Summary
Recent studies substantiate a model of the tunica albuginea of the corpora cavernosa as a bi-layered structure with a 360° complete inner circular layer and a 300° incomplete outer longitudinal coat spanning from the bulbospongiosus and ischiocavernosus proximally and extending continuously into the distal ligament within the glans penis. The anatomical location and histology of the distal ligament invites convincing parallels with the quadrupedal os penis and therefore constitutes potential evidence of the evolutionary process. In the corpora cavernosa, a chamber design is responsible for facilitating rigid erections. For investigating its venous factors exclusively, hemodynamic studies have been performed on both fresh and defrosted human male cadavers. In each case, a rigid erection was unequivocally attainable following venous removal. This clearly has significant ramifications in relation to penile venous surgery and its role in treating impotent patients. One deep dorsal vein, 2 cavernosal veins and 2 pairs of para-arterial veins (as opposed to 1 single vein) are situated between Buck’s fascia and the tunica albuginea. These newfound insights into penile tunical, venous anatomy and erection physiology were inspired by and, in turn, enhance clinical applications routinely encountered by physicians and surgeons, such as penile morphological reconstruction, penile implantation and penile venous surgery.
Keywords: penile anatomy, penile venous anatomy, tunica albuginea, distal ligament, erection hemodynamic mechanism
Background
The animal kingdom has consistently played a predominant role on earth in different eras [1]. Traits of the genitalia best demonstrate how species-level divergence occurs [2,3]. This is most conspicuous in the male genitalia owing to substantial structural and functional variances between species. As the vehicle for delivering sperm cells, this protruding organ has been indispensable to human life [4–6]. It is hardly surprising that this miraculous organ, with its remarkable erectile capability, has throughout human history consistently been considered emblematic of masculinity [7].
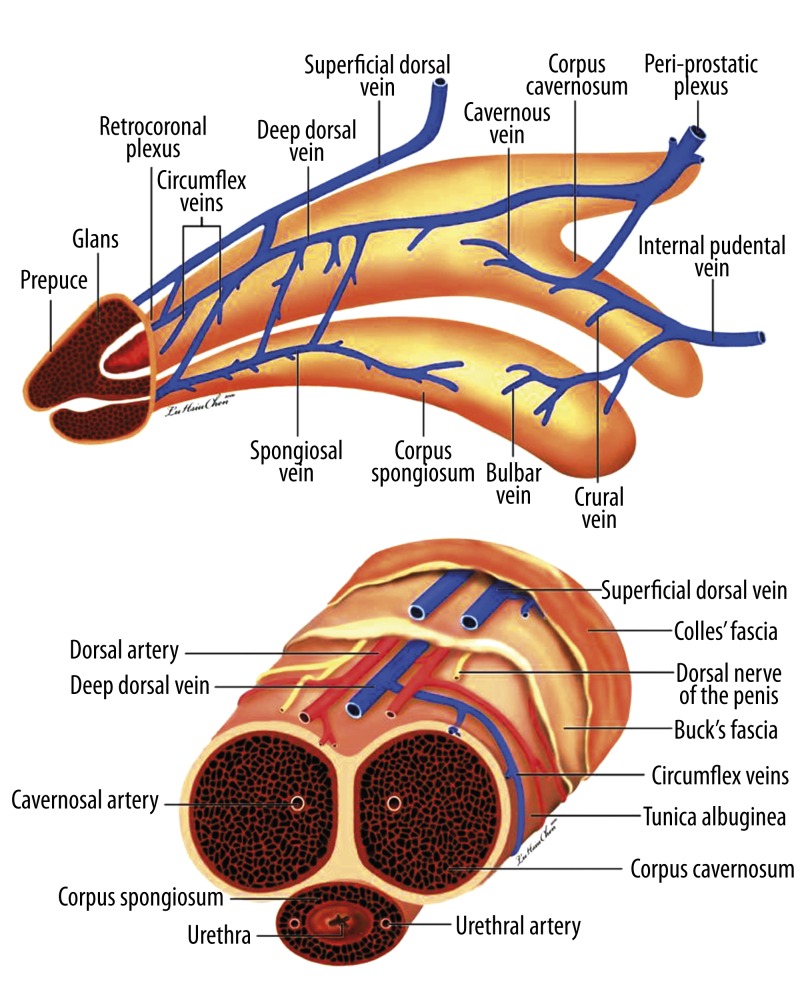
Given its importance, it is unsurprising that the penis has been the subject of exhaustive study, and its anatomy (Figure 1) is generally deemed to be well-established [8–13]. It is commonly believed that the glans penis is composed exclusively of uniform sinusoids. There is a single deep dorsal vein (DDV) and 2 dorsal arteries between the tunica albuginea and Buck’s fascia. Thus the DDV is sandwiched by a pair of dorsal arteries, and the 2:1 ratio of arteries to veins is identical to the umbilical cord’s artery-vein ratio. In addition, the tunica albuginea of the corpora cavernosa is consistently described as a single-layered coat with uniform thickness.
Figure 1.
Schematic illustration of the traditional penile anatomy. (A) Lateral view. The glans penis is exclusively composed of uniform sinusoids only? The deep dorsal vein (DDV) is sandwiched in by a pair of dorsal arteries (DA)? The 2: 1 ratio of arteries to veins is the same as in the umbilicus vessel. (B) Cross-section of a pendulous portion in the human penis. The tunica albuginea of the corpora cavernosa is consistently described as a one-layered coat with uniform thickness. The median septum is complete. There is one single DDV and two DAs between the tunica albuginea and Buck’s fascia. Thus the penile vascular system still complies with the general anatomical rule that veins number more than arteries do.
The conventional anatomical paradigm of the penis may, however, be questionable since it does not yet supply answers to some important questions arising from daily practice. Take, for example, the following case: In 1985, a 32-year-old impotent man sustained a prostrate-depressive course for erectile dysfunction (ED) and had long yearned for a cure for his awkward condition. Sudden loss of body weight had resulted from cancer phobia in the previous month. He asked for reconfirmation of whether a hard ridge that he had palpated a month earlier inside his glans penis from the urethral tip was a cancerous growth, as had been previously suspected by a cancer subspecialist. Upon hearing my reply that there should only be sinusoids within the glans penis, he became convinced of a diagnosis of penile cancer [14]. He had become so desperate that he had ultimately lost interest in daily activities since, in his mind, he had to struggle to survive cancer, with ED being the least of his worries. He suffered from chronic, intractable depression. Could any physician have given him a different answer when the medical literature holds that there are only sinusoids within the glans?
Consider a second example: In 1986, a 3-year-old boy was brought by his mother for medical treatment of urination pain and posthitis resulting from complete phimosis associated with smegma. The mischievous boy asked me why he sometimes had a rigid bone inside his penis, which disappeared after urination. His mother, a veterinarian, reminded me that there is a bone in the canine penis [15]. Is the notion of an os penis in human beings really so outrageous? Perhaps a short aside illustrates why it might indeed be: In 1988, a 27-year-old male sustained a penile fracture when, needing to urinate in the middle of the night, he started moving half-asleep towards the bathroom and his erect penis collided with the wall. These painful accidents are entirely avoidable when the penis is flaccid; they would, however, be an inevitable occurrence were man to possess an os penis.
Consider a third case: In 1987, a pediatric surgeon taught us that plication of Buck’s fascia could correct the penile curvature of his young patient. Might this present a sustainable surgical solution? Fourthly in 2000, a 44-year-old man, a specialist in a form of penile weight practice (which, in Chinese society, is believed to be beneficial for ED and various chronic systemic diseases) consulted our institute because his glans was insufficiently rigid to sustain coitus [16]. He told us the sad story of when, in 1995, he sustained an accident of glanular disruption while attempting to hold a 300-kg weight with his penis in order to attract potential disciples. An emergency repair of the disrupted glans was made by a urologist. His glans penis incurred a 30% volume loss and became considerably weaker afterwards. He was unable to engage in coitus for 5 years because his partner consistently declined his sexual advances in spite of the fact that his erectile function and libido were excellent. Physical examination showed his glans penis to be strange. It had taken on a rubber-like quality, lacking any firm connectivity with the corpora cavernosa. One can only assume that the distal ligament (DL) had been overlooked during the original corrective surgery. It could be hypothesized that without this structure, the glans penis would be too weak either to bear the buckling pressure generated by coitus or to provide sufficient rigidity for intercourse.
Consider a fifth and final case: In 1990, we were approached by a 28-year-old engineer who had been suffering from ED for 7 years and who had already sought consultation with a number of highly credible physicians. His previous doctors had, without exception, diagnosed his ED as having a psychogenic origin, but after multidisciplinary investigations at our institutes we discovered the presence of a veno-occlusive dysfunction [17]. Amazingly, his prolonged condition was corrected by penile venous-stripping surgery. This patient’s diagnosis and successful treatment poses the question – what is the optimal experimental model to isolate the venous factor from psychogenic influences in ED?
The preceding examples demonstrate that it is not uncommon to encounter events in daily practice that seemingly call into question the existing literature’s depiction of both the penile anatomy and erection physiology, highlighting its inability to provide satisfactory answers to medical questions and to act as a foundation for advancing penile surgical strategies. Furthermore, how can an evolutionary process of the animal kingdom be correctly interpreted if human penile anatomy has not been correctly described [18]? Can recent insights into penile anatomy provide answers to common dilemmas and offer evidence of evolutionary differentiation?
This review of penile anatomy and erection physiology is intended for all physicians and surgeons, since both the appreciation of any human-related physiological process and the subsequent derivation of treatment strategies are wholly dependent upon a correct understanding of anatomy and physiology.
The Fibro-Skeleton of the Human Penis: The Tunica Albuginea and its Related Structure
The human penis is a unique structure composed of multiple fascial layers that surround 3 cylinders of erectile sinusoids, including a pair of corpora cavernosa and a single corpus spongiosum. Thus, it consists of the glans penis, the corpus spongiosum (with the bulb of the urethra), the paired corpora cavernosa, the bulbospongiosus, and the ischiocavernosus muscles. Together, they are encased by fascial layers of the tunica albuginea – Buck’s fascia and Colles’ fascia – that separate the corporeal sinusoids from the dermis and skin layer.
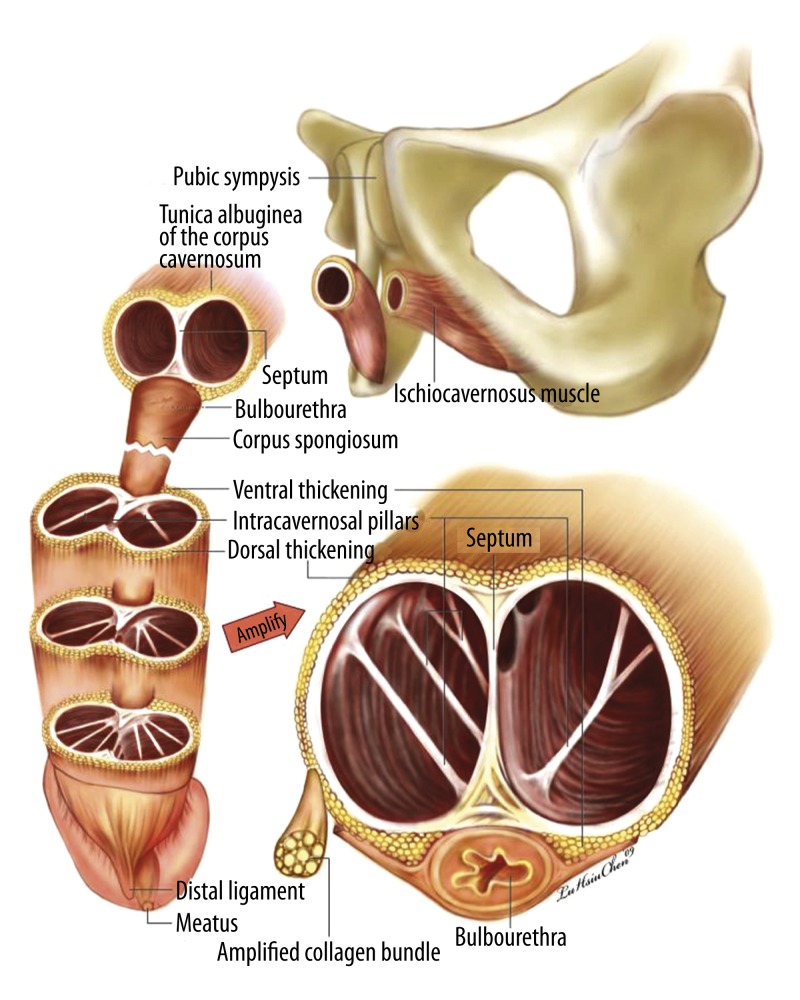
The tunica albuginea of the corpora cavernosa is the essential fibro-skeleton (Figure 2) required for rigidity during sexual intercourse [19]. This design seems indispensable to such a purpose. It has been consistently but wrongly described as a single layer with a uniform circumferential thickness [20]; however, it is in actuality a bi-layered structure in which the inner layer is arranged circumferentially and in which fibers of the outer layer are arrayed longitudinally. The inner layer completely contains and, together with the intracavernosal pillars, supports the sinusoids. There is a paucity of outer layer bundles at the region between the clockwise 5 and 7 o’clock positions, where 2 triangular ligament structures originate [21,22] – these structures, termed the ventral thickening, are a continuation of the left and right bulbospongiosus muscles, respectively. There is close contact of the corpora cavernosa with the corpus spongiosum between them. Between the 11 and 1 o’clock positions on the dorsal aspect, there is also a dorsal thickening of the outer longitudinal tunica, a radiating aspect of the bilateral ischiocavernosus muscles. The continuation of the outer longitudinal layer of the tunica, located at the 12 o’clock position of the distal urethra, is grouped into the glans penis to form the distal ligament (DL). This structure is arranged centrally and acts as a trunk of the glans penis to maintain patency of the distal urethra and preserve the range of ejaculation. At the pendulous portion of the penis the median septum is incomplete, with dorsal fenestration, and differs greatly from the depiction in all textbooks currently available. The extent of fenestration is commensurate with the quantity of the intracavernosal pillars. Thus, in the distal penis the pillars are numerous where the septum is most incomplete, in order to allow for tensile capability.
Figure 2.
Schematic illustration of the fibroskeleton in the human penis. The tunica albuginea of the corpora cavernosa is a bilayered structure. The inner circular layer completely contains the sinusoids and, together with the intracavernosal pillars, supports them. There is a paucity of outer layer bundles at the region between the 5 and 7 o’clock positions where there is close contact with the corpus spongiosum. Distally, they are grouped into the glans penis forming the distal ligament, located at the 12 o’clock position of the distal urethra. The median septum is incomplete with dorsal fenestration at the pendulous portion of the penis and is merely complete where the penile crura are nearly formed.
This anatomical knowledge of the fibro-skeleton is deemed a prerequisite for every physician who attempts to provide a rational solution for any medical event involving the penis. It is also a foundation for any surgeon who performs surgery on the delicate tunica albuginea, such as procedures involved in penile curvature correction [23,24], patch surgery [25], and penile implantation [26].
The Integral Relationship Between the Skeletal and Smooth Muscle Components
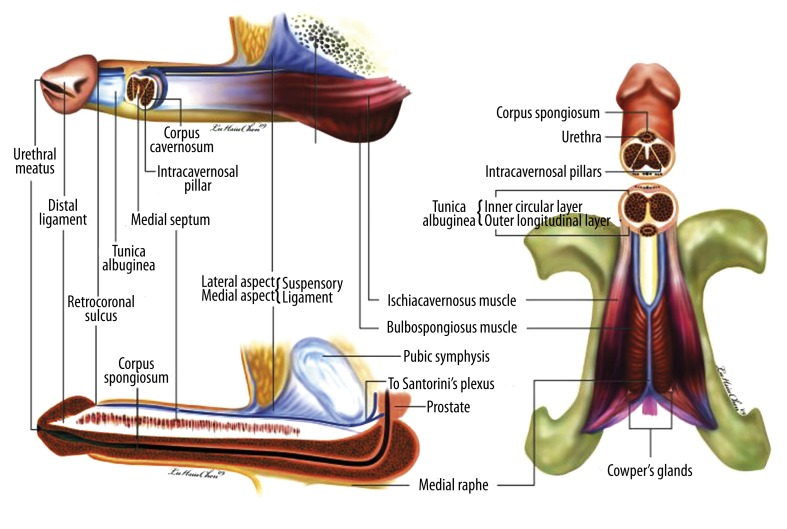
In the human penis, the components of the skeletal muscles include the ischiocavernosus muscle, the bulbospongiosus muscle, and their continuing fibro-skeleton (Figure 3) [27]. The distribution of the tunica albuginea is, however, singularly dependent upon specific anatomical parts, in accordance with which a functional requirement exists – the corpora cavernosa and corpus spongiosum, for instance. Thus, the skeletal ischiocavernosus muscle, bulbospongiosus muscle, and the bi-layered tunica albuginea support and form the corpora cavernosa, the most ideal milieu in the entire human body in which to test Pascal’s law [28], which states that pressure applied to any part of an enclosed fluid at rest is transmitted undiminished to every portion of the fluid and to the walls of the containing vessel. The mono-layered tunica albuginea contains the corpus spongiosum, while the skeletal bulbospongiosus muscle partially encloses it, thereby allowing ejaculation despite the erection being rigid (since tumescence, as opposed to rigidity, is conserved). This is a result of the paucity of the outer longitudinal layer. In the glans penis, however, the skeletal distal ligament is entrapped by the smooth muscle sinusoids and serves as a kind of trunk, allowing the glans to protect the reflexogenic erectile mechanism, thus rendering it essential to coitus.
Figure 3.
Schematic illustration of the three-dimensional human penis. (A) Lateral view. The penis leans upon the suspensory ligament which is an extension of the linea alba. Proximally, it is capped by the glans penis, and the corpus spongiosum is held by the bulbospongiosus muscle in which the fibers are mostly transverse. The corpora cavernosa are surrounded by a bilayered tunica albuginea which is composed of inner circular and outer longitudinal collagen bundles. The intra-cavernosal pillars, which are considerably more numerous distally, are a continuation of the inner circular layer. The corpus cavernosum is entrapped in the ischiocavernosus muscle with the muscle fibers aligned in longitudinal arrangements. (B) Medial view. The distal ligament is segregated from the collagen bundles of the outer longitudinal layer of the tunica albuginea. It is an inelastic fibrous structure which forms the trunk of the glans penis. The incomplete septum is dorsally fenestrated. The corpus spongiosum contains the urethra. (C) Ventral aspect. The three-dimensional structure of the human penis is evident. The ischiocavernosus muscle is paired and situated at the lateral boundary of the perineum. Each segment covers its ipsilateral penile crus. Meanwhile, anterior fibers of the bulbospongiosus muscle partially spread out to encircle the corpus cavernosum (not shown here) and mostly insert into the ventral thickening of the tunica albuginea.
The smooth muscle structure is found inside the vascular walls – the artery, vein, and sinusoidal walls which are intertwiningly formed with smooth muscle cells and fibrous tissue structures throughout the glans penis, the single corpus spongiosum, and the paired corpora cavernosa. The human penis mimics the structure of the human body in which the skeletal muscles and skeleton encompass visceral organs containing smooth muscles. Whether the organ is healthy depends upon the integrity of its muscles.
Any penile surgery, if erectile function is a concern, should spare the muscles, since muscle integrity is essential in determining a penis’s health. This situation is analogous to that of a freely movable finger, which can only be preserved if the arm-sited muscle is spared during attempted hand surgery. Since iatrogenic damage to the muscles resulting from crural detachment or ligation is irreversible and wholly undesirable, this anatomical knowledge is critical to any surgeon [29].
The Os Penis in Quadrupeds and the Distal Ligament in an Upright Animal: Evidence of Evolution
A full exploration of the penile anatomy among animal species provides the framework for an investigation into the processes of species-level divergence [30]. In quadrupeds, such as dogs and rats, an os penis – supported by a pair of well-developed, non-elastic corpora – forms an unvarying and indispensible component of the penis, vital to intercourse. This is necessary for rigorous coitus in dogs, which have a protracted coital time [31]. In rats, a joint-like design between the corpus cavernosum and os penis is optimal for its action of flipping mating in order to remove the semen plug deposited by a previous male competitor (although its coital time lasts only a few seconds) [32]. These multifarious anatomical designs are specifically attuned to the unique physiological functions and requirements of each species, and they accordingly display a great deal of variety.
Although there is no os penis in upright animals such as humans, a closely corresponding distal ligament with the same histology is arranged centrally and acts indispensably as a supporting trunk of the glans penis. The absence of an os penis (and the phenomenon of penile extensibility) in upright animals may have been evolutionary advantageous for locomotion by removing a significant impediment to movement.
The distal ligament is convergent from the outer layer of the tunica albuginea; what we might think of as a kind of spine within the glans penis has been overlooked in the existing literature [33]. Since the distal ligament in human beings is analogous to the quadrupedal os penis not only in terms of the anatomical position but also in the histology component, might this not constitute solid evidence of evolutionary development? Without this important structure, the glans would be too weak to bear the buckling pressure generated during coitus and too limber to maintain an efficacious passage for ejaculation, potentially resulting in poor transmission of intracavernosal pressure along the entirety of the penile shaft during ejaculation. This model of penile anatomy is, accordingly, meaningful, and, in addition to constituting plausible evidence of the evolutionary process, can provide a broader foundation for medical and surgical strategies in clinical practice [34].
New Insights into Penile Venous Anatomy
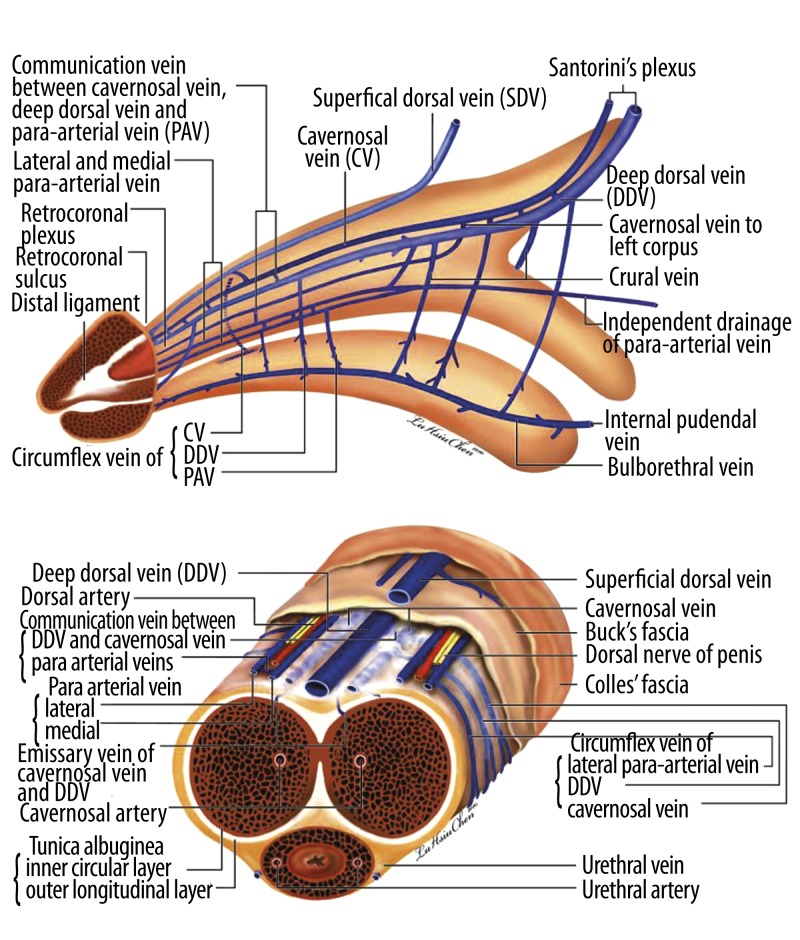
The venous system of the human penis has been widely studied and is generally described as follows: A single superficial dorsal vein (SDV) is located between Buck’s fascia and Colles’ fascia for general venous drainage of superficial tissues, and a single DDV accompanied by a pair of dorsal arteries (DAs) is positioned between the tunica albuginea and Buck’s fascia for blood drainage of the corpora cavernosa, a chamber essential to facilitating a rigid erection [35–38]. Thus, according to the received medical wisdom, the penis, like the umbilical cord, constitutes a rare exception in the human anatomy in that its attendant arteries number fewer than its attendant veins do. A recent study, however, found that each of the DAs is sandwiched by a medial and lateral para-arterial vein (PAV) and that the DDV is accompanied by cavernosal veins (CVs) which are more deeply housed in their own perivascular sheath in accordance with the drain corresponding to the corpus cavernosum (Figure 4) [39]. It is important to recognize that the DDV is not unique in having dedicated emissary and circumflex veins – the CVs and PAVs also possess them.
Figure 4.
Schematic illustration showing advanced anatomy of the erection-related veins in the human penis. (A) Lateral view: The deep dorsal vein is consistently located in the median position and receives blood of the emissary veins from the corpora cavernosa and of the circumflex vein from the corpus spongiosum. It is sandwiched between the cavernosal veins, although these lie at a deeper position. Bilaterally, each dorsal artery is respectively sandwiched by its corresponding medial and lateral para-arterial veins. Note that the lateral para-arterial vein merges with the medial one proximally. The deeper color of the veins indicates the deepest part of the vasculature. (B) Cross section of the mid-penis. Note that the number of veins is seven, not one as was traditionally believed. (Although the number becomes four at the level of the penile hilum because each pair of the nomenclature veins merges.) Erection-related veins are arrayed in an imaginary arc on the dorsal aspect of the tunica albuginea.
The DDV, consistently located in the median position, receives blood through the emissary veins from the corpora cavernosa and through the circumflex vein from the corpus spongiosum. In other words, it remains the common drainage channel of the paired corpora cavernosa and corpus spongiosum. It is flanked by a pair of CVs, although these lie in a deeper position. Bilaterally, each DA is respectively sandwiched by its corresponding medial and lateral PAVs. A slumped-looking communicating vein can clearly be identified between the 2, acting as a kind of hammock or sling for the corresponding dorsal artery [40]. The lateral PAV merges with the medial one proximally. Therefore, there are 7 veins between the tunica albuginea and Buck’s fascia in the pendulous portion of the penis, not 1 as was traditionally believed. (Although the number becomes 4 at the level of the penile hilum, as a merger takes place in each pair of nomenclature veins.)
Hence, the penile vascular system still complies with the general anatomical rule that veins number more than arteries do. Erection-related veins are arrayed in an imaginary arc on the dorsal aspect of the corpora cavernosa. The relationship of the vasculature to the fibro-skeleton is interesting, and the difference between the penile venous and arterial paths is substantial. The veins traverse an oblique path between the inner and outer layers of the tunica albuginea, whereas the arteries take a more direct route. This design is optimal for facilitating penile erection in that the venous vasculature is susceptible to being compressed. As one might expect in erection physiology, there is a description of a subtunical venule space [41], a transitional zone between the inner and outer layers. This outer layer is indispensable for rendering rigidity, yet it has been previously disregarded. Could a proper explanation of erection physiology be made in light of these new insights?
A thorough scrutiny of the penile vasculature reveals a complexity that could plausibly account for physicians overlooking “residual veins” when performing penile venous surgery and in subsequently misinterpreting these veins as “recurrent” in the event of a disappointing postoperative result [42,43].
Pathophysiology of Erectile Dysfunction
The current consensus maintains that erectile function relies upon a number of conditions: a normal hormonal profile, arterial sufficiency, neurological integrity, freedom from the adverse effect of drugs, a lack of systemic chronic disease, the absence of psychological disturbance, and healthy cavernosal sinusoids with integral endothelial function [44–46]. Accordingly, ED could be attributed to any combination of hormonal deficiency, arterial insufficiency, neurological impairment, adverse reaction to drugs, systemic chronic disease, psychological disturbance, or cavernosal defects [47]. However, a recent hemodynamic study of fresh human cadavers indicated that a rigid erection was unequivocally attainable after erection-related veins were removed in all subjects – despite the fact that their sinusoidal tissues were dead [48]. Given that none of the other commonly ascribed contributors to erectile function could be said to affect erectile capability in cadavers, might we not perceive in these findings strong evidence of the pivotal role played by the penile veins? Might we not infer then that a fully rigid erection may depend upon the drainage veins as well, rather than just on the intracavernosal smooth muscle? Therefore, might we not look at venous malfunction [49,50] – a phenomenon manifest in a variety of other disease entities, including hemorrhoids [51], varicose veins of the leg [52], and varicocele testis [53] – with a renewed appreciation for its critical role in ED? It is understandable that veins are susceptible to dysfunction in dependent portions in upright animals, implying that these various diseases could, to a large extent, be avoided if man walked on 4 feet. Could penile venous surgery and morphological reconstruction, therefore, be beneficial for ED patients [54,55]? There is evidence that the mechanism of erections is predominantly a vascular hemodynamic phenomenon. While a rigid erection overall depends on cooperation among healthy sinusoids, a normal tunica, functional arteries, and veno-occlusive drainage, it is logical that the venous factor be included alongside (or even be afforded priority over) other factors.
Conclusions
For physicians, the importance of anatomy is second to none – both in forming treatment strategies and in confronting medical questions in routine practice. These newfound insights into penile tunical, venous anatomy and erection physiology were inspired by, and, in turn, enhance, clinical applications routinely encountered by physicians and surgeons, including penile morphological reconstruction, penile implantation and penile venous surgery.
Acknowledgements
We wish to thank editors Benedict S. A. Murrell and Nicholas E Bagnall for their English editing, Ms Hsiu-Chen Lu for illustrations and photography.
Footnotes
Source of support: This study is supported in part by Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH99-TD-B-111-004)
References
- 1.Nielsen C. Animal evolution: interrelationships of the living phyla. 2nd ed. Oxford: Oxford University Press; 2001. [Google Scholar]
- 2.Mantione K. Estrogen signaling Is conserved during evolution enhancing its biomedical significance. Med Sci Monit. 2005;11(8):LE5. [PubMed] [Google Scholar]
- 3.Tosevski J, Tosevski D. Concealed female external genitals: possible morpho-psychological clue to unique motional and cognitive evolutionary matrix of man. Med Sci Monit. 2006;12(5):HY11–19. [PubMed] [Google Scholar]
- 4.Hosken DJ, Stockley P. Sexual selection and genital evolution. Trends Ecol Evol. 2004;82:87–93. doi: 10.1016/j.tree.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Simmons MN, Jones JS. Male genital morphology and function: an evolutionary perspective. J Urol. 2007;177:1625–31. doi: 10.1016/j.juro.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 6.David E. Transgenics and vertebrate cloning as tools for species conservation. Conserv Biol. 2006;20:723–32. doi: 10.1111/j.1523-1739.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 7.Mattelaer JJ. The phallus in art and culture. 2nd ed. History Office European Association of Urology; 2008. [Google Scholar]
- 8.Aboseif SR, Breza J, Lue TF, et al. Penile venous drainage in erectile dysfunction. Anatomical, radiological and functional considerations. Br J Urol. 1989;64:183–90. doi: 10.1111/j.1464-410x.1989.tb05985.x. [DOI] [PubMed] [Google Scholar]
- 9.Banya Y, Ushiki T, Takagane H, et al. Two circulatory routes within the human corpus cavernosum penis: a scanning electron microscopic study of corrosion casts. J Urol. 1989;142:879–83. doi: 10.1016/s0022-5347(17)38935-8. [DOI] [PubMed] [Google Scholar]
- 10.Moscovici J, Galinier P, Hammoudi S, et al. Contribution to the study of the venous vasculature of the penis. Surg Radiol Anat. 1999;21:193–99. doi: 10.1007/BF01630901. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs AM, Mehringer CM, Rajfer J. Anatomy of penile venous drainage in potent and impotent men during cavernosography. J Urol. 1989;141:1353–56. doi: 10.1016/s0022-5347(17)41305-x. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein AM, Padma-Nathan H. The microarchitecture of the intracavernosal smooth muscle and the cavernosal fibrous skeleton. J Urol. 1990;144:1144–46. doi: 10.1016/s0022-5347(17)39677-5. [DOI] [PubMed] [Google Scholar]
- 13.Gray H. Myology: fascia and muscles of the trunk. In: Williams PL, Dyson M, Warwick R, editors. Gray’s Anatomy. 37th ed. London: Churchill Livingstone; 1989. pp. 587–608. [Google Scholar]
- 14.Putz R, Pabsteds R. Pelvic diaphragm [floor]: male and female external genitalia. In: Putz R, Pabsteds R, editors. Sobotta Atlas of Human Anatomy. 13th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 222–39. [Google Scholar]
- 15.Christensen GC. Angioarchitecture of the canine penis and the process of erection. Am J Anat. 1954;95:227–61. doi: 10.1002/aja.1000950204. [DOI] [PubMed] [Google Scholar]
- 16.Hsu GL, Wen HS, Hsieh CH, et al. Traumatic glans deformity: reconstruction of distal ligamentous structure. J Urol. 2001;166:1390. doi: 10.1016/s0022-5347(05)65781-3. [DOI] [PubMed] [Google Scholar]
- 17.Chen SC, Hsieh CH, Hsu GL, et al. The progression of the penile vein: could it be recurrent? J Androl. 2005;26:56–63. [PubMed] [Google Scholar]
- 18.Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. In: Wein AJ, Kavoussi LR, Novick AC, et al., editors. Campbell-Walsh Urology. 9th ed. Philadelphia: W. B. Saunders; 2007. pp. 718–49. [Google Scholar]
- 19.Hsu GL, Brock GB, Martinez-Pineiro L, et al. The three-dimensional structure of the tunica albuginea: anatomical and ultrastructural levels. Int J Impot Res. 1992;4:117–29. [Google Scholar]
- 20.Eardley I, Sethia K. Anatomy and physiology of erection. In: Eardley I, Sethia K, editors. Erectile dysfunction, current investigation and management. London: Mosby; 2003. pp. 7–23. [Google Scholar]
- 21.Hsu GL, Brock G, Martinez-Pineiro L, et al. Anatomy and strength of the tunica albuginea: its relevance to penile prosthesis extrusion. J Urol. 1994;151:1205–8. doi: 10.1016/s0022-5347(17)35214-x. [DOI] [PubMed] [Google Scholar]
- 22.Hsu GL, Brock G, von Heyden B, et al. The distribution of elastic fibrous elements within the human penis. Br J Urol. 1994;73:566–71. doi: 10.1111/j.1464-410x.1994.tb07645.x. [DOI] [PubMed] [Google Scholar]
- 23.Hsu GL, Chen SH, Weng SS. Outpatient surgery for the correction of penile curvature. Br J Urol. 1997;79:36–39. doi: 10.1046/j.1464-410x.1997.02988.x. [DOI] [PubMed] [Google Scholar]
- 24.Hsu GL, Hsieh CH, Wen HS, et al. Formulas for determining the dimensions of venous graft required for penile curvature correction. Int J Androl. 2006;29:515–20. doi: 10.1111/j.1365-2605.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- 25.Hsu GL, Chen HS, Hsieh CH, et al. Long-term result of an autologous venous grafting for penile morphological reconstruction. J Androl. 2007;28:186–93. doi: 10.2164/jandrol.106.000760. [DOI] [PubMed] [Google Scholar]
- 26.Hsu GL, Hsieh CH, Wen HS. Outpatient penile implantation with the patient under a novel method of crural block. Int J Androl. 2004;27:147–51. doi: 10.1111/j.1365-2605.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 27.Hsu GL, Hsieh CH, Wen HS, et al. Anatomy of the human penis: the relationship of the architecture between skeletal and smooth muscles. J Androl. 2004;25:426–31. doi: 10.1002/j.1939-4640.2004.tb02810.x. [DOI] [PubMed] [Google Scholar]
- 28.Halliday D. Pascal’s principle, fluids. In: Halliday D, Resnick R, Walker J, editors. Fundamentals of Physics. New York: J Wiley; 1997. pp. 355–56. [Google Scholar]
- 29.Mulhall JP, Martin D, Ergin E, et al. Crural ligation surgery for the young male with venogenic erectile dysfunction: technique. Tech Urol. 2001;7:290–93. [PubMed] [Google Scholar]
- 30.Hsu GL, Lin CW, Hsieh CH, et al. Distal ligament in human glans: a comparative study of penile architecture. J Androl. 2005;26:624–28. doi: 10.2164/jandrol.04145. [DOI] [PubMed] [Google Scholar]
- 31.Hart BL. Sexual reflexes and matting behavior in the male dog. J Comp Physiol Psychol. 1967;64:388–99. doi: 10.1037/h0025222. [DOI] [PubMed] [Google Scholar]
- 32.Leipheimer RE, Sachs BD. GABAergic regulation of penile reflexes and copulation in rats. Physiol Behav. 1988;42:351–57. doi: 10.1016/0031-9384(88)90276-4. [DOI] [PubMed] [Google Scholar]
- 33.Brant WO, Bella AI, Lue TF. Anatomy of erectile function. In: Carson CC III, Kirby RS, Goldstein I, Wyllie MG, editors. Textbook of Erectile Dysfunction. 2nd ed. St Heler: Informa HealthCare; 2008. pp. 25–27. [Google Scholar]
- 34.Hsu GL, Hsieh CH, Chen HS, et al. The advancement of pure local anesthesia for penile surgeries: can an outpatient basis be sustainable? J Androl. 2007;28:200–5. doi: 10.2164/jandrol.106.000679. [DOI] [PubMed] [Google Scholar]
- 35.Bookstein JJ, Lurie AL. Selective penile venography: anatomical and hemodynamic observations. J Urol. 1988;140:55–60. doi: 10.1016/s0022-5347(17)41485-6. [DOI] [PubMed] [Google Scholar]
- 36.Fitzpatrick T. The corpus cavernosum intercommunicating venous drainage system. J Urol. 1975;113:494–86. doi: 10.1016/s0022-5347(17)59509-9. [DOI] [PubMed] [Google Scholar]
- 37.Delcour C, Wespes E, Schulman CC, et al. Investigation of the venous system in impotence of vascular origin. Urol Radiol. 1984;6:190–93. doi: 10.1007/BF02923722. [DOI] [PubMed] [Google Scholar]
- 38.Udelson D, L’Esperance J, Morales AM, et al. The mechanics of corporal veno-occlusion in penile erection: a theory on the effect of stretch-associated luminal constrictability on outflow resistance. Int J Impot Res. 2000;12:315–27. doi: 10.1038/sj.ijir.3900628. [DOI] [PubMed] [Google Scholar]
- 39.Hsu GL, Hsieh CH, Wen HS, et al. Penile venous Anatomy: An additional description and its clinical implication. J Androl. 2003;24:921–27. doi: 10.1002/j.1939-4640.2003.tb03145.x. [DOI] [PubMed] [Google Scholar]
- 40.Hsu GL. The hypothesis of human penile anatomy, erection hemodynamic and their clinical applications. Asian J Androl. 2006;8:225–34. doi: 10.1111/j.1745-7262.2006.00108.x. [DOI] [PubMed] [Google Scholar]
- 41.Kirby RS. Male sexual function. In: Tomlinson J, editor. ABC of sexual health. London: BMJ Books; 1999. pp. 29–31. [Google Scholar]
- 42.Freedman AL, Costa Neto F, Mehringer CM, et al. Long-term results of penile vein ligation for impotence from venous leakage. J Urol. 1993;149:1301–3. doi: 10.1016/s0022-5347(17)36374-7. [DOI] [PubMed] [Google Scholar]
- 43.Vale JA, Feneley MR, Lees WR, et al. Venous leak surgery: long-term follow-up of patients undergoing excision and ligation of the deep dorsal vein of the penis. Br J Urol. 1995;76:192–95. doi: 10.1111/j.1464-410x.1995.tb07673.x. [DOI] [PubMed] [Google Scholar]
- 44.Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379–95. doi: 10.1016/j.ucl.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wierzbicki AS, Jackson G. NO problem: arterial and venous endothelial function and erectile dysfunction. Eur Urol. 2011;59:956–58. doi: 10.1016/j.eururo.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 46.Stefano G, Kream R. Reciprocal regulation of cellular nitric oxide formation by nitric oxide synthase and nitrite reductases. Med Sci Monit. 2011;17(10):RA221–26. doi: 10.12659/MSM.881972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaminetsky J. Epidemiology and pathophysiology of male sexual dysfunction. Int J Impot Res. 2008;20:S3–10. doi: 10.1038/ijir.2008.16. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh CH, Wang CJ, Hsu GL, et al. Penile veins play a pivotal role in erection: the hemodynamic evidence. Int J Androl. 2005;28:88–92. doi: 10.1111/j.1365-2605.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 49.Hsu GL, Chen HS, Hsieh CH, et al. Insufficient response to venous surgery: is penile vein recurrent or residual? J Androl. 2006;27:700–6. doi: 10.2164/jandrol.106.000737. [DOI] [PubMed] [Google Scholar]
- 50.Hsu GL, Chen HS, Hsieh CH, et al. Salvaging Penile Venous Stripping Surgery. J Androl. 2010;31:250–60. doi: 10.2164/jandrol.109.008409. [DOI] [PubMed] [Google Scholar]
- 51.Arbman G, Krook H, Haapaniemi S. Closed vs. open hemorrhoidectomy – is there any difference? Dis Colon Rectum. 2000;43:31–34. doi: 10.1007/BF02237240. [DOI] [PubMed] [Google Scholar]
- 52.Jiang P, van Rij AM, Christie RA, et al. Venous physiology in the different patterns of recurrent varicose veins and the relationship to clinical severity. Cardiovasc Surg. 2000;8:130–36. doi: 10.1016/s0967-2109(99)00098-8. [DOI] [PubMed] [Google Scholar]
- 53.Niedzielski J, Paduch DA. Recurrence of varicocele after high retroperitoneal repair: implications of intraoperative venography. J Urol. 2001;165:937–40. [PubMed] [Google Scholar]
- 54.Hsieh CH, Chen HS, Lee WY, et al. Salvage penile tunical surgery. J Androl. 2010;31:450–56. doi: 10.2164/jandrol.109.008573. [DOI] [PubMed] [Google Scholar]
- 55.Hsu GL, Chen HS, Hsieh CH, et al. Clinical experience of a refined penile venous surgery procedure for patients with erectile dysfunction: is it a viable option? J Androl. 2010;31:271–80. doi: 10.2164/jandrol.109.008532. [DOI] [PubMed] [Google Scholar]