Summary
Background
The aim of this study was to evaluate distortion product otoacoustic emissions (DPOAEs) and extended high-frequency (EHF) thresholds in a control group and in patients with normal hearing sensitivity in the conventional frequency range and reporting unilateral tinnitus.
Material/Methods
Seventy patients were enrolled in the study: 47 patients with tinnitus in the left ear (Group 1) and 23 patients with tinnitus in the right ear (Group 2). The control group included 60 otologically normal subjects with no history of pathological tinnitus. Pure-tone thresholds were measured at all standard frequencies from 0.25 to 8 kHz, and at 10, 12.5, 14, and 16 kHz. The DPOAEs were measured in the frequency range from approximately 0.5 to 9 kHz using the primary tones presented at 65/55 dB SPL.
Results
The left ears of patients in Group 1 had higher median hearing thresholds than those in the control subjects at all 4 EHFs, and lower mean DPOAE levels than those in the controls for almost all primary frequencies, but significantly lower only in the 2-kHz region. Median hearing thresholds in the right ears of patients in Group 2 were higher than those in the right ears of the control subjects in the EHF range at 12.5, 14, and 16 kHz. The mean DPOAE levels in the right ears were lower in patients from Group 2 than those in the controls for the majority of primary frequencies, but only reached statistical significance in the 8-kHz region.
Conclusions
Hearing thresholds in tinnitus ears with normal hearing sensitivity in the conventional range were higher in the EHF region than those in non-tinnitus control subjects, implying that cochlear damage in the basal region may result in the perception of tinnitus. In general, DPOAE levels in tinnitus ears were lower than those in ears of non-tinnitus subjects, suggesting that subclinical cochlear impairment in limited areas, which can be revealed by DPOAEs but not by conventional audiometry, may exist in tinnitus ears. For patients with tinnitus, DPOAE measures combined with behavioral EHF hearing thresholds may provide additional clinical information about the status of the peripheral hearing.
Keywords: tinnitus, otoacoustic emission, extended high frequency audiometry
Background
Subjective tinnitus results from an abnormal neural activity elicited at any level of the auditory pathways, and it is interpreted in the auditory cortex as a perception of sound without any physical stimulus arising in the ear canal [1]. The majority of cases of tinnitus are associated with hearing loss [2]. It has been postulated that unilateral tinnitus correlates with neural activity of the gamma band in the auditory cortex, opposite to the site of tinnitus perception [3–8]. The percentage of tinnitus patients without hearing impairment varies from 8% for individuals with pure-tone thresholds ≤20 dB HL for all standard audiometric frequencies up to 8 kHz, to about 30% for patients with average threshold at 1, 2, 4, and 6 kHz ≤25 dB HL [9]. Even though it is generally agreed that tinnitus is induced or triggered by abnormal events in the cochlea, there is little support in the literature for the perception of tinnitus being solely related to cochlear mechanisms. The Discordant Damage theory of Jastreboff provides one of several possible explanations of the existence of tinnitus in patients with normal hearing [1]. According to this theory, the presence of a limited area of damaged outer hair cells (OHCs), which may not be detected in a conventional audiogram, with intact inner hair cells, can result in unbalanced neural activity between Type I and Type II fibers. Consequently, this unbalanced activity, after being further enhanced at different stages of the auditory pathway, is perceived as tinnitus.
Since cochlear function may play an important role in the generation of tinnitus perception, the assessment of the inner ear is important for the evaluation of tinnitus patients. Cochlear function may be tested objectively and non-invasively using otoacoustic emissions (OAEs). Numerous studies on OAEs in tinnitus patients used distortion product OAEs (DPOAEs) because those can be measured over a wide range of primary frequencies (f1 and f2) and their levels (L1 and L2). The majority of DPOAE data in tinnitus patients with normal hearing indicated that, despite the similarities in the audiograms, mean DPOAE levels were in general lower in tinnitus patients than in the controls [10–15]. The generation mechanisms of DPOAEs are attributed to 2 cochlear processes: 1) a nonlinear interaction of the primary tones, mainly at the cochlear site in and around the region basal to the f2 location, and 2) a reflection of the traveling wave from the location corresponding to the distortion product frequency of 2f1–f2[16,17]. It has been postulated that the state of the basal cochlear region influences OAEs measured more apically [18–22]. For example, Dreisbach et al. [23] confirmed an earlier report by Arnold et al. [22] by showing that in subjects with normal hearing in the conventional frequency range (0.25–8 kHz), a decrease of hearing sensitivity in the extended high-frequency (EHF) region resulted in diminished DPOAEs measured at lower frequencies.
The EHF audiometric data for tinnitus patients are limited [24]. A recent study by Sanches et al. [25] included adults with hearing thresholds ≤25 dB HL at frequencies from 0.25 to 8 kHz, and the EHF testing covered the frequency range up to 20 kHz. A control group comprised subjects ranging in age from 22 to 40 years. The tinnitus group included patients up to 56 years of age with either bilateral or unilateral tinnitus; results for both ears of each of those subjects were included in the tinnitus group data. The DPOAE growth functions were measured at the f2 frequencies of 2 and 4 kHz. The EHF results revealed higher hearing thresholds in the tinnitus groups than in the control group at all frequencies tested. The slope of the DPOAE growth function for f2=4 kHz was more shallow for the tinnitus group than for the controls.
The mechanism of tinnitus generation in patients with normal audiograms in the conventional region (up to 8 kHz) remains unclear. One can hypothesize that possible causes of tinnitus in those patients may be linked to the cochlear impairment in the most basal region, which is routinely not tested by the EHF audiometry, and/or to subclinical OHC damage corresponding to the conventional frequency region. The aim of the current study was to evaluate DPOAEs and EHF thresholds in a control group and in patients with normal hearing sensitivity in the conventional frequency range and reporting unilateral tinnitus of greater than 6-months duration. Inclusion criteria regarding the age and hearing sensitivity of the subjects and the laterality of tinnitus were rigorously defined to limit potential confounding factors.
Material and Methods
Subjects
Our study enrolled 70 patients, whose main complaint was tinnitus, attending audiology clinics of the Institute of Physiology and Pathology of Hearing in Warsaw, ranging in age from 14 to 40 years. Data were collected for subjects who had normal otoscopic inspection, normal results of immittance audiometry, hearing thresholds ≤20 dB HL from 0.25 to 8 kHz and ≤65 dB HL in the EHF region (up to 16 kHz), no hyperacusis, and reported constant unilateral tinnitus of greater than 6-months duration. The Minimum Masking Level (MML) for white noise stimuli was established for each patient to confirm the lateralization of tinnitus [9]. We excluded patients who began to hear tinnitus in the opposite ear when it was masked on one side, because those patients were believed to have bilateral, but asymmetrical (louder on one side), rather than unilateral, tinnitus. There were 47 patients with tinnitus in the left ear (Group 1) and 23 patients with tinnitus in the right ear (Group 2). The estimation of tinnitus pitch was performed for all 70 tinnitus patients (Groups 1 and 2) using a two-alternative forced-choice procedure [27]. The vast majority of the patients (63 out of 70) matched their tinnitus to a tone or a narrow-band noise with frequency above 3 kHz. Among those 63 individuals, the tinnitus pitch was matched above 8 kHz in 48 patients, and the most common frequency matched was around 12.5 kHz. This finding prompted the inclusion of EHF audiometry in the study protocol.
Sixty healthy and otologically normal subjects, ranging in age from 14 to 40 years, were included in the control group. They were recruited mainly from the personnel of the Institute in Warsaw and their families. Five medical students also participated. The control subjects had no history of hearing problems, excessive noise exposure, or of recurrent otitis media in childhood. Those individuals reported that they had never experienced tinnitus lasting longer than 5 min, and thus had no history of pathological tinnitus according to generally accepted tinnitus classification [26]. The audiometric inclusion criteria for control subjects were the same as those for the tinnitus patients. Each participant signed a consent form prior to the enrollment in the study. Descriptive statistics of the subjects’ groups is presented in Table 1.
Table 1.
Descriptive statistics of the subjects enrolled in the study.
| Number of subjects | Number of females | Number of males | Age: Mean (s.d.) in years | |
|---|---|---|---|---|
| Group 1 (LE tinnitus) | 47 | 24 | 23 | 28.1 (7.4) |
| Group 1 (RE tinnitus) | 23 | 16 | 7 | 29.3 (7.4) |
| Controls | 60 | 32 | 28 | 28.6 (7.5) |
Measurements
Hearing threshold measurements were performed in a sound-treated booth using the Madsen Orbiter 922 audiometer equipped with circumaural earphones (Sennheiser HDA 200) and calibrated according to the manufacturer’s guidelines and the ISO standard for the EHF range [28,29]. Pure-tone thresholds were measured at all standard frequencies from 0.25 to 8 kHz, and at 10, 12.5, 14, and 16 kHz. Immittance testing was done using the Madsen Zodiac 901 middle ear analyzer. The Madsen Celesta 503 system was used to record DPOAEs while subjects sat comfortably in a sound-treated booth. The levels of the primary tones L1 and L2 were set at 65 and 55 dB SPL, respectively. The f2/f1 ratio was kept at approximately 1.2. The DPOAEs were measured at 9 2f1–f2 frequencies, with the f2 frequency decreasing from approximately 9 to 0.5 kHz with the steps of 2 points per octave. The spectral analysis of the microphone signal was based on 128 averages for primary frequencies below 1 kHz and on 64 averages for higher frequencies. The system provided an estimate of the background noise based on the average of spectral components in the vicinity of the 2f1–f2. A valid DPOAE data point measurement required at least 3 dB signal-to-noise ratio (SNR) and a DPOAE level above −15 dB SPL.
Statistics
Normality test (Kolmogorov-Smirnov) performed for audiometric data within each group of subjects failed for almost all test frequencies, indicating that the results varied significantly from the pattern of normal distribution. Therefore, those results were analyzed using the non-parametric Mann-Whitney test. The median audiometric thresholds of each group at 10, 12.5, 14, and 16 kHz were compared. A comparison of mean DPOAEs between groups was done using the t test. All statistical analyses were performed using SigmaPlot (version 12.0), Systat Software, Inc., with the significance level set at p≤0.05. A Bonferroni adjustment of critical p-values was applied both for EHF audiometric data and DPOAE levels due to performing multiple comparisons.
Results
Group 1 (Left ear tinnitus) versus the control group
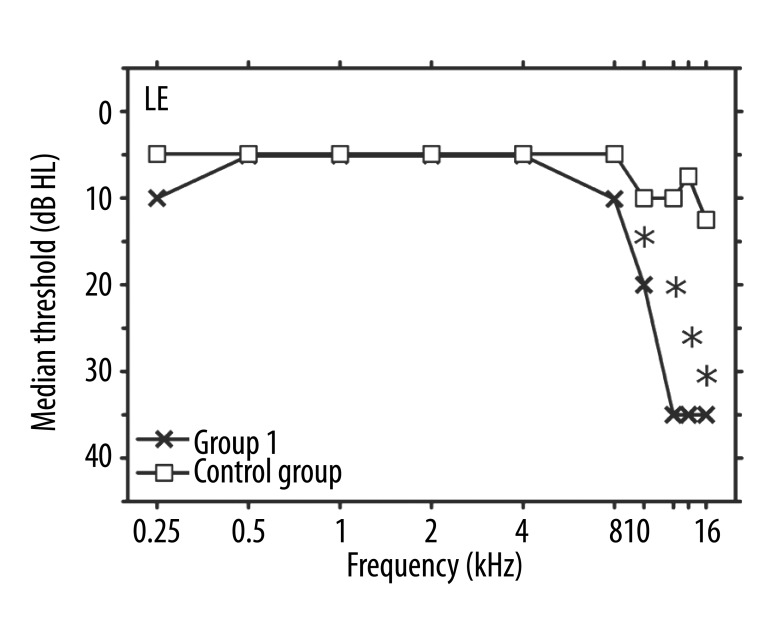
A comparison of audiometric data for Group 1 and the controls revealed that the median thresholds in the left ears of tinnitus patients were higher than those in the control subjects at all 4 EHFs (p<0.001). No statistical differences were found in the conventional frequency range (from 0.25 to 8 kHz), and the median values of the 2 groups were exactly the same for frequencies between 0.5 and 4 kHz (Figure 1).
Figure 1.
Median values of hearing thresholds (in dB HL) vs. frequency (in kHz) in left ears. Crosses: Group 1 (unilateral tinnitus in LE); squares: the control group. Asterisks indicate significant differences between the median values for the two groups.
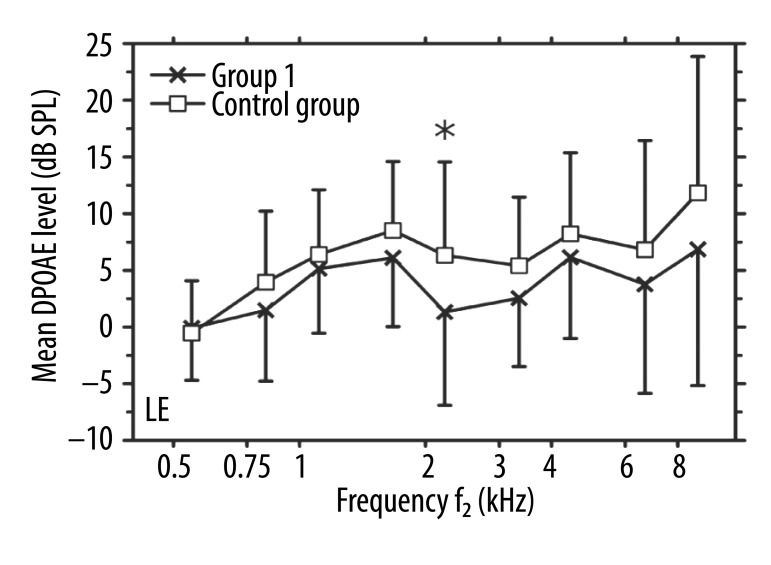
The analyses of individual DPOAE results led to excluding several data points with SNRs below the required criterion of at least 3 dB. This was especially the case for the lowest primary frequencies (around 500 Hz) due to an increase of the background noise in that region. Specifically, in 26 out of 47 ears of Group 1 and in 24 out of 60 ears of the control group, DPOAE levels exhibited SNR <3 dB in the 500-Hz region. For other primaries, the number of DPOAE data points excluded from further analyses was smaller than 5 or 2 per group for Group 1 and the controls, respectively. The mean DPOAE levels in the left ears were lower in patients from Group 1 than those in the controls for all primary frequencies, except for the lowest pair (Figure 2). The difference between the group mean values varied from 1.3 to 5.1 dB, but reached statistical significance only for 1 primaries in the 2-kHz region (p=0.016).
Figure 2.
Mean DPOAE levels (in dB SPL) vs. f2 frequency (in kHz) in left ears. Crosses: Group 1 (unilateral tinnitus in LE); squares: the control group. Error bars indicate one standard deviation from the means. An asterisk indicates a significant difference between the mean values for the two groups.
Group 2 (Right ear tinnitus) versus the control group
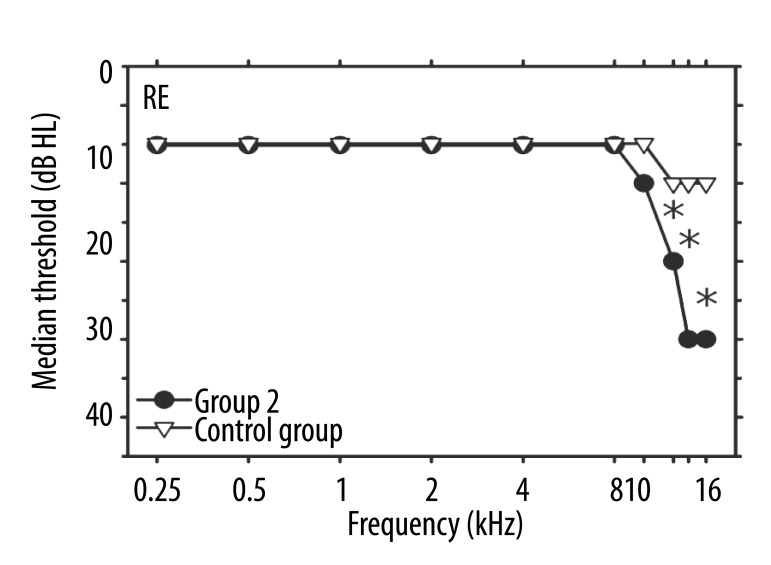
Median hearing thresholds in the right ears of patients in Group 2 were higher than those in the right ears of the control subjects in the EHF range, and showed significant differences at 12.5 kHz (p=0.008) and at 14 and 16 kHz with p<0.001 (Figure 3). In the conventional frequency range, the median values of the 2 groups were exactly the same. An additional analysis compared the hearing thresholds in ears with tinnitus (the left ears of Group 1 and the right ears of Group 2) and showed no differences between the median hearing sensitivity data in those cases (Figures 1, 3).
Figure 3.
Median values of hearing thresholds (in dB HL) vs. frequency (in kHz) in right ears. Circles: Group 2 (unilateral tinnitus in RE); triangles: the control group. Asterisks indicate significant differences between the median values for the two groups.
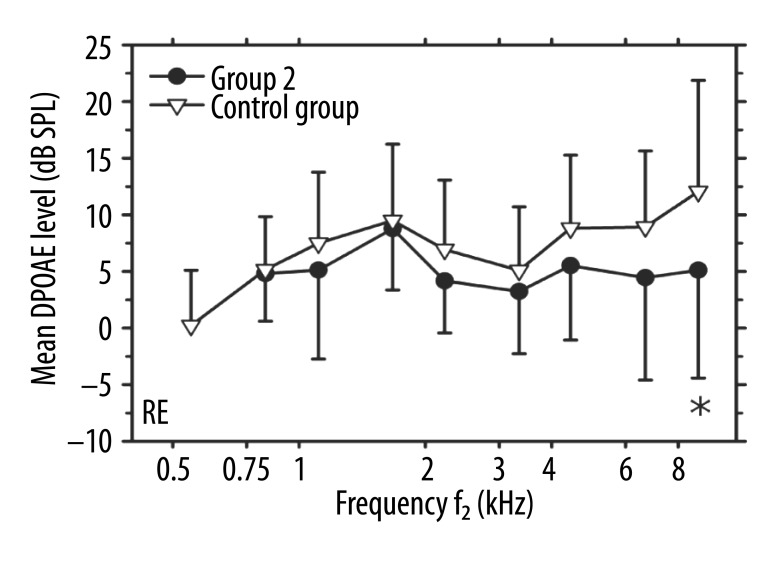
Only 8 out of 23 right ears of Group 2 exhibited DPOAEs with SNR ≥3 dB in the 500-Hz region. Those data failed the normality test and therefore were excluded from further analysis. For other primaries, at least 22 ears fulfilled the DPOAE inclusion criteria. The mean DPOAE levels in the right ears were lower in patients from Group 2 than those in the controls for the primary frequencies above 2 kHz and around 1.1 kHz (Figure 4). At those frequencies, the difference between the group mean values varied from 1.8 to 7.0 dB, but reached statistical significance just for 1 primaries in the 8-kHz region (p=0.048). The analysis comparing the DPOAE levels in ears with tinnitus (the left ears of Group 1 and the right ears of Group 2) showed no differences between the mean values in those cases (Figures 2, 4).
Figure 4.
Mean DPOAE levels (in dB SPL) vs. f2 frequency (in kHz) in right ears. Circles: Group 2 (unilateral tinnitus in RE); triangles: the control group. Error bars indicate one standard deviation from the means. An asterisk indicates a significant difference between the mean values for the two groups.
Discussion
Numerous hypotheses regarding mechanisms of tinnitus generation have been suggested [2]. Due to the heterogeneity observed across the population of tinnitus patients, it is agreed that no single model or hypothesis may explain the presence of tinnitus in all patients. Moreover, multiple mechanisms may be present in a single individual with tinnitus. Generally, sensorineural hearing loss is ascribed a major role as an initiating event that triggers neurophysiological processes, which are perceived as tinnitus. On the other hand, it is also known that some tinnitus patients do not show any hearing loss in the audiogram, which is routinely measured using pure-tones with frequencies up to 8 kHz. Such patients were included in the current study. Moreover, 2 groups of patients were formed based on the laterality of their tinnitus to exclude those potential participants who may have bilateral, but asymmetrical tinnitus. The aim of the study was to explore several potential hypotheses of tinnitus origin in normally hearing patients. The first concept is related to limitations of the audiogram, which alone does not always indicate peripheral damage. This hypothesis is supported by animal models. For example, studies in chinchillas showed that damage to ≈20% of OHCs was not detected in the behavioral threshold measures [30]. Also, a study of Kujawa and Liberman [31] in noise-exposed mice supported the possibility of the presence of a sub-clinical hearing loss with normal hearing thresholds. However, peripheral neurodegeneration can lead to changes in brainstem circuitry and cortical reorganization. These changes may contribute to post-exposure perceptual anomalies, including tinnitus. A recent study by Schaette and McAlpine [32] found that patients with tinnitus and normal audiograms up to 8 kHz might present a sub-clinical hearing loss, manifested by significantly reduced amplitude of the Auditory Brainstem Response Wave I. A proposed computational model implied that tinnitus in these patients can arise from a homeostatic response of neurons in the central auditory system to a reduced auditory nerve input in the absence of elevated hearing thresholds.
The main finding of the current study was that median hearing thresholds in tinnitus ears (left ears in Group 1 and right ears in Group 2) were higher than those in non-tinnitus control subjects at 12.5, 14 and 16 kHz, and also at 10 kHz in Group 1 (Figures 1, 3). Those data support a hypothesis that cochlear damage in the basal region may provoke a series of changes along the auditory pathway, resulting in the perception of tinnitus. Similar findings were recently reported by Sanches et al. [26]. The vast majority of tinnitus patients report high-frequency locus of their tinnitus. Such a notion is likely related to a high-frequency hearing loss and is supported by the results of the current study. Almost 70% of the patients matched the pitch of their tinnitus above 8 kHz, and many of them to the 12-kHz region (the region exhibiting elevated hearing thresholds in tinnitus patients) when compared to the control subjects (Figures 1, 3).
The comparison between left and right ears regarding the prevalence of tinnitus has been reported in many studies [27,33]. In general, the percentage of left-sided tinnitus is greater than right-sided, similarly to the data of the current study. The differences in the anatomical structures and physiology of the right and left central nervous system could suggest a cause [34]. For example, the left ear seems to be more susceptible to a wide-range of cochlear insults such as noise [35] and ototoxic drugs [36].
The second finding of the study was that, in general, the mean DPOAE levels in tinnitus ears (the left ears in Group 1 and the right ears in Group 2) were lower than those in the controls (Figures 2, 4). However, differences between the mean values of each tinnitus group when compared to the controls reached statistical significance just for 1 pair of primaries per subjects’ group. Cochlear regions are independent of each other and cannot be treated as sources of OAE generation. Therefore, a conservative statistical analysis of DPOAE data was used by applying the Bonferroni correction, which decreased the p-value for the group of 9 comparisons (9 DPOAE data points were collected for each subject) by a factor of 9. Consequently, several notable between-group differences were marked as non-significant.
There are several possible explanations for a general trend of decreased DPOAEs in tinnitus ears than in the controls. The results of several previous studies suggest that the basal region of the cochlea may to some extent contribute to DPOAEs measured at lower frequencies [22,23]. The EHF audiometric data in the tinnitus ears (Figures 1, 3) may indicate the OHC impairment in the most basal region, and thus resulting in reduced contribution to more apically generated DPOAEs. In addition, it has been hypothesized that OAEs are more sensitive to OHC dysfunction than traditional behavioral measures. The sensitivity of DPOAEs may depend to a large extent on the magnitude and pattern of OHC loss. Thus, for OAEs to be more sensitive than audiometry in measurement of OHC function, there may need to be small regions of normal OHCs that can mediate the behavioral task scattered throughout regions of significant OHC loss. Harding et al. [38] reported that DPOAEs in chinchillas detected accumulated OHC losses that were greater than 10% over a broad region, while damage to OHCs of ≈20% might not be detected in the behavioral threshold measures [30]. Therefore, it may be postulated that limited areas of OHC impairments existed in the cochleae of tinnitus patients included in Groups 1 and 2. Such sub-clinical pathologies, which may potentially be revealed by even slightly reduced DPOAEs (Figures 2, 4), but not by conventional audiometry (up to 8 kHz), can contribute to tinnitus generation in those ears. Moreover, it could be hypothesized that OHC impairment (indicated by DPOAE reduction) may be present together with other subclinical pathologies, such as loss of normally high-threshold spiral ganglion cells, resulting in tinnitus.
Conclusions
The data of this study showed that patients with normal hearing sensitivity in the conventional frequency range (up to 8 kHz), and reporting unilateral tinnitus, present alterations of the auditory system in the affected side, manifested as elevated EHF thresholds. In addition, DPOAE levels in tinnitus ears were lower than those in ears of non-tinnitus subjects, even though the differences in mean DPOAE levels were non-significant for the majority of the data points. Alternative DPOAE protocols (eg, using lower level primaries and smaller frequency steps between successive primary pairs) may potentially enhance the separation between tinnitus and non-tinnitus ears of normally-hearing patients. Hearing loss in the EHF region may potentially be a predictor of already existing sub-clinically reduced OHC function in more apical regions resulting in tinnitus. For patients with tinnitus, DPOAE measures at conventional frequencies, combined with behavioral EHF hearing thresholds, may provide additional clinical information about the status of the peripheral hearing.
Acknowledgements
The authors would like to thank Maria Custer MS, who assisted with the submission of the revised manuscript.
List of acronyms and abbreviations
- OAE
otoacoustic emission
- DPOAE
distortion product otoacoustic emission
- EHF
extended high frequency
- OHC
outer hair cell
Footnotes
Source of support: Departmental sources
References
- 1.Jastreboff PJ. Tinnitus as a phantom perception: theories and clinical implications. In: Vernon JA, Møller AR, editors. Mechanisms of Tinnitus. London: Allyn and Bacon; 1995. pp. 73–94. [Google Scholar]
- 2.Baguley DM. Mechanisms of tinnitus. Br Med Bull. 2002;63:195–212. doi: 10.1093/bmb/63.1.195. [DOI] [PubMed] [Google Scholar]
- 3.Llinás RR, Ribary U, Jeanmonod D, et al. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96:15222–27. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Loo E, Gais S, Congedo M, et al. Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS One. 2009;9(4):e7396. doi: 10.1371/journal.pone.0007396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisz N, Müller S, Schlee W, Dohrmann K, et al. The neural code of auditory phantom perception. J Neurosci. 2007;27:1479–84. doi: 10.1523/JNEUROSCI.3711-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giraud AL, Chéry-Croze S, Fischer G, et al. A selective imaging of tinnitus. Neuroreport. 1999;10:1–5. doi: 10.1097/00001756-199901180-00001. [DOI] [PubMed] [Google Scholar]
- 7.Lockwood AH, Salvi RJ, Coad ML, et al. The functional neuroanatomy of tinnitus: evidence of limbic system links and neural plasticity. Neurology. 1998;50:114–20. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- 8.Smits M, Kovacs S, de Ridder D, et al. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology. 2007;49:669–79. doi: 10.1007/s00234-007-0231-3. [DOI] [PubMed] [Google Scholar]
- 9.Vernon JA, Meikle MB. Tinnitus masking. In: Tyler R, editor. Tinnitus Handbook. New York: Thomson Learning; 2000. pp. 313–56. [Google Scholar]
- 10.Ami M, Abdullah A, Awang MA, et al. Relation of distortion product otoacoustic emission with tinnitus. Laryngoscope. 2008;118:712–17. doi: 10.1097/MLG.0b013e318161e521. [DOI] [PubMed] [Google Scholar]
- 11.Bartnik G, Hawley ML, Rogowski M, et al. Distortion product otoacoustic emission levels and Input/Output-Growth functions in normal-hearing individuals with tinnitus and/or hyperacusis. Semin Hear. 2007;28:303–18. [Google Scholar]
- 12.Granjeiro RC, Kehrle HM, Bezerra RL, et al. Transient and distortion product evoked oto-acoustic emissions in normal hearing patients with and without tinnitus. Otolaryngol Head Neck Surg. 2008;138:502–6. doi: 10.1016/j.otohns.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Onishi ET, Fukuda Y, Suzuki FA. Distortion product otoacoustic emissions in tinnitus patients. Int Tinnitus J. 2004;10:13–16. [PubMed] [Google Scholar]
- 14.Ozimek E, Wicher A, Szyfter W, Szymiec E. Distortion product otoacoustic emission (DPOAE) in tinnitus patients. J Acoust Soc Am. 2006;119:527–38. doi: 10.1121/1.2141297. [DOI] [PubMed] [Google Scholar]
- 15.Paglialonga A, Del Bo L, Ravazzani P, Tognola G. Quantitative analysis of cochlear active mechanisms in tinnitus subjects with normal hearing sensitivity: multiparametric recording of evoked otoacoustic emissions and contralateral suppression. Auris Nasus Larynx. 2010;37:291–98. doi: 10.1016/j.anl.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Kalluri R, Shera CA. Distortion-product source unmixing: a test of the two-mechanism model for DPOAE generation. J Acoust Soc Am. 2001;109:622–37. doi: 10.1121/1.1334597. [DOI] [PubMed] [Google Scholar]
- 17.Withnell RH, Lodde J. In search of basal distortion product generators. J Acoust Soc Am. 2006;120:2116–23. doi: 10.1121/1.2338291. [DOI] [PubMed] [Google Scholar]
- 18.Avan P, Bonfils P, Loth D, et al. Quantitative assessment of human cochlear function by evoked otoacoustic emissions. Hear Res. 1991;52:99–112. doi: 10.1016/0378-5955(91)90191-b. [DOI] [PubMed] [Google Scholar]
- 19.Avan P, Bonfils P, Loth D, et al. Transient-evoked otoacoustic emissions and high-frequency acoustic trauma in the guinea pig. J Acoust Soc Am. 1995;97:3012–20. doi: 10.1121/1.411866. [DOI] [PubMed] [Google Scholar]
- 20.Withnell RH, Yates GK, Kirk DL. Changes to low-frequency components of the TEOAE following acoustic trauma to the base of the cochlea. Hear Res. 2000;139:1–12. doi: 10.1016/s0378-5955(99)00132-x. [DOI] [PubMed] [Google Scholar]
- 21.Avan P, Elbez M, Bonfils P. Click-evoked otoacoustic emissions and the influence of high-frequency hearing losses in humans. J Acoust Soc Am. 1997;101:2771–77. doi: 10.1121/1.418564. [DOI] [PubMed] [Google Scholar]
- 22.Arnold DJ, Lonsbury-Martin BL, Martin GK. High-frequency hearing influences lower-frequency distortion-product otoacoustic emissions. Arch Otolaryngol Head Neck Surg. 1999;125:215–22. doi: 10.1001/archotol.125.2.215. [DOI] [PubMed] [Google Scholar]
- 23.Dreisbach LE, Torre P, III, Kramer SJ, et al. Influence of ultrahigh-frequency hearing thresholds on distortion-product otoacoustic emission levels at conventional frequencies. J Am Acad Audiol. 2008;19:325–36. doi: 10.3766/jaaa.19.4.5. [DOI] [PubMed] [Google Scholar]
- 24.Barnea G, Attias J, Gold S, Shahar A. Tinnitus with normal hearing sensitivity: extended high-frequency audiometry and auditory-nerve brain-stem-evoked responses. Audiology. 1990;29:36–45. doi: 10.3109/00206099009081644. [DOI] [PubMed] [Google Scholar]
- 25.Sanches SGG, Sanchez TG, Carvallo RMM. Influence of cochlear function on auditory temporal resolution in tinnitus patients. Audiol Neurotol. 2010;15:273–81. doi: 10.1159/000272939. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell CR, Vernon JA, Creedon TA. Measuring tinnitus parameters: Loudness, pitch and maskability. J Am Acad Audiol. 1993;4:139–51. [PubMed] [Google Scholar]
- 27.Davis A, Rafaie EA. Epidemiology of tinnitus. In: Tyler R, editor. Tinnitus Handbook. New York: Thomson Learning; 2000. pp. 1–23. [Google Scholar]
- 28.International Organization for Standardization. Part 5: Reference equivalent threshold sound pressure levels for pure tones in the frequency range 8 kHz to 16 kHz. Geneva: ISO; 1998. Acoustics. Reference zero for the calibration of audiometric equipment. ISO/TR 389-5. [Google Scholar]
- 29.Schmuziger N, Probst R, Smurzynski J. Test-retest reliability of pure-tone thresholds from 0.5 to 16 kHz using Sennheiser HDA 200 and Etymotic Research ER-2 earphones. Ear Hear. 2004;25:127–32. doi: 10.1097/01.aud.0000120361.87401.c8. [DOI] [PubMed] [Google Scholar]
- 30.Clark WW, Kim DO, Zurek PM, Bohne BA. Spontaneous otoacoustic emissions in chinchilla ear canals: correlation with histopathology and suppression by external tones. Hear Res. 1984;16:299–314. doi: 10.1016/0378-5955(84)90119-9. [DOI] [PubMed] [Google Scholar]
- 31.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaette R, McAlpine D. Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31:13452–57. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meikle M, Taylor-Walsh E. Characteristics of tinnitus and related observations in over 1800 tinnitus clinic patients. J Laryngol Otol Suppl. 1984;9:17–21. doi: 10.1017/s1755146300090053. [DOI] [PubMed] [Google Scholar]
- 34.Kannan PM, Lipscomb DM. Bilateral hearing asymmetry in a large population. J Acoust Soc Am. 1974;55:1092–94. doi: 10.1121/1.1914657. [DOI] [PubMed] [Google Scholar]
- 35.Nageris BI, Raveh E, Zilberberg M, Attias J. Asymmetry in noise-induced hearing loss: relevance of acoustic reflex and left or right handedness. Otol Neurotol. 2007;28:434–37. doi: 10.1097/mao.0b013e3180430191. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt CM, Knief A, Lagosch AK, et al. Left-right asymmetry in hearing loss following cisplatin therapy in children – the left ear is slightly but significantly more affected. Ear Hear. 2008;29:830–37. doi: 10.1097/AUD.0b013e31818005a4. [DOI] [PubMed] [Google Scholar]
- 37.Harding GW, Bohne BA, Ahmad M. DPOAE level shifts and ABR threshold shifts compared to detailed analysis of histopathological damage from noise. Hear Res. 2002;174:158–71. doi: 10.1016/s0378-5955(02)00653-6. [DOI] [PubMed] [Google Scholar]