Summary
Background
The peptide glucagon-like peptide-1 (GLP-1) is a hormone secreted by intestinal L cells in response to food intake. GLP-1 has been proposed as the basis of emerging therapy for patients with type 2 diabetes. However, the effects of GLP-1 on vascular injury in diabetes have not been identified. Advanced glycation end products (AGEs) induce endothelial cell apoptosis and have been implicated in the process of vascular complications from diabetes.
Material/Methods
The aim of this work was to investigate whether and how GLP-1 protects endothelial cells from apoptosis induced by AGEs. Human umbilical vein endothelial cells (HUVECs) were treated with AGEs (200 μg/mL) for 48 h in the presence or absence of GLP-1. Cell morphology, viability, apoptosis, ratio of Bcl-2 protein to Bax protein, cytochrome c release, and activity of caspase-9 and −3 were determined.
Results
Treatment of cells with AGEs led to cell morphology changes and decreased cell viability, resulting in apoptosis. GLP-1 alone increased cell viability in a concentration-dependent manner. GLP-1 partially inhibited AGEs-induced apoptosis in HUVECs. GLP-1 increased Bcl-2/Bax ratio, reduced cytochrome c levels in the cytoplasm, and reduced the activity of caspase-9 and −3 in AGEs-treated HUVECs.
Conclusions
AGEs induces apoptosis via the mitochondrion-cytochrome c-caspase protease pathway, and GLP-1 protects endothelial cells by interfering with this mechanism. GLP-1 may represent an anti-apoptotic agent in the treatment of vascular complications arising from diabetes.
Keywords: diabetes, peptide glucagon-like peptide-1, advanced glycation end products, endothelium, apoptosis, Bcl-2
Background
Cardiovascular complications are the leading cause of morbidity and mortality in patients with diabetes. Apoptosis of vascular endothelial cells has been considered to be an important event in the process of vascular dysfunction. A number of studies have shown that advanced glycation end products (AGEs), formed by non-enzymatic glycation reactions between sugars and macromolecules, are crucially involved in diabetic vascular complications [1,2]. The large accumulation of AGEs in patients with diabetes can trigger the process of inflammation, elicit oxidative stress generation, induce the apoptosis of vascular endothelial cells, and finally result in the onset and progression of diabetic vascular complications [3–5]. Several critical steps have been reported to be involved in AGEs-induced apoptosis in HUVECs, including increased pro-apoptotic protein levels and activation of caspase protease [6]. Therefore, inhibition of AGEs-induced apoptosis of endothelial cells may be an effective therapeutic approach to prevention and treatment of diabetic complications [7].
Glucagon-like peptide-1 (GLP-1), a brain-gut insulinotropic peptide, has been proposed as a prospective target for clinical treatment of type 2 diabetes mellitus (T2DM) [8]. Most studies focus on the insulinotropic effects of GLP-1 on insulin-secreting cells but there is a growing body of evidence demonstrating that GLP-1 also has cardiovascular effects [9–11]. GLP-1 can regulate vascular tone and promote vascular endothelial cell proliferation [10,12,13]. Recent studies have shown that in HUVECs, GLP-1 not only suppresses AGEs-induced upregulation of reactive oxygen species and inflammation [14], but also inhibits H2O2-induced cellular senescence [15]. In addition, GLP-1 protects beta cells from the dangerous effects of AGEs [16]. Therefore, GLP-1 may have potential protective effects against different extracellular stimuli in different cell types. Further, GLP-1 exerts anti-apoptotic effects in different cell types, including pancreatic β-cells [17–19], neuronal cells [20–22], cardiomyocytes [23], and cholangiocytes [24]. However, it remains unknown whether GLP-1 can inhibit AGEs-induced endothelial cell apoptosis.
Therefore, in the present study we used an in vitro model to investigate the effects of GLP-1 on the apoptosis of human umbilical vein endothelial cells (HUVECs) induced by AGEs and its possible mechanism(s).
Material and Methods
Materials
D-glucose, bovine serum albumin (BSA), and GLP-1 (7–36 amide) were purchased from Sigma (St. Louis, MO, USA). Antibodies against Bcl-2, Bax, cytochrome c, and β-actin were purchased from Santa Cruz Biotechnology Inc. (Delaware, CA, USA). Annexin V-FITC/PI Apoptosis Detection Kit was purchased from Invitrogen (Carlsbad, CA, USA). Endothelial cell growth factor and caspase-3 and −9 activity assay kits were obtained from BD Biosciences (Franklin Laker, NJ, USA). BCA protein assay kit was purchased from Fisher Scientific (Pittsburgh, PA, USA). M199 culture medium and fetal bovine serum (FBS) were purchased from Gibco BRL (Grand Island, NY, USA).
Cell culture
HUVECs were isolated from umbilical cord, as previously described [25]. HUVEC medium consisted of M199 medium, supplemented with 20% FBS and 50 μg/mL endothelial cell growth factor. These cells were tested positive for von Willebrand factor antigen by immunofluorescence. Cells from the third to fifth passages were used for experiments.
Preparation of AGE-BSA
AGE-modified BSA (AGE-BSA), an AGE used to study the toxicity of AGEs on a number of cells types, was prepared as described previously [26]. Briefly, 1.0 g BSA was dissolved in 10 mL of 0.5 mol/L phosphate-buffered saline (PBS; pH=7.4) with 3.0 g D-glucose. The sample was filter-sterilized using a 0.22-μm millipore filter and incubated at 37°C for 12 weeks under sterile conditions in the dark. After incubation, the sample was dialyzed against PBS to remove unbound sugars. In the meantime, non-glycated BSA was prepared simultaneously by the same method, but without using D-glucose. AGE-BSA was identified using a fluorescence spectrophotometer. Protein concentration was measured using the BCA protein assay.
Cell treatment
HUVECs were grown to 90% confluence, followed by an incubation overnight in serum-deficient media containing 0.5% FBS. Cells were incubated with or without 200 μg/mL AGE-BSA (this concentration corresponds to the serum concentration of AGEs in patients with diabetes and is broadly used in the literature [6,27,28]) in the presence or absence of the indicated concentrations of GLP-1 for 48 h. Control cells were treated with BSA alone (at a final concentration of 200 μg/mL).
Cell viability
Cell viability was measured with the MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay. HUVECs cultured in 96-well plates (8×103 cells/well) were treated as indicated in the legend to Figure 1 and incubated for 48 h. After treatment, 5 mg/mL MTT was added to the cell culture. After 4-hour incubation, the medium was removed, the cells were solubilized in DMSO, and absorption was measured at 570 with a microplate reader.
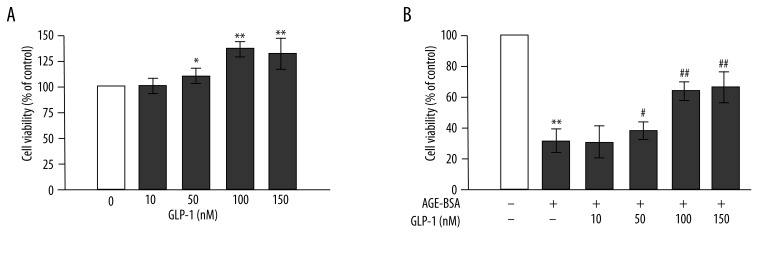
Figure 1.
Peptide glucagon-like peptide-1 (GLP-1) increases cell viability and reduces advanced glycation end products (AGEs)-induced toxicity in human umbilical vein endothelial cells (HUVECs). (A) GLP-1 increases cell viability. Cells were incubated with GLP-1 (10, 50, 100, 150 nmol/L) for 48 h. Total cell viability was measured with the MTT assay. (B) GLP-1 (50, 100, 150 nmol/L) reduces AGEs-induced cell toxicity. n=3, * p<0.05, and ** p<0.01 vs. control; # p<0.05 and ## p<0.01 vs. AGEs-treated cells.
Hoechst 33258 staining
Cells cultured on glass coverslips were fixed, permeabilized, and then stained with Hoechst 33258 at a dilution of 1:200 (1 mg/mL stock solution) for 5 min in the dark. Apoptotic cells were counted under a fluorescence microscope. At least 1000 cells were counted for each experimental condition.
Flow cytometry analysis
After treatment, cells were evaluated by double staining with FITC-conjugated Annexin V and propidium iodide (PI), according to the manufacturer’s instructions. Cells were washed twice with PBS and stained with Annexin V and PI for 15 min at room temperature. Flow cytometric analyses were performed on a FACSCalibur flow cytometer (BD Biosciences), and the data were analyzed with the Cell Quest analysis program. Apoptotic cells were defined as cells that were negative for PI (indicating an intact plasma membrane) and positive for Annexin V-FITC.
Western blotting analysis
Cells with various treatments were lysed in protein lysis buffer (1% SDS in 25 mmol/L Tris-HCl, pH=7.4, 1 mmol/L EDTA, 100 mmol/L NaCl, 1 mmol/L phenylmethanesulfonyl fluoride (PMSF), 10 μg/mL leupeptin, and 10 μg/mL pepstatin). Cell lysates were frozen and thawed 3 times and were further centrifuged at 14,000 g for 10 min at 4°C to pelletize the insoluble material. The supernatant contained the cell extracts and the protein concentration was measured using the BCA protein assay. Equal amounts of protein (20 μg) from each sample were separated on 12% SDS-polyacrylamide gel and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked in 5% non-fat milk and incubated with either anti-Bcl-2 (1:1,000), anti-Bax (1:2000), anti–cytochrome c (1:2000), or anti-β-actin (1:2000) antibody. Secondary specific horseradish-peroxidase – linked antibodies were added for 1 h, and immune complexes were detected by ECL chemiluminescence.
Measurement of caspase-3 and −9 activity
The caspase-3 and −9 activity assays were performed according to the manufacturer’s instructions. Cells were washed twice with PBS and pelleted with centrifugation. Cell pellets were then resuspended with iced lysis buffer for 10 min. After centrifugation, cell extracts were transferred to fresh tubes. Specific substrates of caspase-3 or −9 were added, and the tubes were incubated at 37°C overnight. Caspase-3 and −9 activities were read in a microplate reader at 405 nm.
Statistical analyses
Results are representative of at least three experiments. All analyses were carried out with SPSS 13.0 software. Data are expressed as mean ± standard deviation (SD). Differences between groups were tested by one-way ANOVA followed by a Student-Newman-Keuls test. Statistical significance was defined as two-sided p<0.05.
Results
Effects of GLP-1 on AGEs-induced toxicity in HUVECs
First, we evaluated the effect of GLP-1 (10, 50, 100, 150 nmol/L) on cell viability with the MTT assay. As shown in Figure 1A, GLP-1 increased cell viability in a concentration-dependent manner. Moreover, GLP-1 had similar activity at concentrations of 100 and 150 nmol/L.
In additional experiments, HUVECs were incubated with 200 μg/mL AGEs for 48 h in the presence or absence of different concentrations of GLP-1 (10, 50, 100, 150 nmol/L). Compared with control cells, HUVECs treated with AGEs (200 μg/mL) showed significantly reduced cell viability (p<0.01). GLP-1 partially restored cell viability in a dose-dependent manner, with a peak at 100 nmol/L (p<0.01 vs. AGEs-treated cells) (Figure 1B). Therefore, all subsequent experiments were performed with 100 nmol/L of GLP-1. These data demonstrated that GLP-1 increases cell viability and partially prevents AGEs-induced toxicity.
Effects of GLP-1 on AGEs-induced apoptosis in HUVECs
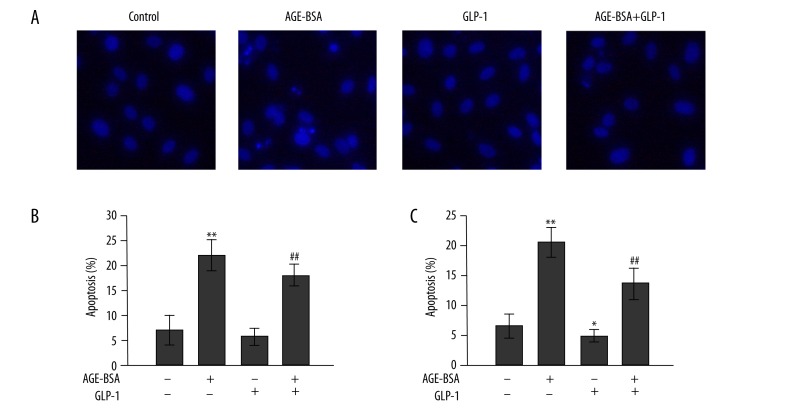
Induction of apoptosis in AGEs-induced HUVECs was detected with Hoechst 33258 staining and Annexin-FITC/PI assays. Cells showed the typical morphological changes of apoptosis, including condensation of chromatin and nuclear fragmentation after incubation with 200 μg/mL AGEs for 48 h. These morphological changes were strongly attenuated in the presence of GLP-1 and AGEs (Figure 2A). As shown in Figure 2B, AGEs significantly induced cell apoptosis as compared with controls. However, adding GLP-1 to the culture medium partially reversed AGEs-induced apoptosis. In addition, the proportion of apoptotic cells was quantified by Annexin-FITC/PI assay (Figure 2C). After cells were exposed to AGEs for 48 h, the percentage of early apoptotic cells was increased by 13%; this increment was blunted when cells were incubated with AGEs and GLP-1.
Figure 2.
Effects of GLP-1 on AGEs-induced apoptosis in HUVECs. Cells were treated with 200 μg/mL AGEs or non-glycated BSA in the presence or absence of GLP-1. (A) Morphological changes in HUVECs. Cells were detected by Hoechst 33258 staining. Apoptotic cells were observed as blue intact round nuclei and fragmented/condensed nuclei. Original magnification, ×200. Quantification of apoptotic cells with Hoechst 33258 staining (B) and Annexin-FITC/PI assay (C). n=3, * p<0.05, and ** p<0.01 vs. control; ## p<0.01 vs. AGEs-treated cells.
GLP-1 upregulates expression of Bcl-2/Bax protein ratio in AGEs-treated HUVECs
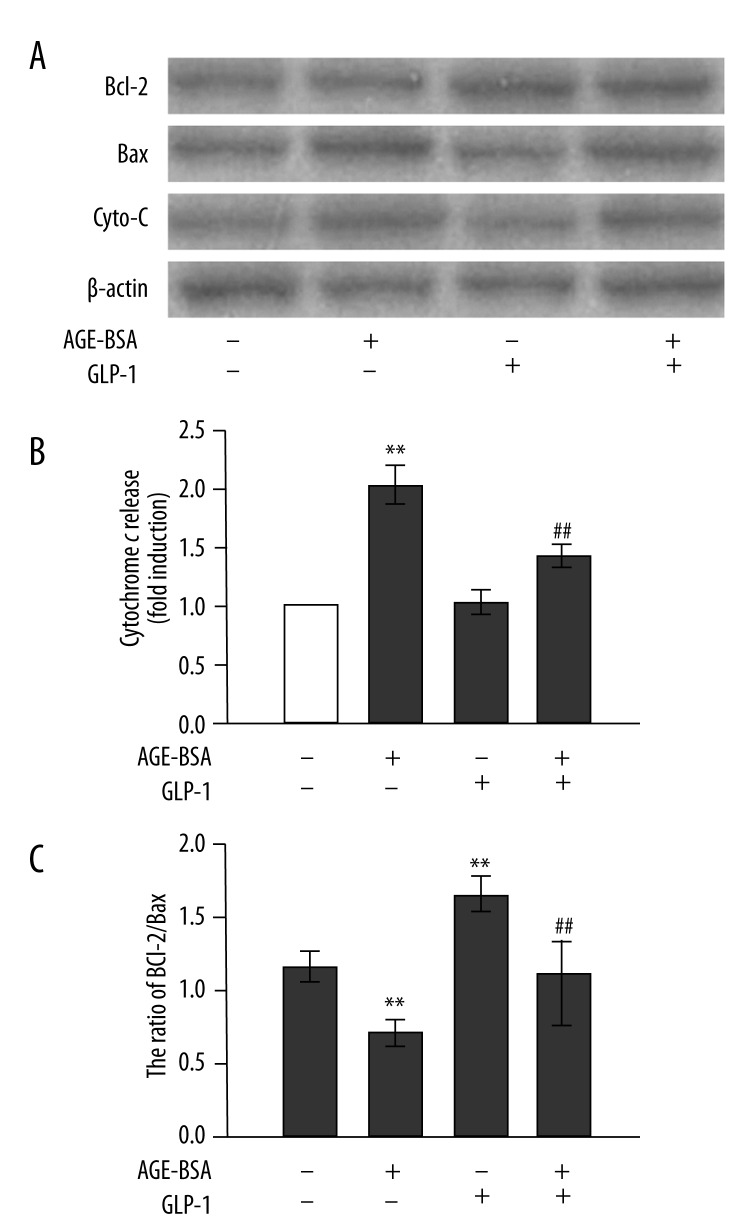
To investigate the cellular mechanism by which GLP-1 prevented the AGEs-induced apoptosis of endothelial cells, we analyzed the Bcl-2/Bax ratio in HUVECs induced by AGEs (Figure 3A, C). AGEs increased the expression of the pro-apoptotic protein Bax but failed to influence the anti-apoptotic protein Bcl-2 expression. The Bcl-2/Bax ratio was significantly reduced in HUVECs incubated with AGEs for 48 h. This decrement was completely blunted in the presence of GLP-1 and AGEs. Compared with control cells, GLP-1 alone significantly increased the Bcl-2/Bax protein ratio by upregulating Bcl-2 expression and downregulating Bax expression. These results indicate that GLP-1 can attenuate AGEs-induced apoptosis in HUVECs by altering the expression of apoptosis-related proteins.
Figure 3.
Inhibitory effects of GLP-1 on AGEs-induced apoptotic protein expression and mitochondrial permeability in HUVECs. (A) Western blot analysis of apoptosis-provoking proteins in response to AGEs (200 μg/mL) and GLP-1 (100 nmol/L) for 48 h. (B) Relative levels of cytochrome c (in the cytosolic fraction) normalized to levels of β-actin. (C) Ratio of Bcl-2 protein to Bax protein. n=3, ** p<0.01 vs. control; ## p<0.01 vs. AGEs-treated cells.
GLP-1 reduces the release of cytochrome c in AGEs-treated HUVECs
To determine whether mitochondrial release of cytochrome c, a critical event in the progression of apoptosis, contributes to the anti-apoptotic actions of GLP-1, cytosolic fractions were isolated from lysates of HUVECs. Compared with control cells, AGEs-treated HUVECs exhibited increased cytochrome c release from mitochondria. However, this increment was blunted in the presence of GLP-1 and AGEs (Figure 3B).
GLP-1 sequentially inhibits the AGEs-induced activation of caspase-9 and −3 in HUVECs
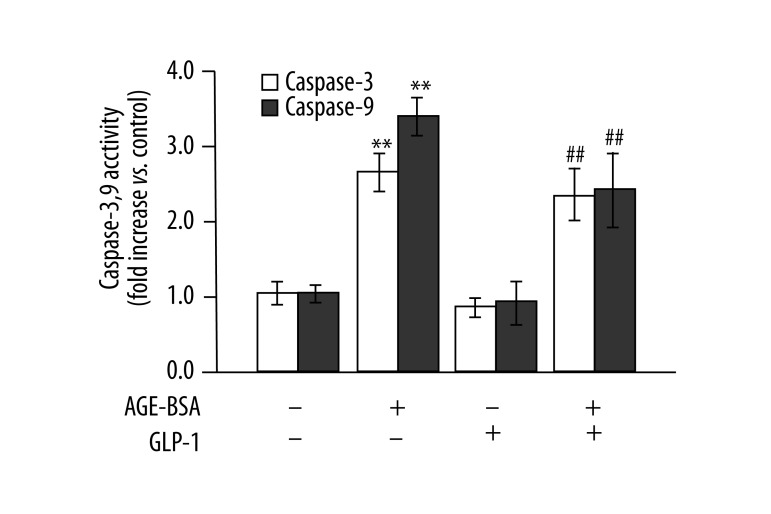
Cytochrome c is required to activate caspase-9 and −3. Caspase-9 and −3 can in turn activate apoptotic signaling and lead to apoptosis. Therefore, we further investigated whether GLP-1 acted by suppressing the activation of caspase-9 and −3. As illustrated in Figure 4, AGEs significantly increased the activity of caspase-9 and −3, and this alteration was partially inhibited in the presence of GLP-1 and AGEs, demonstrating that GLP-1 has an inhibitory effect on AGEs-induced caspase activation in the apoptosis of HUVECs.
Figure 4.
Inhibitory effects of GLP-1 on AGEs-induced caspase-9 and −3 activation. n=3, ** p<0.01 vs. control; ## p<0.01 vs. AGEs-treated cells.
Discussion
GLP-1 and its receptor agonists represent promising new therapeutic compounds not only for the treatment of T2DM, but also for the prevention of cardiovascular morbidity and mortality associated with T2DM. GLP-1 improves vascular dysfunction, such as inducing vasodilatation [29] and promoting vascular endothelial cell proliferation [12]. AGEs can be considered as a marker for the development of vascular complications in diabetes [30], as the presence of diabetic complications correlates with elevated serum AGEs levels. Evidence is growing that endothelial apoptosis induced by AGEs plays an important role in the pathogenesis of diabetes-accelerated atherosclerosis. However, it remains unknown whether GLP-1 could inhibit AGEs-induced apoptosis of HUVECs. In this in vitro study, the MTT assay showed that GLP-1 could effectively attenuate the reduction in cell viability induced by AGEs. Furthermore, GLP-1 greatly inhibited chromatin condensation and nuclear fragmentation induced by AGEs. Finally, GLP-1 led to a strong reduction in the number of apoptotic cells. These data suggest that GLP-1 can inhibit AGEs-induced apoptosis of HUVECs in vitro.
We next sought to uncover the mechanism(s) underlying the inhibition of apoptosis by GLP-1. It is well accepted that members of the Bcl-2 family represent central regulators of cell death. The pro-apoptotic protein Bax is essential for permeabilization of the mitochondrial outer membrane, leading to cytochrome c release, and subsequently the activation of caspases [31]. Nevertheless, Bcl-2, an anti-apoptotic protein, inhibits this process by inhibiting the translocation of Bax upstream of mitochondria and thus reducing the activity of caspases [31,32]. Caspase-9 and −3, the initiator caspase and the executor caspase, respectively, both amplify the pro-apoptotic signal and result in apoptosis [33]. Here, in line with the results of other studies [6], AGEs increased the expression of the pro-apoptotic protein Bax, activated caspase-9 and −3, and subsequently induced the apoptosis of HUVECs. Our data also show that AGEs increased cytochrome c release into the cytosol. However, all these activities induced by AGEs were attenuated in the presence of GLP-1. The mechanisms by which GLP-1 protects HUVECs against the apoptotic effects of AGEs could be, at least in part, through the upregulation of the Bcl-2/Bax protein ratio and the activation of the mitochondrial pathway.
The anti-apoptotic actions of GLP-1 via alteration of the Bcl-2 family proteins–caspase protease pathway may be effective in not only endothelial cells, but also in other cell types. For instance, GLP-1 upregulates Bcl-2 and inhibits Bax expression in cholangiocytes [24] and neuronal cells [20,34]. In addition, GLP-1 prevents Bax/Bcl-2 protein ratio increase, cytochrome c release, and caspase-3 activity increase induced by staurosporine in cardiomyocytes [23]. Moreover, GLP-1 also induces Bcl-2 upregulation [35], BAD inactivation [36], and caspase-3 activity reduction [19] in pancreatic β-cells. Here, we have confirmed and expanded these data by demonstrating that GLP-1 prevents the reduction of the Bcl-2/Bax protein ratio and the release of cytochrome c, and reverses the increase in caspase-9 and −3 activity induced by AGEs in HUVECs.
Conclusions
This is the first report to reveal the GLP-1 exhibits protection against AGEs-induced endothelial cell apoptosis. GLP-1 may exert anti-apoptotic effects against AGEs through a pathway involving the mitochondrion, cytochrome c, and caspase protease. Our findings support the notion that new agents based on GLP-1 and its mechanism of action, such as GLP-1 analogs, may act as double-edged swords, simultaneously ameliorating hyperglycemia and protecting against vascular injury in patients with T2DM.
Acknowledgments
We would like to express our gratitude to all those who have helped us in the preparation of this manuscript. We acknowledge Professor Lin-lang Guo for his assistance with the written English in this manuscript. The study was supported by the Natural Science Foundation of Guangdong Province (S2011010002074).
Footnotes
Source of support: The study was supported by the Natural Science Foundation of Guangdong Province (S2011010002074), China
References
- 1.Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005;11:2279–99. doi: 10.2174/1381612054367300. [DOI] [PubMed] [Google Scholar]
- 2.Meerwaldt R, van der Vaart MG, van Dam GM, et al. Clinical Relevance of Advanced Glycation Endproducts for Vascular Surgery. Eur J Vasc Endovasc. 2008;36:125–31. doi: 10.1016/j.ejvs.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Warboys CM, Toh HB, Fraser PA. Role of NADPH oxidase in retinal microvascular permeability increase by RAGE activation. Invest Ophthalmol Vis Sci. 2009;50:1319–28. doi: 10.1167/iovs.08-2730. [DOI] [PubMed] [Google Scholar]
- 4.Yamagishi S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol. 2011;46:217–24. doi: 10.1016/j.exger.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res. 2004;53:131–42. [PubMed] [Google Scholar]
- 6.Zhou YJ, Wang JH, Zhang J. Hepatocyte growth factor protects human endothelial cells against advanced glycation end products-induced apoptosis. Biochem Biophys Res Commun. 2006;344:658–66. doi: 10.1016/j.bbrc.2006.03.167. [DOI] [PubMed] [Google Scholar]
- 7.Vasan S, Foiles P, Founds H. Therapeutic potential of breakers of advanced glycation end product-protein crosslinks. Arch Biochem Biophys. 2003;419:89–96. doi: 10.1016/j.abb.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aaboe K, Krarup T, Madsbad S, Holst JJ. GLP-1: physiological effects and potential therapeutic applications. Diabetes Obes Metab. 2008;10:994–1003. doi: 10.1111/j.1463-1326.2008.00853.x. [DOI] [PubMed] [Google Scholar]
- 10.Nystrom T. The potential beneficial role of glucagon-like peptide-1 in endothelial dysfunction and heart failure associated with insulin resistance. Horm Metab Res. 2008;40:593–606. doi: 10.1055/s-0028-1082326. [DOI] [PubMed] [Google Scholar]
- 11.Nystrom T, Gutniak MK, Zhang QM, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol-Endoc M. 2004;287:E1209–15. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 12.Erdogdu O, Nathanson D, Sjoholm A, et al. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol. 2010;325:26–35. doi: 10.1016/j.mce.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Xiao-Yun X, Zhao-Hui M, Ke C, et al. Glucagon-like peptide-1 improves proliferation and differentiation of endothelial progenitor cells via upregulating VEGF generation. Med Sci Monit. 2011;17(2):BR35–41. doi: 10.12659/MSM.881383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem Biophys Res Commun. 2010;391:1405–8. doi: 10.1016/j.bbrc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 15.Oeseburg H, de Boer RA, Buikema H, et al. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol. 2010;30:1407–14. doi: 10.1161/ATVBAHA.110.206425. [DOI] [PubMed] [Google Scholar]
- 16.Puddu A, Storace D, Durante A, et al. Glucagon-like peptide-1 counteracts the detrimental effects of Advanced Glycation End-Products in the pancreatic beta cell line HIT-T 15. Biochem Biophys Res Commun. 2010;398:462–66. doi: 10.1016/j.bbrc.2010.06.100. [DOI] [PubMed] [Google Scholar]
- 17.Cunha DA, Ladriere L, Ortis F, et al. Glucagon-like peptide-1 agonists protect pancreatic beta-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes. 2009;58:2851–62. doi: 10.2337/db09-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blandino-Rosano M, Perez-Arana G, Aguilar-Diosdado M, et al. Anti-proliferative effect of pro-inflammatory cytokines in cultured beta cells is associated with extracellular signal-regulated kinase 1/2 pathway inhibition: protective role of glucagon-like peptide −1. J Mol Endocrinol. 2008;41:35–44. doi: 10.1677/JME-07-0154. [DOI] [PubMed] [Google Scholar]
- 19.Tews D, Lehr S, Hartwig S, et al. Anti-apoptotic action of exendin-4 in INS-1 beta cells: comparative protein pattern analysis of isolated mitochondria. Horm Metab Res. 2009;41:294–301. doi: 10.1055/s-0028-1105911. [DOI] [PubMed] [Google Scholar]
- 20.Liu JH, Yin F, Guo LX, et al. Neuroprotection of geniposide against hydrogen peroxide induced PC12 cells injury: involvement of PI3 kinase signal pathway. Acta Pharmacol Sin. 2009;30:159–65. doi: 10.1038/aps.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura R, Okouchi M, Fujioka H, et al. Glucagon-like peptide-1 (GLP-1) protects against methylglyoxal-induced PC12 cell apoptosis through the PI3K/Akt/mTOR/GCLc/redox signaling pathway. Neuroscience. 2009;162:1212–19. doi: 10.1016/j.neuroscience.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Poornima I, Brown SB, Bhashyam S, et al. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circ Heart Fail. 2008;1:153–60. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravassa S, Zudaire A, Carr RD, Diez J. Antiapoptotic effects of GLP-1 in murine HL-1 cardiomyocytes. Am J Physiol Heart Circ Physiol. 2011;300:H1361–72. doi: 10.1152/ajpheart.00885.2010. [DOI] [PubMed] [Google Scholar]
- 24.Marzioni M, Alpini G, Saccomanno S, et al. Exendin-4, a glucagon-like peptide 1 receptor agonist, protects cholangiocytes from apoptosis. Gut. 2009;58:990–97. doi: 10.1136/gut.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horiuchi S, Araki N, Morino Y. Immunochemical approach to characterize advanced glycation end products of the Maillard reaction. Evidence for the presence of a common structure. J Biol Chem. 1991;266:7329–32. [PubMed] [Google Scholar]
- 27.Han Y, Liu Y, Mi Q, et al. Pyridoxine improves platelet nitric oxide synthase dysfunction induced by advanced glycation end products in vitro. Int J Vitam Nutr Res. 2010;80:168–77. doi: 10.1024/0300-9831/a000019. [DOI] [PubMed] [Google Scholar]
- 28.Xu B, Chibber R, Ruggiero D, et al. Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. Faseb J. 2003;17:1289–91. doi: 10.1096/fj.02-0490fje. [DOI] [PubMed] [Google Scholar]
- 29.Goyal S, Kumar S, Bijjem KV, Singh M. Role of glucagon-like peptide- 1 in vascular endothelial dysfunction. Indian J Exp Biol. 2010;48:61–69. [PubMed] [Google Scholar]
- 30.Mendez JD, Xie J, Aguilar-Hernandez M, Mendez-Valenzuela V. Trends in advanced glycation end products research in diabetes mellitus and its complications. Mol Cell Biochem. 2010;341:33–41. doi: 10.1007/s11010-010-0434-5. [DOI] [PubMed] [Google Scholar]
- 31.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–64. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dejean LM, Ryu SY, Martinez-Caballero S, et al. MAC and Bcl-2 family proteins conspire in a deadly plot. Biochim Biophys Acta. 2010;1797:1231–38. doi: 10.1016/j.bbabio.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsden VS, O’Connor L, O’Reilly LA, et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 2002;419:634–37. doi: 10.1038/nature01101. [DOI] [PubMed] [Google Scholar]
- 34.Qin Z, Sun Z, Huang J, et al. Mutated recombinant human glucagon-like peptide-1 protects SH-SY5Y cells from apoptosis induced by amyloid-beta peptide (1–42) Neurosci Lett. 2008;444:217–21. doi: 10.1016/j.neulet.2008.08.047. [DOI] [PubMed] [Google Scholar]
- 35.Natalicchio A, De Stefano F, Orlando MR, et al. Exendin-4 prevents c-Jun N-terminal protein kinase activation by tumor necrosis factor-alpha (TNFalpha) and inhibits TNFalpha-induced apoptosis in insulin-secreting cells. Endocrinology. 2010;151:2019–29. doi: 10.1210/en.2009-1166. [DOI] [PubMed] [Google Scholar]
- 36.Quoyer J, Longuet C, Broca C, et al. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J Biol Chem. 2010;285:1989–2002. doi: 10.1074/jbc.M109.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]