term pathological hypoxia following femoral neck fractures might play a positive or negative role on the fate of the femoral head. Additionally, previous findings suggest that genetic preconditions might explain individual differences [28–30]. Clinically, excessive intra-capsular pressure following femoral neck fractures could cause tamponade effect, which reduces local blood supply. Such a tamponade effect could be reversed via capsulotomy; however, other studies have indicated that the effect of capsulotomy on ONFH incidence was undetermined [31]. Definitely, improved quality of reduction for displaced femoral neck fractures is the only undisputed determinant for prevention of traumatic ONFH [10].
The limitation of the current study is that biomechanical properties of the hip joint of canines are different from human beings, but the anatomy and blood supply of the hip in dogs and humans are very similar [32]. The majority of blood supply to the femoral head is from lateral and medial circumflex femoral arteries (Figure 5); vessels through the ligamentum teres to the head are comprised of double veins and an artery. Additionally, peculiar branches to the canine femoral head include the caudal gluteal artery, the cranial gluteal artery, and the iliolumbar artery, but all these arteries are negligible [33,34]. The femoral head bridges to the trochanter region directly without a real neck in canines; therefore, all transverse femoral neck fractures passing through the narrow connection are considered to maintain a similar pattern. Although the present study was comparative and positive controls were set, it should be noted that the follow-up was relatively short, which might yield a lower incidence of fracture-induced ONFH. According to or results, the incidence of fracture-induced ONFH cannot be reduced via immediate or early reduction and fixation in the canine model of femoral neck fractures. Although the prognosis of artificial joint replacement for advanced ONFH has been promoted [35], associated factors are to be revealed in the near future which effectively prevent ONFH.
Figure 5.
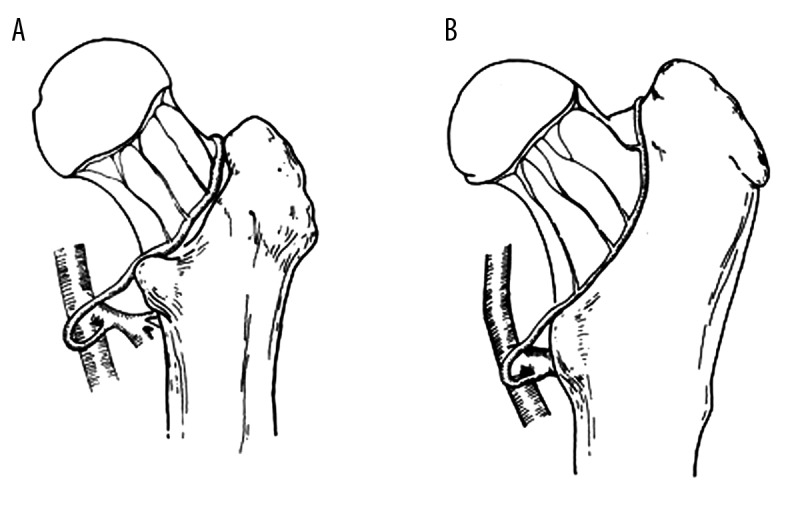
Schematic diagram of main blood supply to the femoral head in human and canine. The main blood supply to the femoral head comes from lateral and medial circumflex femoral artery, which form extra-articular anastomosis ring at the base in human (A) and canine (B).
Conclusions
Based on the canine model of femoral neck fractures, the occurrence of fracture-induced osteonecrosis of the femoral head cannot be reduced via shortening of ISI. The incidence of traumatic ONFH was similar when the fractures were reduced and stabilized immediately, early and late.
Acknowledgement
We sincerely thank Dr. Tian-Yi Wu, who is from Nanjing Medical University, for his contributions to the schematic diagram.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Source of support: The current research was financially supported by a Creative Research Scholarship for Ph.D. Candidates of Shanghai Jiao Tong University School of Medicine (No. BXJ2011038), and Key Project of Science and Technology Commission Foundation of Shanghai (No. 11411950400)
References
- 1.Roshan A, Ram S. The neglected femoral neck fracture in young adults: Review of a challenging problem. Clin Med Res. 2008;6:33–39. doi: 10.3121/cmr.2008.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fortina M, Carta S, Crainz E, et al. Management of displaced intracapsular femoral neck fracture in young adult: Why complications are still so high? Case report of posttraumatic avascular necrosis in a 30-year-old man and a brief review. J Trauma. 2009;67:E163–66. doi: 10.1097/TA.0b013e31814b9319. [DOI] [PubMed] [Google Scholar]
- 3.Varshney MK, Kumar A, Khan SA, Rastogi S. Functional and radiological outcome after delayed fixation of femoral neck fractures in pediatric patients. J Orthopaed Traumatol. 2009;10:211–16. doi: 10.1007/s10195-009-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray TJ. Femoral neck fractures fixation. Clinical decision making. Clin Orthop Relat Res. 1997;339:20–31. doi: 10.1097/00003086-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Ly TV, Swiontkowski MF. Treatment of femoral neck fractures in young adults. Instr Course Lect. 2009;58:69–81. [PubMed] [Google Scholar]
- 6.Bachiller F, Caballer AP, Portal LF. Avascular necrosis of the femoral head after femoral neck fracture. Clin Orthop Relat Res. 2002;399:87–109. doi: 10.1097/00003086-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Damany DS, Parker MJ, Chojnowski A. Complications after intracapsular hip fractures in young adults. A meta-analysis of 18 published studies involving 564 fractures. Injury. 2005;36:131–41. doi: 10.1016/j.injury.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Kuroda Y, Akiyama H, Kawanabe K, et al. Treatment of experimental osteonecrosis of the hip in adult rabbits with a single injection of recombinant human FGF-2 micropheres. J Bone Miner Metab. 2010;28:608–16. doi: 10.1007/s00774-010-0172-5. [DOI] [PubMed] [Google Scholar]
- 9.Malizos KN, Karantanas AH, Varitimidis SE, et al. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol. 2007;63:16–28. doi: 10.1016/j.ejrad.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Kyle RF. Fractures of the femoral neck. Instr Course Lect. 2009;58:61–68. [PubMed] [Google Scholar]
- 11.Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE. Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Am. 1994;76:15–25. doi: 10.2106/00004623-199401000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Nikolopoulos KE, Papadakis SA, Kateros KT, et al. Long-term outcome of patients with avascular necrosis after internal fixation of femoral neck fractures. Injury. 2003;34:525–28. doi: 10.1016/s0020-1383(02)00367-4. [DOI] [PubMed] [Google Scholar]
- 13.Davidovitch RI, Jordan CJ, Egol KA, Vrahas MS. Challenges in the treatment of femoral neck fractures in the nonelderly adults. J Trauma. 2010;68:236–42. doi: 10.1097/TA.0b013e3181c428ce. [DOI] [PubMed] [Google Scholar]
- 14.Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291:1738–43. doi: 10.1001/jama.291.14.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Upadhyay A, Jain P, Mishra P, et al. Delayed internal fixation of fractures of the neck of the femur in young adults. A prospective, randomised study comparing closed and open reduction. J Bone Joint Surg Br. 2004;86:1035–40. doi: 10.1302/0301-620x.86b7.15047. [DOI] [PubMed] [Google Scholar]
- 16.Dolk T. Operation in hip fracture patients: Analysis of the time factor. Injury. 1990;21:369–72. doi: 10.1016/0020-1383(90)90121-a. [DOI] [PubMed] [Google Scholar]
- 17.Asnis SE, Wanek-Sgaglione L. Intracapsular fractures of the femoral neck. J Bone Joint Surg Am. 1994;76:1793–803. doi: 10.2106/00004623-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Butt MF, Dhar SA, Gani N, et al. Delayed fixation of displaced femoral neck fractures in younger adults. Injury. 2008;39:238–43. doi: 10.1016/j.injury.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Huang CH. Treatment of neglected femoral neck fractures in young adults. Clin Orthop Relat Res. 1986;206:117–26. [PubMed] [Google Scholar]
- 20.Bejar J, Peled E, Boss JH. Vasculature deprivation-induced osteonecrosis of the rat femoral head as a model for therapeutic trials. Theor Biol Med Model. 2005;2:24. doi: 10.1186/1742-4682-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harper WM, Barnes MR, Gregg PJ. Femoral head blood flow in femoral neck fractures. An analysis using intra-osseous pressure measurement. J Bone Joint Surg Br. 1991;73:73–75. doi: 10.1302/0301-620X.73B1.1991780. [DOI] [PubMed] [Google Scholar]
- 22.Mark C, James B. Occult hypoxia after femoral neck fracture and elective hip surgery. Clin Orthop Relat Res. 2000;370:265–71. doi: 10.1097/00003086-200001000-00027. [DOI] [PubMed] [Google Scholar]
- 23.Potier E, Ferreira E, Andriamanalijaona R, et al. Hypoxia affects mesenchymal stem cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078–87. doi: 10.1016/j.bone.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner L, Arnhold S, Brixius K, et al. Human mesenchymal stem cells: Influence of oxygen pressure on proliferation and chondrogenic differentiation in fibrin in vitro. J Biomed Mater Res. 2010;93A:930–40. doi: 10.1002/jbm.a.32577. [DOI] [PubMed] [Google Scholar]
- 25.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–53. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 26.Salim A, Randall P, Morgan EF, et al. Transient changes in oxygen tension inhibit osteogenic differentiation and Rux2 expression in osteoblast. J Biol Chem. 2004;279(38):40007–16. doi: 10.1074/jbc.M403715200. [DOI] [PubMed] [Google Scholar]
- 27.Holzwarth C, Vaegler M, Gieseke F, et al. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:11. doi: 10.1186/1471-2121-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu YF, Chen WM, Lin YF, et al. Type II collagen gene variants and inherited osteonecrosis of the femoral head. N Eng J Med. 2005;352:2294–301. doi: 10.1056/NEJMoa042480. [DOI] [PubMed] [Google Scholar]
- 29.Zalavras CG, Vartholomatos G, Dokou E, Malizos KN. Genetic background of osteonecrosis. Associated with thrombophilic mutations? Clin Orthop Relat Res. 2004;422:251–55. [PubMed] [Google Scholar]
- 30.Hadjigeorgiou G, Dardiotis E, Dardioti M, et al. Genetic association studies in osteonecrosis of the femoral head: mini review of the literature. Skeletal Radiol. 2008;37:1–7. doi: 10.1007/s00256-007-0395-2. [DOI] [PubMed] [Google Scholar]
- 31.Archdeacon MT, Cannada LK, Herscovici D, Jr, et al. Prevention of complications after treatment of proximal femoral fractures. Instr Course Lect. 2009;58:13–19. [PubMed] [Google Scholar]
- 32.Malizos KN, Quarles LD, Seaber AV, et al. An experimental canine model of osteonecrosis of the repair process. J Orthop Res. 1993;11:350–57. doi: 10.1002/jor.1100110306. [DOI] [PubMed] [Google Scholar]
- 33.Kaderly RE, Anderson WD, Anderson BG. Extraosseous vascular supply to the mature dog’s coxofemoral joint. Am J Vet Res. 1982;43:1208–14. [PubMed] [Google Scholar]
- 34.Kaderly RE, Anderson BG, Anderson WD. Intracapsular and intraosseous vascular supply to the mature dog’s coxofemoral joint. Am J Vet Res. 1983;44:1805–12. [PubMed] [Google Scholar]
- 35.Madadi F, Eajazi A, Kazemi SM, et al. Total hip arthroplasty in advanced osteonecrosis: The short-term results by metal-on-metal hip resurfacing. Med Sci Monit. 2011;17(2):CR78–82. doi: 10.12659/MSM.881391. [DOI] [PMC free article] [PubMed] [Google Scholar]