Summary
Sedentary activity is a modifiable life-style behavior and a key component in the etiology of atherosclerotic cardiovascular disease (ACVD). US adults and children spend more than half their waking time in sedentary pursuits. Sedentary activity has been shown to result in impaired insulin sensitivity, impaired metabolic function and attenuated endothelial function, which are classic markers of ACVD. Sedentary activity is defined as ‘sitting without otherwise being active.’ This behavior promotes reduced muscular activity of the lower extremities which decreases leg blood flow, increases blood pooling in the calf, augments mean arterial pressure, and deforms arterial segments resulting in low mean shear stress (SS). SS activates distinct physiological mechanisms which have been proposed to be protective against ACVD; specifically through a SS-induced endothelium-derived nitric oxide mechanism. Reduced bioavailability of nitric oxide creates a pro-oxidant milieu resulting in increased oxidative stress. There is sufficient evidence which demonstrates that endothelial function is attenuated in the presence of oxidative stress. Sedentary activity results in low SS in the lower extremities which may result in increased oxidative stress and impaired endothelial function. This review furthers the use of sitting as model to study the effects of inactivity, discusses possible physiological mechanisms and suggests future directions.
Keywords: nitric oxide, oxidative stress, shear rate, sitting
Background
Life-style factors are significant components in the etiology of atherosclerotic cardiovascular disease (ACVD), malignant neoplasms, and cerebrovascular disease which are the leading causes of death in this country [1,2]. In fact, it is estimated that 50% of deaths attributable to ACVD are preventable through life-style modification [2]. Diet and physical inactivity are only second to tobacco in life-style contributors for all-cause mortality [2]. Along these lines, physical activity is used as a primary intervention to help prevent and treat these diseases [3]. However, adults and children in the US spend ~55% of their waking time in sedentary pursuits [4]; which may be a greater contributing factor to ACVD than the actual lack of physical activity (PA) [5].
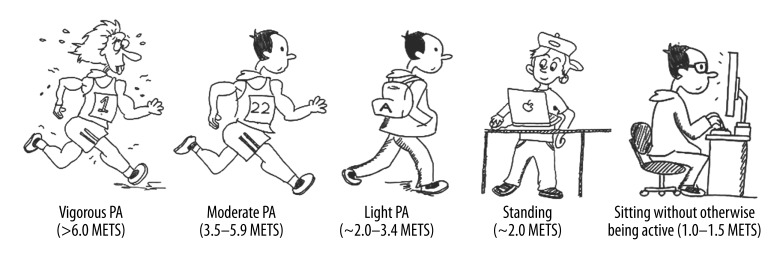
PA can be expressed as a continuum with one end being vigorous intensity PA and the opposite being sitting without otherwise being active (Figure 1). The PA continuum also represents a spectrum of muscular contraction intensity and frequency, and thus energy expenditure. Typically, physical inactivity has been used as an umbrella term for the lower end of the continuum. Sedentary activity can be thought of as the farthest point of the PA continuum. It is critical to differentiate between physical inactivity and sedentary activity when physiological research questions are considered at the lowest end of the continuum. Sedentary comes from the Latin word Seder which means “to sit.” As discussed by Owen and colleagues (6), increased sitting is very different from a lack of exercise. In theory, a person can be sitting while performing arm or leg exercises or movements, which would increase energy expenditure (e.g., weight lifting, rowing, cycling). The definition of sedentary activity that seems most appropriate to use while conducting physiological research is ‘sitting without being otherwise active,’ a definition which has been previously used by Owen and colleagues [7]. Examples of sedentary activities are sitting at work, sitting in an automobile, or other activities that range between 1 to 1.5 METs [8]. In this context, sedentary activity is relatively easy to simulate in the laboratory and may represent the best inactivity model due to its simplicity and practical application. This review will address the considerations for physical inactivity as it relates to ACVD, and discuss how different experimental models of physical inactivity affect endothelial function. Sedentary activity, as defined above, is a very common form of inactivity as time spent watching television and performing computer-related activities have increased [4]. Sitting as a model introduces distinctly different physiological mechanisms (e.g. low shear stress, bent artery system, hydrostatic load, pooling of blood) when compared to traditional physical inactivity models. The use of sedentary activity models in the laboratory may also limit time dependent physiological adaptations which take place as a result of traditional chronic models (e.g. spinal cord injury, bed rest, etc.). To that extent this paper will further the use of sitting as a model of inactivity and propose how sitting influences hemodynamic alterations (specifically low shear stress) within the vasculature.
Figure 1.
Continuum of physical activity.
Endothelial Dysfunction in ACVD
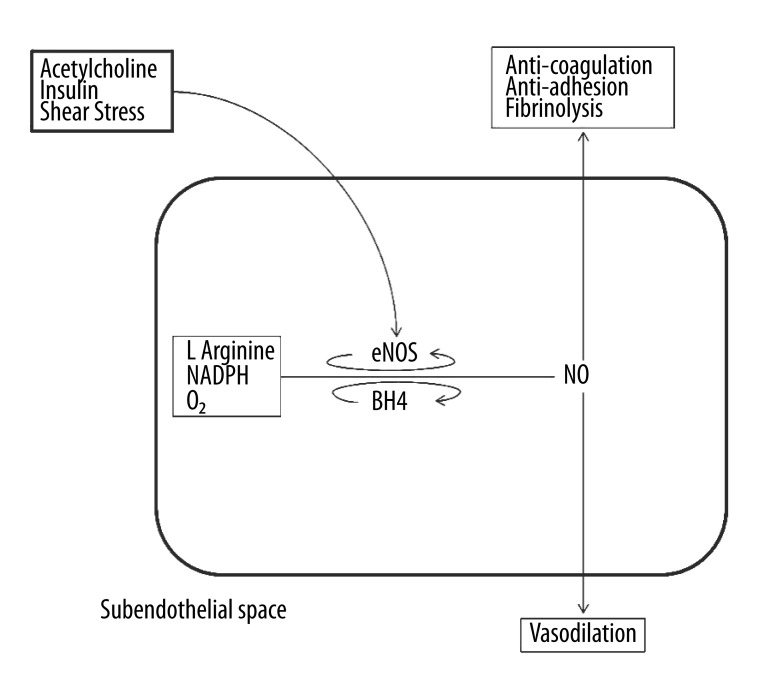
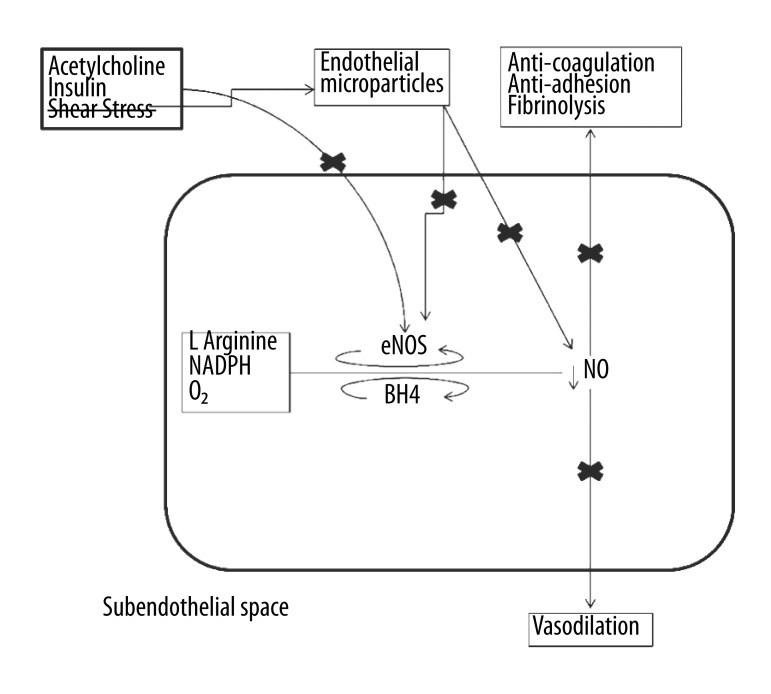
The endothelium is a single layer of cells lining nearly all of the vascular system and it performs anti-atherogenic functions; such as anti-coagulation, fibrinolysis, anti-inflammation, anti-adhesion, and regulates permeability as well as vasomotor control [9]. One of the primary etiologies of endothelial dysfunction is an imbalance between pro- and anti-oxidants thereby producing oxidative stress, which is the primary etiology in cardiovascular disease [10]. When the endothelium is compromised or its functions are attenuated, its anti-atherogenic protective activities are diminished, thus promoting atherosclerosis [11]. Nitric oxide is the key to endothelial function, involved with all the anti-atherogenic properties of the endothelium [12–15]. Both chemical and physical factors stimulate endothelium-derived nitric oxide production (16). Figure 2A describes how nitric oxide is produced in an endothelial cell. The synthesis of nitric oxide from L-arginine, molecular oxygen, and NADPH is catalyzed by the phosphorylation of endothelial nitric oxide synthase (eNOS); and dependent on other cofactors (tetrahydrobiopterin, flavin adenine dinucleotide & flavin mononucleotide). Shear stress from arterial blood flow, insulin, and agonists (i.e. acetylcholine (ACh)) initiate pathways to phosphorylate eNOS. Both insulin and shear stress work through a calcium-independent signaling pathway comprising phosphatidylinositol-3-kinase (PI-3 kinase); whereas ACh works through a calcium-dependent pathway. Figure 2B describes how low or turbulent shear stress directly diminishes the production of nitric oxide through decreased eNOS phosphorylation and indirectly lowers nitric oxide bioavailability via the production of endothelial microparticles (small vesicles released from apoptotic or activated endothelial cells). The lack of nitric oxide bioavailability and the resulting oxidative stress create a pro-atherogenic environment in the endothelium resulting in attenuated endothelial function.
Figure 2A.
Normal functioning of the shear stress, acetylcholine and insulin mediated NO mechanism in and around the endothelial cell. In the presence of laminar shear stress, insulin and acetylcholine, nitric oxide is produced from L-Arginine. Nitric oxide functions as an anti-oxidant molecule. It also performs the functions of anti-coagulation, anti-adhesion, anti-fibrinolysis and vasodilation in the endothelial environment
Figure 2B.
Low, oscillatory or turbulent shear stress results in endothelial injury and release of endothelial microparticles. Altered shear stress directly and indirectly impairs the nitric oxide bioavailability resulting in oxidative stress and decline in anti-atherosclerotic functions thus resulting in endothelial dysfunction and pro-atherosclerotic environment. Crossed out ‘shear stress’ implies non-laminar shear stress. ‘X’ marks represent decline in or blocking of a particular function.
Endothelial function can be assessed using various methods. The common methods used in activity restriction experiments include flow mediated dilation (FMD) [17], venous occlusion plethysmography [18], and iontophoresis [19]. FMD is a non-invasive procedure using high-resolution ultrasound and flow Doppler technology to image both conduit artery diameter and blood velocity. The FMD response is primarily dependent on endothelium-derived nitric oxide and based on the principle that augmented shear stress along the endothelium elicits vasodilation (20). Venous occlusion plethysmography, regarded as the gold standard for the assessment of vascular function, [21] uses a strain gauge to measure the change in limb volume while arterial inflow is maintained and venous return is contained within the limb. Similar to FMD, this technique is also primarily mediated by nitric oxide [22]. Iontophoresis involves delivering vasodilator substances, such as acetylcholine, across the skin using a weak electrical current which increases perfusion of the skin microvasculature. The increase in perfusion is measured by laser Doppler. This technique has been shown to correlate well with other measures of endothelial function [23].
Endothelial function and activity restriction
Both insulin sensitivity [24] and increased blood flow [25,26] are factors that promote a healthy endothelium and increase nitric oxide (Figure 2A). Insulin sensitivity has been the variable of interest in physical inactivity studies [17,27,28]; with a common decrease in insulin sensitivity. The role of blood flow (or shear stress) has also been addressed in models of physical inactivity [29–32]. It has primarily been observed in relation to arterial remodeling and vascular adaptations in models which are chronic states of physical inactivity (e.g. spinal cord injury) which permit time for structural and functional vascular adaptations [29,31,33].
In the intact organism, PA increases blood flow to various tissues in the body. Shear stress is the resulting tangential force due to blood flow across the endothelium and is essential for the release of vasoactive substances (i.e. nitric oxide), gene expression, cell morphology, and cell metabolism [34]. Shear stress also preserves endothelial cell stability and prevents apoptosis [25], maintains endothelial integrity, and prevents cell proliferation [35]. Correspondingly, a reduction in blood flow or insulin sensitivity reduces nitric oxide bioavailability and attenuates endothelial function, thus creating a pro-atherogenic environment.
Experimental Physical Inactivity Models in the Study of Endothelial Function
The experimental physical inactivity models utilized in human studies examining activity restriction and its effects on endothelial function in the laboratory setting are summarized in Table 1. The summary is organized by experimental model and provides a list of variables investigated as well as the results. The most common experimental models include step counts, bed rest, dry water immersion, and sitting. Below, we discuss these studies, specifically those focused on outcome variables which directly or indirectly influence endothelial function. We choose not to discuss head low bed rest and spinal cord injury inactivity models because they cause distinct physiological changes (e.g. reduced plasma volume, reduced or absent sympathetic drive etc.) [36,37] which may not be directly applicable to apparently healthy individuals under normal circumstances.
Table 1.
Different typed of in vivo models for studying physical inactivity.
| Authors and year | Inactivity model | Outcome measures | Results |
|---|---|---|---|
| Demiot, Dignat-George et al. 2007 | 56 days of bed rest | Endothelial dependent vasodilation of the microcirculation using iontophoresis | Endothelial dependent vasodilation of the microcirculation decreased and number of circulating endothelial cells significantly increased |
| Hamburg, McMackin et al. 2007 | 5 days bed rest, 30 minutes per day out of bed allowed | Glucose tolerance, vascular function, inflammatory markers, total cholesterol, triglycerides, reactive hyperemia, and blood pressure, | 67% increase in insulin response to glucose loading-> insulin resistance (HPMA-IR and ISI0,120), high total cholesterol and triglycerides. Decreased reactive hyperemia in forearm and calf, decreased brachial artery diameter and increased systolic blood pressure. No change in inflammatory markers |
| Hitosugi, Niwa et al. 2000 | 2 hours of quiet sitting, at 22–24°C, 40–50% humidity | Blood rheologic changes | Blood viscosity, blood count, blood chemistry. Increased thrombosis tendency in the leg (hematocrit from leg vein). No systemic changes. |
| Homans 1954 | 14 hour flight, automobile drive; case studies of individual patients | Case studies | Deep vein thrombosis in the calf |
| Krogh-Madsen, Thyfault et al. 2010 | Decreasing steps from ~10000 day−1 to less than 1500−1 day for two weeks | Insulin resistance, VO2max, inflammatory markers, muscle mass | 17% reduction in glucose infusion rate during the hyperinsulinemic-euglycemic clamp, peripheral insulin resistance,7% decrease in VO2max, significant decrease in lower extremity lean mass, no change in inflammatory markers like TNF alpha, IL-6, etc. |
| Navasiolava, Dignat-George et al. 2010 | 7 days of dry water immersion (+ 2 days pre ambulatory control and 2 days recovery | Endothelial function | Decreased endothelium dependent vasodilation at the skin level and increased circulating endothelial micro particles. |
| Olsen, Krogh-Madsen et al. 2008 |
Sub group 1: Decreasing steps 6203 to 1394 day−1 Sub group 2: Decreasing steps 10501 to 1344 day−1 – one week |
Step count, fat mass, oral glucose tolerance test, oral fat tolerance test, | Reduced step count, 7% increase in abdominal fat mass, attenuated Insulin sensitivity and post prandial lipid |
| Shvartz, Gaume et al. 1983 |
Group A: 5 hr. quite sitting preceded by 30 min of recumbency and 20 min standing Group B: 70 min sitting preceded by recumbency only |
Hemodynamic variables in the lower extremity | Calf blood pooling, decreased thigh blood flow during sitting, at 1 hr. responses similar in A and B. In group A, 5 hr. sitting significantly increased calf pooling (17%) and decreased calf blood flow (13%), increase in diastolic and mean arterial pressure. |
Experimental model of physical inactivity: Step counts
Pedometers and accelerometers are common instruments used to quantify the number of steps taken over the course of a given time frame. Tudor-Locke and colleagues [38] have classified 10,000–12,499 steps·day−1 as ‘active’ and <5000 steps·day−1 as ‘sedentary’. In a restricted step count study, Krogh-Madsen and colleagues [27] instructed subjects to decrease their steps from ~10,000 steps·day−1 to below 1,500 steps·day−1 for 2 weeks. As a result, they found significant decreases in insulin stimulated Akt phosphorylation and peripheral insulin sensitivity, a decrease in maximal oxygen consumption, and a decrease in lean leg mass. Similarly, Olsen and colleagues [28] asked apparently healthy adults to reduce their steps from 6,000 steps·day−1 to <1,400 steps·day−1 for two weeks and found decreased insulin sensitivity, attenuated post-prandial lipid metabolism, and a 7% increase in abdominal fat measured by a magnetic resonance scanner [28]. Although the aforementioned studies did not directly measure endothelial function, it is widely accepted that decreased insulin sensitivity results in endothelial dysfunction [39].
Experimental model of physical inactivity: Bed rest
Bed rest has been the conventional model for physical inactivity. In addition to replicating physical inactivity, [17] it has been utilized to observe alerted gravitational states [40], as well as for the study of cardiovascular [41] and skeletal [42] physiological responses to physical inactivity. Hamburg and colleagues subjected 20 adults to 5 days of complete bed rest [17]. Following the intervention, significant increases in LDL cholesterol, blood pressure, blood glucose, insulin resistance, and a significant decrease in microvascular function measured by venous occlusion plethysmography were observed. Conduit artery function (brachial artery FMD) was also assessed; however, it was not found to be impaired following bed rest. These results indicate that 5 days of bed rest may preferentially affect the microvasculature while conduit artery function remains unaltered or that the traditional measurement of FMD was not sensitive to the vascular changes [17]. Although, the authors postulated that insulin resistance is the primary mechanism responsible for the decline in vascular function, they also suggested that low vascular shear stress may have contributed to the decreased microvascular function [17].
Arterial function in the arms is the most common site for assessing endothelial function. However, the brachial artery may not be the optimal site to measure endothelial function following sedentary activity interventions because the lower limbs are primarily the inactive limbs during these interventions. In most protocols [17,18], subjects were allowed to use their arms during bed rest which may have assisted in the preservation of brachial artery FMD. Due to periodic arm movements, the brachial artery may not accurately reflect the local condition of decreased blood flow in the legs during bed rest [43]. In a bed rest study, Sonne and colleagues had first degree relatives of type 2 diabetics, subjects with low birth weight, and healthy controls undergo 10 days of bed rest with 15 minutes of activity for personal hygiene allowed each day [18]. Subjects were asked to refrain from tilting the upper body more than 60° throughout the intervention. Controls and low birth weight subjects experienced a reduction in insulin-mediated vasodilation in the forearm. However, endothelium-dependent vasodilation in the brachial artery remained unaffected after bed-rest in all groups. The authors concluded this may have occurred because arm movements were not controlled during the study, or because the inactivity stimulus was insufficient [18]. Bed rest has also been shown to increase circulating endothelial cells in eight healthy adults [44] following a 13–52 day protocol. It was speculated that low shear stress during bed rest initiated endothelial cell apoptosis which augmented circulating endothelial cells. In this context, the endothelium has been identified as an ideal site for treatment during and following prolonged physical inactivity [44]. FMD responses following bed rest and deconditioning have been somewhat counterintuitive. An augmented FMD response has been observed after a 25–52 day bed rest intervention [31] which had typically been attributed to the structural and functional adaptations of the vasculature (e.g. decreased conduit artery diameter, increased reactivity to nitric oxide, increased endothelium independent vasodilation and FMD) [29,31]. There have been several studies on bed ridden hospital patients and apparently healthy individuals which have found that bed rest results in insulin resistance [45–47] by decreasing skeletal muscle energy expenditure [48]. It is logical to speculate that endothelial function decreased as a result of augmented insulin resistance following these interventions.
The bed rest model appears to be a viable method for studying the effects of physical inactivity on vascular function; however careful consideration should be taken into account when interpreting changes in local endothelial function. Albeit, the vast majority of working adults sit during the work day and sit during leisure time [49]; thereby making bed rest models less applicable to the general sedentary population. Regardless of the model used, it is critical that a sensitive and accurate method specific to that model be used for assessing endothelial function following physical inactivity.
Experimental model of physical inactivity: Dry water immersion
In dry water immersion, subjects are submerged in thermoneutral water but kept dry by a thin, elastic, waterproof film which separates the subjects from contact with the water. The film is thin and large enough to ensure that the water’s hydrostatic pressure is equally distributed throughout the surface of the body and the subject appears to be freely suspended in water [19]. Navasiolava and colleagues studied the effect of 7 days of dry water immersion on endothelial integrity and function [19]. Following the intervention, calf blood flow and endothelium-dependent vasodilation at the skin-level were significantly diminished following the intervention. In addition, plasma vascular endothelial growth factor (a potent angiogenic cytokine) was significantly lower and endothelium derived microparticles were significantly greater compared to baseline. Despite the inability to measure shear stress during the intervention, Navasiolava and colleagues hypothesized that low shear stress may be a possible mechanism which caused the observed endothelial damage [19]. Interestingly, endothelium derived microparticles appear attenuate endothelial function [16] by augmenting oxidative stress [50] following periods of inactivity.
Experimental model of physical inactivity: Sitting
Physiological and hemodynamic effects of sitting have been observed by several investigators [43,51–53]. Padilla and colleagues had subjects sit in the upright position for three hours and measured popliteal artery shear stress during the intervention and popliteal artery FMD following the intervention [51]. When compared to the baseline supine position, it only took 30 minutes of sitting to decrease mean, maximum, and minimum shear. However, FMD after the three hour intervention remained unchanged suggesting that the popliteal artery is not affected by these shear patterns or the stimulus was not sufficient enough to observe a change in popliteal artery FMD. Shvartz and colleagues had their subjects sit on a chair with a standard seat cushion and without arm-rests or lumbar support [52]. The thigh-torso angle was adjusted to 104° for each subject and feet were placed on simulated paddles so as to replicate an operator’s seat in a truck or a van. Using this model, Shvartz and colleagues concluded that at least 60 minutes of sitting is necessary to observe an increase in calf blood pooling and mean arterial pressure and a decrease in calf blood flow [53]. Newcomer et al. (2008) showed that the shear rate in the femoral artery was significantly lower in sitting and standing as compared to the supine position; and femoral artery shear rate was lower than the brachial artery across all postures [43]. Sitting may also limit the time dependent structural and functional adaptations (e.g. decreased conduit artery diameter, increased reactivity to nitric oxide, increased endothelium independent vasodilation and FMD) that have been shown with other models of inactivity [31,54] thereby enabling evaluation of the acute impact of sedentary activity. In a review of physical inactivity models and adaptations, Thijssen and colleagues [29] concluded that mean blood pressure does not change in inactivity models. Conversely, Padilla and colleagues [51] and Shvartz and colleagues [52] have shown that short duration sitting increased mean arterial blood pressure. Thus sitting seems to create a distinct physiological milieu as compared to other models. Given these changes and the amount of time the population spends in the seated position, it appears logical to use this model in further studies examining the physiological effects of sedentary activity.
Discussion
A vast majority of the literature utilizing chronic physical inactivity models have investigated insulin-related and blood flow related mechanisms, which have been shown to alter vascular function [17,27–29,31]. However, our team, as well as other investigators [43,51–53], believe that hemodynamic responses including low blood flow due to sitting warrants further attention based on the prominent role of shear stress in the pathogenesis of ACVD [55].
The nature and magnitude of shear stress influences the structure of the vessel and function of the endothelial cells [34]. Areas of high shear stress (>15 dynes/cm3) have preserved endothelial function and are relatively protected from atherosclerosis; whereas arterial segments with low shear stress (<4 dynes/cm3) are exposed to the pathology of the disease [55]. There is also a strong correlation between areas of low shear stress (i.e. arterial branch points) and endothelial dysfunction [56]. Thus, low mean shear stress has been identified as one of the etiologies of atherosclerosis and cardiovascular disease. Shear stress associated with exercise appears to augment the bioavailability of nitric oxide [15], which is important for the prevention of atherosclerosis [16]. Exercise episodically increases shear stress and subsequently improves endothelial function [57]. However, the increase in shear stress appears to be ephemeral [59] and it seems logical that long bouts of sedentary activity maintain a state of low shear stress which prohibits an increase in endothelial function. Indeed, after only 30 minutes of sitting, antegrade shear is reduced [51] and following only one hour of sitting, blood pools in the leg (53), thigh blood flow decreases [53], and blood viscosity increases [59]. In this context, repeated sedentary activity appears to expose to the endothelium to a pro-atherogenic milieu, whereas repeated bouts of activity interrupt the harmful hemodynamic environment associated with sedentary activity.
Today, most jobs and leisure time activities involve hours of continuous sitting [4,60]. The underlying nature of sitting does not promote muscular contractions, augmented energy expenditure, or increased blood flow. Sitting also changes the angle at which major arteries (femoral and popliteal) run; as compared to a standing or supine posture. Bends within the arterial tree alter flow patterns which have been shown to affect the atherosclerotic process [61,62]. Due to the predominantly seated posture during sedentary activity, turbulent blood flow might be augmented in deformed arterial segments of the lower extremities [56]. The turbulent flow may also be an underlying mechanism for the prevalence of atherosclerosis in the femoro-popliteal arterial segment [63]. Additionally, shear rate (estimate of shear stress without accounting for blood viscosity) is lower in the femoral artery versus the brachial artery in the supine, standing, and seated positions [43]. It could be hypothesized that repeated sedentary activity presents a chronic stimuli in the lower extremity which promotes the development of atherosclerosis. In the seated posture, blood pools in the leg, and both peripheral resistance and blood pressure in the leg increase [52,53,64]. As previously mentioned, Padilla and colleagues [51] observed changes in popliteal artery shear following 30 minutes of sitting; however, these changes were not detected in the post-intervention popliteal artery FMD, even after normalizing FMD to the shear stimulus. These data indicate that three hours of sitting provides an insufficient stimulus to evoke changes in popliteal artery function assessed by FMD or that the popliteal artery does not respond to this type of an intervention. It may also be possible that since they repositioned their subjects prone for evaluating FMD, the stimulus was lost. However, it is clear from the above observations that sitting upright causes low mean shear stress in the legs as compared to the supine position [43,51], which over time may influence endothelial function.
Here, we provide evidence for why we think low mean shear stress, attributable to sedentary activity, plays a key role in affecting the pathogenesis of ACVD. Based on previously discussed literature, our hypothesis is that low mean shear stress due to sedentary activity causes an elevation of oxidative stress which promotes atherogenesis. Low shear stress can lead to decreased endothelial nitric oxide synthase expression [55] which leads to decreased bioavailability of nitric oxide and oxidative stress (Figure 2B). Along these lines, sedentary mice have been shown to have an increased superoxide production [65]. In this study, inactivity promoted NADPH oxidase activity leading to increased oxidative stress [66]. Evidence indicates that oscillatory shear stress creates a pro-oxidant environment in the endothelial cell [66,67]. Although oxidative stress has been shown to be increased in other physical inactivity models [68,69], sitting may present a different physiological challenge. Muscle contractions increase local blood flow [70] and shear stress [71] and may negate the harmful effects of prolonged sitting. Recently, Szostak and Laurant have also proposed that low shear stress associated with inactivity increases oxidative stress in humans [72]. We further their hypothesis by specifically postulating that sitting induces low mean shear stress which elevates oxidative stress and promotes atherosclerosis.
Conclusions
Several models of physical inactivity have been used in research laboratory settings to identify possible mechanisms which may be associated with ACVD. Of these models, sitting represents the most applied experimental model of inactivity because of the abundant number of people who engage in prolonged sitting at work or during leisure time. Sitting replicates the exact “activity” commonly found in inactivity; nothing has to be simulated. Sitting appears to influence endothelial function due to multiple hemodynamic changes. In particular, sitting alters shear stress which predisposes the lower limb vasculature to increased oxidative stress, endothelial dysfunction, and subsequently atherosclerosis.
Future directions
Currently, there is no scientific literature on the dose response relationship between sitting time and endothelial function. It is possible that repeated bouts of sitting and the associated low mean shear stress predispose the lower extremity to atherosclerosis. Various studies where sedentary activity is compared to sitting and contracting lower extremity muscles (e.g., sitting on a therapy ball, ankle exercises, etc.), could be conducted to determine if elevating blood flow to the legs during sitting lowers oxidative stress and improves local endothelial function. Investigating the role of prolonged and repeated sedentary activity on oxidative stress might be a key link for the treatment and prevention of ACVD. Appropriate models should be used to study the dose response of sitting on low shear stress, oxidative stress, local and systemic endothelial function. Sedentary activity is an independent cardiovascular risk factor and more human studies need to be conducted to explore its pathological effects which influence the progression of ACVD so that accurate public health guidelines can be established.
Acknowledgements
The authors thank Ning Ding for assistance with the figures.
Footnotes
Conflict of interest
The authors report no conflict of interest.
Source of support: Self financing
Sources of funding
No funding was received. BDJ is supported by the Mayo Foundation.
References
- 1.Heron M. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. 8. Vol. 59. National Vital Statistics System; 2011. Deaths: Leading causes for 2007; pp. 1–96. [PubMed] [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.Balady GJ, Williams MA, Ades PA, et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update. A scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115:2675–82. doi: 10.1161/CIRCULATIONAHA.106.180945. [DOI] [PubMed] [Google Scholar]
- 4.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Healy GN, Dunstan DW, Salmon J, et al. Television time and continuous metabolic risk in physically active adults. Med Sci Sports Exerc. 2008;40(4):639–45. doi: 10.1249/MSS.0b013e3181607421. [DOI] [PubMed] [Google Scholar]
- 6.Owen N, Healy G, Matthews C, Dunstan D. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105–13. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen N, Sugiyama T, Eakin EE, et al. Adults’ Sedentary Behavior: Determinants and Interventions. Am J Prev Med. 2011;41(2):189–96. doi: 10.1016/j.amepre.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 9.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(Suppl 231):III-27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 10.Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73(3):411–18. doi: 10.1253/circj.cj-08-1102. [DOI] [PubMed] [Google Scholar]
- 11.Sitia S, Tomasoni L, Atzeni F, et al. From endothelial dysfunction to atherosclerosis. Autoimmunity Reviews. 2010;9(12):830–34. doi: 10.1016/j.autrev.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Holtz J, Förstermann U, Pohl U, et al. Flow-dependent, endothelium-mediated dilation of epicardial coronary arteries in conscious dogs: effects of cyclooxygenase inhibition. J Cardiovasc Pharmacol. 1984;6(6):1161–69. [PubMed] [Google Scholar]
- 13.Wang J, Wolin MS, Hintze TH. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circulation Research. 1993;73(5):829–38. doi: 10.1161/01.res.73.5.829. [DOI] [PubMed] [Google Scholar]
- 14.Hambrecht R, Adams V, Erbs S, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107(25):3152–58. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 15.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium derived nitric oxide function in humans. J Physiol. 2004;561(1):1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallance P, Chan N. Endothelial function and nitric oxide: clinical relevance. Heart. 2001;85(3):342–50. doi: 10.1136/heart.85.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamburg NM, McMackin CJ, Huang AL, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007;27(12):2650–56. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonne MP, Højbjerre L, Alibegovic AC, et al. Endothelial function after 10 days of bed rest in individuals at risk for type 2 diabetes and cardiovascular disease. Exp Physiol. 2011;96(10):1000–9. doi: 10.1113/expphysiol.2011.058511. [DOI] [PubMed] [Google Scholar]
- 19.Navasiolava NM, Dignat-George F, Sabatier F, et al. Enforced physical inactivity increases endothelial microparticle levels in healthy volunteers. Am J Physiol Heart Circ Physiol. 2010;299(2):H248–56. doi: 10.1152/ajpheart.00152.2010. [DOI] [PubMed] [Google Scholar]
- 20.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol. 2001;52(6):631–46. doi: 10.1046/j.1365-2125.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blitzer ML, Lee SD, Creager MA. Endothelium-derived nitric oxide mediates hypoxic vasodilation of resistance vessels in humans. Am J Physiol Heart Circ Physiol. 1996;271(3):H1182–85. doi: 10.1152/ajpheart.1996.271.3.H1182. [DOI] [PubMed] [Google Scholar]
- 23.Turner J, Belch JJF, Khan F. Current concepts in assessment of microvascular endothelial function using laser Doppler imaging and iontophoresis. Trends Cardiovasc Med. 2008;18(4):109–16. doi: 10.1016/j.tcm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Serne EH, Stehouwer CDA, ter Maaten JC, et al. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation. 1999;99(7):896–902. doi: 10.1161/01.cir.99.7.896. [DOI] [PubMed] [Google Scholar]
- 25.Davies PF. Flow-mediated endothelial mechanotransduction. Physiological Reviews. 1995;75(3):519–60. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gokce N, Keaney J, Jr, Vita J. Endotheliopathies: clinical manifestations of endothelial dysfunction. Thrombosis and Hemorrhage. 1998:901–24. [Google Scholar]
- 27.Krogh-Madsen R, Thyfault JP, Broholm C, et al. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol. 2010;108(5):1034–40. doi: 10.1152/japplphysiol.00977.2009. [DOI] [PubMed] [Google Scholar]
- 28.Olsen RH, Krogh-Madsen R, Thomsen C, et al. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA. 2008;299(11):12613. doi: 10.1001/jama.299.11.1259. [DOI] [PubMed] [Google Scholar]
- 29.Thijssen DHJ, Green DJ, Hopman MTE. Blood vessel remodeling and physical inactivity in humans. J Appl Physiol. 2011;111(6):1836–45. doi: 10.1152/japplphysiol.00394.2011. [DOI] [PubMed] [Google Scholar]
- 30.Thijssen DHJ, Maiorana AJ, O’Driscoll G, et al. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol. 2010;108(5):845–75. doi: 10.1007/s00421-009-1260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bleeker MWP, De Groot PCE, Rongen GA, et al. Vascular adaptation to deconditioning and the effect of an exercise countermeasure: results of the Berlin Bed Rest study. J Appl Physiol. 2005;99(4):1293–300. doi: 10.1152/japplphysiol.00118.2005. [DOI] [PubMed] [Google Scholar]
- 32.de Groot PCE, van Kuppevelt DHJM, Pons C, Snoek G, et al. Time course of arterial vascular adaptations to inactivity and paralyses in humans. Med Sci Sports Exerc. 2003;35(12):1977–85. doi: 10.1249/01.MSS.0000099088.21547.67. [DOI] [PubMed] [Google Scholar]
- 33.de Groot PCE, Poelkens F, Kooijman M, Hopman MTE. Preserved flow-mediated dilation in the inactive legs of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol. 2004;287(1):H374–80. doi: 10.1152/ajpheart.00958.2003. [DOI] [PubMed] [Google Scholar]
- 34.Davies PF, Dewey CF, Jr, Bussolari SR, et al. Influence of hemodynamic forces on vascular endothelial function. In vitro studies of shear stress and pinocytosis in bovine aortic cells. J Clin Invest. 1984;73(4):1121–29. doi: 10.1172/JCI111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levesque M, Nerem R, Sprague E. Vascular endottielial cell proliferation in culture and the influence of flow. Biomaterials. 1990;11(9):702–7. doi: 10.1016/0142-9612(90)90031-k. [DOI] [PubMed] [Google Scholar]
- 36.Claydon VE, Krassioukov AV. Clinical correlates of frequency analyses of cardiovascular control after spinal cord injury. Am J Physiol Heart Circ Physiol. 2008;294(2):H668–78. doi: 10.1152/ajpheart.00869.2007. [DOI] [PubMed] [Google Scholar]
- 37.Platts SH, Martin DS, Stenger MB, et al. Cardiovascular adaptations to long-duration head-down bed rest. Aviat Apace Environ Med. 2009;80(Suppl 1):A29–36. doi: 10.3357/asem.br03.2009. [DOI] [PubMed] [Google Scholar]
- 38.Tudor-Locke C, Bassett JDR. How many steps/day are enough?: Preliminary pedometer indices for public health. Sports Medicine. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 39.Arcaro G, Cretti A, Balzano S, et al. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105(5):576–82. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]
- 40.Pavy-Le Traon A, Heer M, Narici MV, et al. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986–2006) Eur J Appl Physiol. 2007;101(2):143–94. doi: 10.1007/s00421-007-0474-z. [DOI] [PubMed] [Google Scholar]
- 41.Takenaka K, Suzuki Y, Kawakubo K, et al. Cardiovascular effects of 20 days bed rest in healthy young subjects. Acta Physiol Scand Suppl. 1994;616:59–63. [PubMed] [Google Scholar]
- 42.Leblanc AD, Schneider VS, Evans HJ, et al. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990;5(8):843–50. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 43.Newcomer S, Sauder C, Kuipers N, et al. Effects of posture on shear rates in human brachial and superficial femoral arteries. Am J Physiol Heart Circ Physiol. 2008;294(4):H1833–39. doi: 10.1152/ajpheart.01108.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demiot C, Dignat-George F, Fortrat JO, et al. WISE 2005: chronic bed rest impairs microcirculatory endothelium in women. Am J Physiol Heart Circ Physiol. 2007;293(5):H3159–64. doi: 10.1152/ajpheart.00591.2007. [DOI] [PubMed] [Google Scholar]
- 45.Yanagibori R, Suzuki Y, Kawakubo K, et al. Carbohydrate and lipid metabolism after 20 days of bed rest. Acta Physiol Scand Suppl. 1994;616:51–57. [PubMed] [Google Scholar]
- 46.Stuart CA, Shangraw RE, Prince MJ, et al. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism. 1988;37(8):802–6. doi: 10.1016/0026-0495(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 47.Nygren J, Thorell A, Efendic S, et al. Site of insulin resistance after surgery: the contribution of hypocaloric nutrition and bed rest. Clin Sci (Lond) 1997;93(2):137–46. doi: 10.1042/cs0930137. [DOI] [PubMed] [Google Scholar]
- 48.Thyfault JP, Krogh-Madsen R. Metabolic disruptions induced by reduced ambulatory activity in free living humans. J Appli Physiol. 2011;111(4):1218–24. doi: 10.1152/japplphysiol.00478.2011. [DOI] [PubMed] [Google Scholar]
- 49.Tudor-Locke C, Leonardi C, Johnson WD, Katzmarzyk PT. Time Spent in Physical Activity and Sedentary Behaviors on the Working Day: The American Time Use Survey. J Occup Environ Med. 2011;53(12):1382–87. doi: 10.1097/JOM.0b013e31823c1402. [DOI] [PubMed] [Google Scholar]
- 50.Brodsky SV, Zhang F, Nasjletti A, Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol. 2004;286(5):H1910–15. doi: 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- 51.Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol. 2009;297(3):H1103–8. doi: 10.1152/ajpheart.00167.2009. [DOI] [PubMed] [Google Scholar]
- 52.Shvartz E, Reibold R, White R, Gaume J. Hemodynamic responses in orthostasis following 5 hours of sitting. Aviat Space Environ Med. 1982;53(3):226–31. [PubMed] [Google Scholar]
- 53.Shvartz E, Gaume J, White R, Reibold R. Hemodynamic responses during prolonged sitting. J App Physiol. 1983;54(6):1673–80. doi: 10.1152/jappl.1983.54.6.1673. [DOI] [PubMed] [Google Scholar]
- 54.de Groot PCE, Bleeker MWP, Hopman MTE. Magnitude and time course of arterial vascular adaptations to inactivity in humans. Exerc Sport Sci Rev. 2006;34(2):65–71. doi: 10.1249/00003677-200604000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282(21):2035–42. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 56.Ku DN. Blood flow in arteries. Ann Rev Fluid Mech. 1997;29(1):399–434. [Google Scholar]
- 57.Tinken TM, Thijssen DHJ, Hopkins N, et al. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55(2):312–18. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 58.Johnson BD, Wallace JP. A comparison of postexercise shear rate patterns following different intensities and durations of running in healthy men. Clin Physiol Funct Imaging. 2012;32(3):234–40. doi: 10.1111/j.1475-097X.2011.01116.x. [DOI] [PubMed] [Google Scholar]
- 59.Hitosugi M, Niwa M, Takatsu A. Rheologic changes in venous blood during prolonged sitting. Thromb Res. 2000;100(5):409–12. doi: 10.1016/s0049-3848(00)00348-0. [DOI] [PubMed] [Google Scholar]
- 60.Church TS, Thomas DM, Tudor-Locke C, et al. Trends over 5 decades in US occupation-related physical activity and their associations with obesity. PLoS One. 2011;6(5):e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liepsch D. An introduction to biofluid mechanics – basic models and applications. J Biomech. 2002;35(4):415–35. doi: 10.1016/s0021-9290(01)00185-3. [DOI] [PubMed] [Google Scholar]
- 62.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arterioscler Thromb Vasc Biol. 1985;5(3):293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 63.Taylor G, Calo A. Atherosclerosis of arteries of lower limbs. BMJ. 1962;1(5277):507–10. doi: 10.1136/bmj.1.5277.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pekarski S. A gravitational hypothesis of essential hypertension as a natural adaptation to increased gravitational stress caused by regular, prolonged sitting typical of modern life. Med Sci Monit. 2004;10(6):HY27–32. [PubMed] [Google Scholar]
- 65.Laufs U, Wassmann S, Czech T, et al. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25(4):809–14. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 66.De Keulenaer GW, Chappell DC, Ishizaka N, et al. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82(10):1094–101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 67.Slager C, Wentzel J, Gijsen F, et al. The role of shear stress in the generation of rupture-prone vulnerable plaques. Nat Clin Pract Cardiovasc Med. 2005;2(8):401–7. doi: 10.1038/ncpcardio0274. [DOI] [PubMed] [Google Scholar]
- 68.Dalla Libera L, Ravara B, Gobbo V, et al. A transient antioxidant stress response accompanies the onset of disuse atrophy in human skeletal muscle. J App Physiol. 2009;107(2):549–57. doi: 10.1152/japplphysiol.00280.2009. [DOI] [PubMed] [Google Scholar]
- 69.Margaritis I, Rousseau A, Marini J, Chopard A. Does antioxidant system adaptive response alleviate related oxidative damage with long term bed rest? Clin Biochem. 2009;42(4–5):371–79. doi: 10.1016/j.clinbiochem.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 70.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97(1):393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- 71.Prior BM, Yang H, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol. 2004;97(3):1119–28. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 72.Szostak J, Laurant P. The forgotten face of regular physical exercise: a’natural’anti-atherogenic activity. Clin Sci (Lond) 2011;121:91–106. doi: 10.1042/CS20100520. [DOI] [PubMed] [Google Scholar]