Summary
Background
Continuous positive airway pressure (CPAP) is the most effective method for treating obstructive sleep apnea syndrome (OSAS) and alleviating symptoms. Improved sleep quality with effective CPAP therapy might also contribute to attenuated systemic inflammation and improved endothelial function, with subsequent reduction of cardiovascular risk.
The aim of this study was to assess the effect of 3-month CPAP therapy on brachial artery flow-mediated dilation (FMD) and plasma C-reactive protein (CRP) levels in patients with OSAS.
Material/Methods
Our study group consisted of 38 male patients with no prior history of cardiovascular disease. Twenty patients with an Apnea-Hypopnea Index (AHI) ≥15 were assigned to receive CPAP treatment and 18 subjects with an AHI<5 were included in the control group. Six patients failed to comply with the CPAP treatment. Measurement of FMD and blood analysis was performed at baseline and 3 months after CPAP therapy.
Results
Baseline FMD values were negatively correlated with age, BMI, AHI, DSI,% of time <90% Sa02, and CRP (p<0.05). Plasma CRP values were positively correlated with BMI, AHI, DSI and% of time <90% Sa02 (p<0.05). In the group of patients who complied with the CPAP treatment, there was a significant increase in the FMD values (9.18±0.55 vs. 6.27±0.50) and a decrease in the levels of CRP (0.67±0.15 vs. 0.84±0.18) (p<0.05).
Conclusions
Appropriate CPAP therapy improved both CRP and FMD values, suggesting its potentially beneficial role in reducing cardiovascular risk in OSAS patients.
Keywords: CPAP, sleep apnea, inflammation
Background
The obstructive sleep apnea syndrome (OSAS) is a highly prevalent sleep disorder characterized by recurrent episodes of upper airway obstruction and subsequent recurrent arousal during sleep [1]. It is estimated that up to 5% of adults in Western countries have OSAS [2].
According to published data, OSAS is an independent risk factor for hypertension and coronary artery disease [3,4]. Emerging studies suggest that the repetitive episodes of hypoxia and reoxygenation, in a manner similar to that of the ischemia/reperfusion injury model, promote the activation of proinflammatory pathways and disrupt normal endothelial function [5,6].
Brachial artery flow-mediated dilation (FMD) is a validated and widely used research tool for the quantification of endothelial function [7,8]. FMD values are diminished in patients with severe OSAS [9–11].
CRP is an acute phase protein and plays an important role in innate immunity. It is a sensitive marker of inflammation and an important marker of future cardiovascular risk [12,13]. Elevated CRP levels have been reported by several researchers in OSAS patients [14–16].
Brachial artery FMD and CRP are valuable markers of the atherogenic process in OSAS. CPAP is the most effective method for treating OSAS and alleviating symptoms [17]. We hypothesized that the proper use of CPAP should also help improve the levels of FMD and CRP and thus reduce the risk for adverse cardiovascular events.
The aim of this study was to assess the effect of 3-month CPAP therapy on FMD and plasma CRP levels in patients with OSAS.
Material and Methods
This study was conducted in the sleep laboratory of the department of clinical therapeutics at Alexandra Hospital; Athens Medical School. Informed consent was obtained from each patient and approval was also obtained for the conduct of this study from the Hospital Ethics Committee.
Male patients with suspected OSAS, receiving no medication, and known not to suffer from diabetes mellitus, arterial hypertension, dyslipidemia, or inflammatory, cardiovascular, neuromuscular or pulmonary diseases were recruited into the study.
All patients underwent full overnight polysomnography. According to the standard criteria of the American Academy of Sleep Medicine: 1) apnea was defined as a complete cessation of airflow for at least 10 seconds; 2) hypopnea was defined as a reduction in airflow of at least 50%, a <50% reduction associated with electroencephalographic arousals, or a >3% decrease in oxygen saturation; and 3) the apnea-hypopnea index (AHI) referred to the average number of apneas and hypopneas per hour of sleep. Subjects with an AHI ≥15 were assigned to receive CPAP treatment and formed our study group. Subjects with an AHI<5 were defined as not having OSAS and were included in the control group. Patients with an AHI 5–15 were not eligible for CPAP treatment and were excluded from the study analysis.
A high-resolution 12.0-MHz transducer ultrasound was used to measure brachial artery diameter at rest and during reactive hyperemia. All patients fasted for at least 8 hours prior to the measurement. The assessment was carried out in a quiet room, with a stable temperature at 22 to 24°C, by an experienced physician who was blinded to the patient’s sleep recordings and blood analysis. Reactive hyperemia was induced by inflation of a blood pressure cuff placed on the lower part of the arm and inflated to 250 mm Hg, followed by release after 5 minutes. Brachial artery diameter was measured 40 and 60 seconds after cuff deflation. Flow-mediated dilation (FMD) was calculated as the ratio of change in diameter (maximum of 2 measurements – baseline) over baseline value. Endothelial function measurements were reassessed after 3 months of CPAP therapy.
Patients’ smoking status, body mass index (BMI) (calculated as kilograms per meter squared), arterial blood pressure, and heart rate were recorded at baseline and 3 months after CPAP treatment. Non-smokers were defined as those patients who had never smoked. Furthermore blood analysis including: glucose, total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), triglycerides and CRP, was performed prior and 3 months after CPAP.
A standard statistical software package SPSS (SPSS Inc, Chicago, IL) was used in the analysis. Descriptive statistics were calculated for all variables. Difference regarding smoking status between OSA and control subjects was assessed with the chi-square test. The t test was used to detect differences in age, BMI, blood pressure, heart rate, lipidemic profile, sleep parameters, FMD and CRP values between OSA patients and control subjects. The association between baseline CRP and FMD values with age, BMI, and sleep parameters was assessed with the Pearson correlation test. Paired-samples t test was used for comparison of the patients’ FMD and CRP values at baseline and 3 months after CPAP therapy. P values less than 0.05 were considered statistically significant.
Results
A total of 48 patients were recruited during our study period. Ten patients had an AHI between 5 and 15 and were excluded from the study, 18 subjects were included in the control group (AHI<5), and 20 patients with AHI ≥15 formed the OSAS group in need of CPAP treatment.
Baseline characteristics of OSAS and control subjects are presented in Table 1. There were no significant differences between control subjects and OSAS patients with regard to the mean age, BMI, blood pressure, heart rate, lipid markers, glucose and smoking status. Compared to the controls, the OSAS group had significantly lower FMD values (6.72±0.86 vs. 9.59±1.15) and higher plasma CRP levels (0.82±0.16 vs. 0.29±0.14) (p=0.00).
Table 1.
Baseline characteristics of control subjects and patients with OSAS.
| Control (N=18) | OSAS (N=20) | P-Value | |
|---|---|---|---|
| Age(years) | 48.33±7.67 | 54.30±10.84 | 0.06 |
| Smoking(N/%) | 14 (77.80%) | 15 (75.00%) | 0.67 |
| BMI | 30.00±2.14 | 31.30±2.00 | 0.06 |
| Systolic BP(mmHg) | 126.57±10.64 | 124.35±4.84 | 0.40 |
| Diastolic BP(mmHg) | 78.03±5.86 | 77.02±5.90 | 0.60 |
| Heart rate/min | 79.57±7.83 | 77.49±8.90 | 0.45 |
| Cholesterol (mg/dl) | 204.67±47.49 | 225.50±43.52 | 0.17 |
| LDL(mg/dl) | 134.00±33.36 | 142.75±29.78 | 0.40 |
| HDL(mg/dl) | 48.17±5.09 | 51.25±9.93 | 0.23 |
| Triglycerides (mg/dl) | 145.83±64.97 | 146.80±60.50 | 0.96 |
| Glucose (mg/dl) | 97.33±9.24 | 97.10±12.89 | 0.95 |
| AHI | 2.67±1.41 | 25.35±15.05 | 0.00 |
| DSI | 1.50±0.51 | 21.80±11.28 | 0.00 |
| % of time <90% Sa02 | 0.67±0.49 | 7.40±4.62 | 0.00 |
| CRP(mg/dl) | 0.29±0.14 | 0.82±0.16 | 0.00 |
| FMD | 9.59±1.15 | 6.72±0.86 | 0.00 |
Baseline FMD values were negatively correlated with age, BMI, AHI, DSI,% of time <90% Sa02, and CRP (p<0.05). Plasma CRP values were positively correlated with BMI, AHI, DSI, and% of time <90% Sa02 (p<0.05) (Table 2). After adjustment for age and BMI, FMD values retained their correlation with AHI (B=−0.08, p=0.00), DSI (B=−0.10, p=0.00) and% of time <90% Sa02 (B=−0.28, p=0.00). After adjustment for BMI, CRP values also retained their correlation with% of time <90% SaO2 (B=0.04, p=0.00), DSI (B=0.01, p=0.00) and AHI (B=0.01, p=0.00).
Table 2.
Association between baseline CRP and FMD with age, BMI, and sleep parameters.
| FMD | CRP | ||
|---|---|---|---|
| Age | r | −0.38 | 0.16 |
| P-value | 0.02 | 0.32 | |
| BMI | r | −0.47 | 0.52 |
| P-value | 0.00 | 0.00 | |
| AHI | r | −0.81 | 0.77 |
| P-value | 0.00 | 0.00 | |
| DSI | r | −0.83 | 0.80 |
| P-value | 0.00 | 0.00 | |
| % of time <90% Sa02 | r | −0.82 | 0.77 |
| P-value | 0.00 | 0.00 | |
| CRP | r | −0.79 | 1.00 |
| P-value | 0.00 | . |
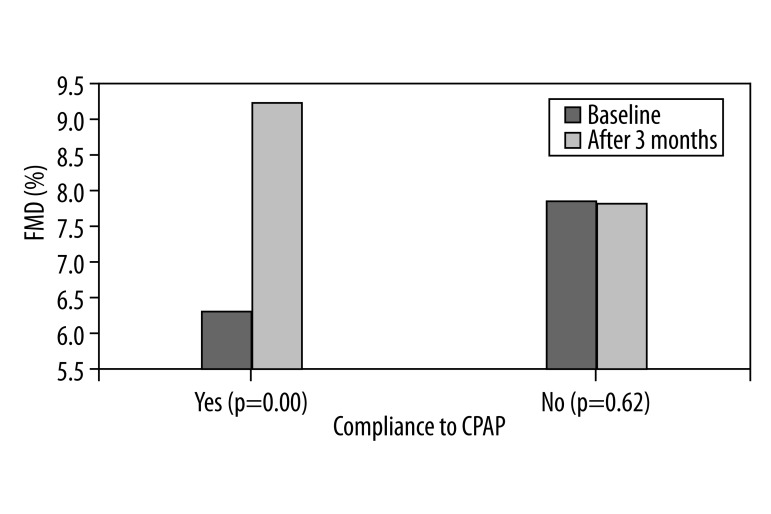
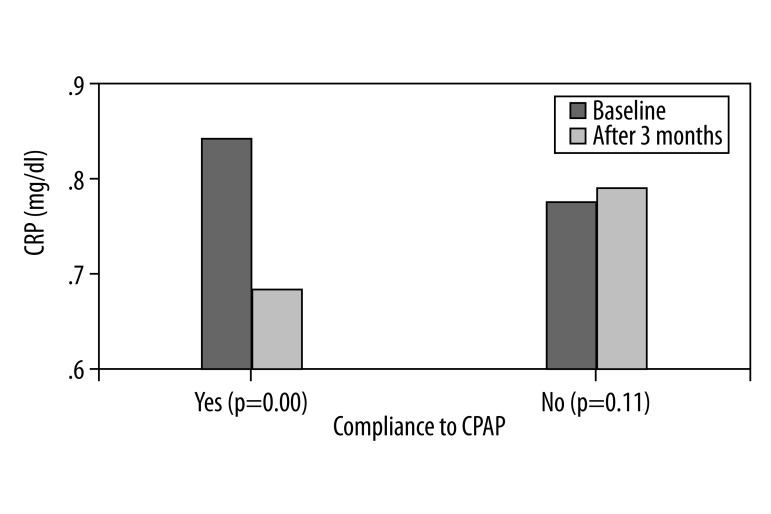
Out of 20 patients in the OSAS group, 6 failed to comply with the CPAP treatment and returned the devise within 2 weeks. During the study period, patients’ BMI and smoking status did not change. In the group of patients who complied with the CPAP treatment, there was a significant increase in the FMD values (9.18±0.55 vs. 6.27±0.50) and a decrease in the levels of CRP (0.67±0.15 vs. 0.84±0.18) (p=0.00). No changes were detected in the group of patients who failed to comply with CPAP (Figures 1, 2).
Figure 1.
Bar charts represent mean value of FMD at baseline and 3 months after in OSA patients compliant and none compliant with CPAP.
Figure 2.
Bar charts represent mean value of CRP at baseline and 3 months after in OSA patients compliant and none compliant with CPAP.
Discussion
The measurement of endothelium-dependent dilation in response to reactive hyperemia is a non-invasive and validated method for the assessment of the endothelial function [18]. Alterations in endothelium dependent dilatation have been documented in patients with coronary artery disease and diabetes and, recently, OSAS [19–22]. The regulation of vasomotor tone, as measured by the change in the forearm blood flow after transient ischemia, appears to be regulated by the availability of nitric oxide. Researchers have shown that in the OSAS the pathophysiologic stressors resulting from repetitive episodes of hypoxemia/reoxygenation downregulate the activity of the endothelial nitric oxide synthase and upregulate the expression of various vasoactive substances such as endothelin-1 and angiotensin II [23–25].
In this study, FMD values appeared to be significantly higher in the control group compared to the OSAS patients, and were negatively correlated with the AHI, DSI and% of time <90% Sa02. In accordance with our results, previous studies have also shown that endothelial dysfunction, as measured by FMD, was correlated with the severity of OSAS [9–11]. Furthermore, FMD values were negatively correlated with age. In a recent study by Yim-Yeh et al., age proved to be an independent predictor of FMD [10].
Recently, data have emerged regarding the potential beneficial effects of CPAP on endothelial function. A study by Ip et al. demonstrated that FMD was significantly improved after 4 weeks of CPAP treatment, while Bayram et al. found that the improvement in the endothelial function was sustained after 6 months of treatment in complaint patients [26,27]. Our results also confirm the beneficial role of CPAP on FMD values after 3 months of treatment.
Recently, a large amount of evidence has demonstrated the pivotal role of inflammation in cardiovascular disease. In view of this, C-reactive protein has gained increasing attention as an independent risk factor for coronary disease [28]. In patients with OSAS, circulating levels of inflammatory markers appeared to be elevated, suggesting the presence of systemic inflammation [29,30]. In agreement with previous reports, we showed that CRP levels were positively correlated with the OSAS severity [14–16]. Furthermore, CRP was correlated with BMI, an observation consistent with the ability of adipose tissue to release certain cytokines, including Interleukin-6, and to stimulate CRP synthesis [31].
Although both FMD and CRP are involved in the pathophysiology of cardiovascular complications, the association between these 2 parameters in OSAS remains elusive. Two separate studies by Chung et al. and Verma et al. failed to find a relationship between FMD and CRP in healthy subjects [32,33]. Nevertheless, a study by Nystrom et al in patients with coronary artery disease revealed a correlation between endothelial dysfunction (measured with FMD) and the levels of CRP [34]. This study demonstrated a negative correlation between CRP and FMD. These discrepancies may be attributed to differences in methodology and inclusion criteria among published studies.
This study also demonstrated that appropriate use of CPAP therapy can significantly decrease the levels of CRP. Previous studies have also presented similar results, thus denoting the possible beneficial role of CPAP in reducing systemic inflammation and cardiovascular risk in OSAS patients [35–39].
Conclusions
The limitations of this study should be noted. The study population consisted of patients with no prior history of cardiovascular or pulmonary disease, thus the study results should be interpreted with caution in patients beyond this particular group. Furthermore, female patients were excluded from the study analysis to avoid potential sex-based differences in hormone secretion that could affect both CRP and endothelial function.
This single-center study demonstrated that male OSAS patients with no prior history of cardiovascular disease had significantly higher CRP levels and lower FMD values compared to control subjects – a finding indicative of the possible presence of subclinical atherosclerosis and subsequent increased risk for developing cardiovascular disease. Appropriate CPAP therapy improved both CRP and FMD values, suggesting its potentially beneficial role in reducing cardiovascular morbidity and mortality in OSAS patients. Further well-designed prospective studies are needed to elucidate the effect of CPAP on the reduction of cardiovascular risk.
Footnotes
Source of support: Departmental sources
References
- 1.Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132:325–37. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 5.Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite mediated reperfusion injury. J Biol Chem. 1996;271:29223–30. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 6.Liao JK, Zulueta JJ, Yu FS, et al. Regulation of bovine endothelial constitutive nitric oxide synthase by oxygen. J Clin Invest. 1995;96:2661–66. doi: 10.1172/JCI118332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos-García D, Rodríguez-Yáñez M, Arias-Rivas S, Blanco M. Brachial arterial flow mediated dilation: utility in clinical and experimental practice. Rev Neurol. 2011;53:351–60. [PubMed] [Google Scholar]
- 8.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sert Kuniyoshi FH, Singh P, Gami AS, et al. Patients with obstructive sleep apnea exhibit impaired endothelial function after myocardial infarction. Chest. 2011;140:62–67. doi: 10.1378/chest.10-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yim-Yeh S, Rahangdale S, Nguyen AT, et al. Obstructive sleep apnea and aging effects on macrovascular and microcirculatory function. Sleep. 2010;33:1177–83. doi: 10.1093/sleep/33.9.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yim-Yeh S, Rahangdale S, Nguyen AT, et al. Vascular dysfunction in obstructive sleep apnea and type 2 diabetes mellitus. Obesity (Silver Spring) 2011;19:17–22. doi: 10.1038/oby.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madjid M, Willerson JT. Inflammatory markers in coronary heart disease. Br Med Bull. 2011;100:23–38. doi: 10.1093/bmb/ldr043. [DOI] [PubMed] [Google Scholar]
- 13.Rus H, Niculescu FI. Inflammation, aspirin, and the risk of cardiovascular disease. N Engl J Med. 1997;337:423. author reply 423–24. [PubMed] [Google Scholar]
- 14.Kokturk O, Ciftci TU, Mollarecep E, Ciftci B. Elevated C-reactive protein levels and increased cardiovascular risk in patients with obstructive sleep apnea syndrome. Int Heart J. 2005;46:801–9. doi: 10.1536/ihj.46.801. [DOI] [PubMed] [Google Scholar]
- 15.Firat Guven S, Turkkani MH, Ciftci B, et al. The relationship between high-sensitivity C-reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath. 2012;16:217–21. doi: 10.1007/s11325-011-0492-2. [DOI] [PubMed] [Google Scholar]
- 16.Lui MM, Lam JC, Mak HK, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135:950–56. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 17.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011;86:549–54. doi: 10.4065/mcp.2010.0810. quiz 554–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 19.Simova I, Katova T, Denchev S. Diagnostic accuracy of flow-mediated dilatation and intima-media thickness for the presence of significant coronary artery disease. J Am Soc Hypertens. 2009;3:388–94. doi: 10.1016/j.jash.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su Y, Liu XM, Sun YM, et al. Endothelial dysfunction in impaired fasting glycemia, impaired glucose tolerance, and type 2 diabetes mellitus. Am J Cardiol. 2008;102:497–98. doi: 10.1016/j.amjcard.2008.03.087. [DOI] [PubMed] [Google Scholar]
- 22.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–10. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 23.Shirai M, Pearson JT, Shimouchi A, et al. Changes in functional and histological distributions of nitric oxide synthase caused by chronic hypoxia in rat small pulmonary arteries. Br J Pharmacol. 2003;139:899–910. doi: 10.1038/sj.bjp.0705312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips BG, Narkiewicz K, Pesek CA, et al. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens. 1999;17:61–66. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- 25.Møller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens. 2003;16:274–80. doi: 10.1016/s0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- 26.Ip MSM, Tse HF, Lam B, Tsang KWT, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–53. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 27.Bayram NA, Ciftci B, Keles T, et al. Endothelial function in normotensive men with obstructive sleep apnea before and 6 months after CPAP treatment. Sleep. 2009;32:1257–63. doi: 10.1093/sleep/32.10.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–64. doi: 10.1161/01.cir.0000018948.95175.03. 2002. [DOI] [PubMed] [Google Scholar]
- 30.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:566–70. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 31.Browning LM, Krebs JD, Magee EC, et al. Circulating markers of inflammation and their link to indices of adiposity. Obes Facts. 2008;1:259–65. doi: 10.1159/000169832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung S, Yoon IY, Shin YK, et al. Endothelial dysfunction and C-reactive protein in relation with the severity of obstructive sleep apnea syndrome. Sleep. 2007;30:997–1001. doi: 10.1093/sleep/30.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma S, Wang CH, Lonn E, et al. Cross-sectional evaluation of brachial artery flow-mediated vasodilation and C-reactive protein in healthy individuals. Eur Heart J. 2004;25:1754–60. doi: 10.1016/j.ehj.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 34.Nyström T, Nygren A, Sjöholm A. Persistent endothelial dysfunction is related to elevated C-reactive protein (CRP) levels in Type II diabetic patients after acute myocardial infarction. Clin Sci (Lond) 2005;108:121–28. doi: 10.1042/CS20040243. [DOI] [PubMed] [Google Scholar]
- 35.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of Creactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 36.Schiza SE, Mermigkis C, Panagiotis P, et al. C-reactive protein evolution in obstructive sleep apnoea patients under CPAP therapy. Eur J Clin Invest. 2010;40:968–75. doi: 10.1111/j.1365-2362.2010.02348.x. [DOI] [PubMed] [Google Scholar]
- 37.Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest. 2009;136:125–29. doi: 10.1378/chest.08-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korostovtseva LS, Sviryaev YV, Zvartau NE, et al. Prognosis and cardiovascular morbidity and mortality in prospective study of hypertensive patients with obstructive sleep apnea syndrome in St Petersburg. Russia Med Sci Monit. 2011;17(3):CR146–53. doi: 10.12659/MSM.881448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zirlik S, Hauck T, Fuchs FS, et al. Leptin, obestatin and apelin levels in patients with obstructive sleep apnoea syndrome. Med Sci Monit. 2011;17(3):159–64. doi: 10.12659/MSM.881450. [DOI] [PMC free article] [PubMed] [Google Scholar]