Summary
Background
We examined the cerebrospinal fluid (CSF) markers of subarachnoid hemorrhage (SAH)-induced and idiopathic normal pressure hydrocephalus (INPH) to investigate the pathophysiology and mechanism of communicating hydrocephalus compared to obstructive hydrocephalus.
Material/Methods
We obtained CSF samples from 8 INPH, 10 SAH-induced hydrocephalus, and 6 unmatched patients with non-hemorrhagic obstructive hydrocephalus during their ventriculoperitoneal shunt operations. Transforming growth factor (TGF)-β1, tumor necrosis factor (TNF)-α, vascular endothelial growth factor (VEGF), and total tau in the CSF were analyzed via enzyme-linked immunosorbent assay.
Results
The mean VEGF levels in the CSF of patients with SAH-induced hydrocephalus, INPH, and obstructive hydrocephalus were 239±131, 239±75, and 163±122 pg/mL, respectively. The total tau concentrations in the CSF of the groups were 1139±1900, 325±325, and 1550±2886 pg/mL, respectively. TNF-α values were 114±34, 134±38, and 55±16 pg/mL, respectively. TGF-β1 values were 953±430, 869±447, and 136±63 pg/mL, respectively. A significant difference in TNF-α and TGF-β1 levels was observed only between SAH-induced and chronic obstructive hydrocephalus, and between INPH and chronic obstructive hydrocephalus (p<0.01).
Conclusions
No significant differences in the 4 CSF biomarker levels were observed between INPH and SAH-induced hydrocephalus, whereas CSF TNF-α and TGF-β1 levels were increased compared to those in patients with chronic obstructive hydrocephalus. Post-SAH hydrocephalus and INPH are probably more destructive to neural tissues, and then stimulate the inflammatory reaction and healing process, compared with obstructive hydrocephalus.
Keywords: cerebrospinal fluid, subarachnoid hemorrhage, normal pressure hydrocephalus, transforming growth factor-β1, tumor necrosis factor-α, vascular endothelial growth factor, total tau
Background
Many biochemical products are exchanged within the central nervous system (CNS) through the cerebrospinal fluid (CSF), which is the main component of the CNS’s extracellular fluid. CSF may reflect the pathophysiology of various neurological disorders that occur in the CNS, as well as in healthy conditions, because the CSF plays a specific role for physiological control in the brain to maintain a stable CNS condition [1]. Various biochemical materials, such as neuropeptides, neurotransmitters, proteins, enzymes, and metabolic by-products, have been assayed in the CSF of patients with psychiatric, neurochemical, dementia, neuroinflammatory, and traumatic disorders [2–4]. Previous studies have reported that either transforming growth factor (TGF)-β [5,6] or vascular endothelial growth factor (VEGF) [6] is increased in the CSF of patients with post-hemorrhagic hydrocephalus, whereas tau [7] and tumor necrosis factor (TNF)-α [8] are increased in the CSF of patients with normal pressure hydrocephalus (NPH). Thus, these proteins have emerged as promising biological markers of chronic hydrocephalus [9].
NPH is characterized by a symptom triad of gait disturbance, cognitive dysfunction, and urinary incontinence in patients with an enlarged ventricular system despite normal CSF pressure [10]. It is a critical pathophysiological feature of NPH that the CSF dynamics are interrupted with reduced absorption through the arachnoid villi, a compensatory CSF flow into the periventricular white matter, and transcapillary CSF absorption [11,12]. The hydrocephalus of NPH was first considered idiopathic. However, an etiology can be identified in some cases, suggesting a previous history of elevated intracranial pressure. The possible etiologies include, but are not limited to, subarachnoid hemorrhage (SAH), trauma, meningitis, and tumors. Among them, SAH is one of the common causes of NPH, along with idiopathic NPH (INPH). Chronic hydrocephalus develops in 10–20% of patients who survive aneurysmal SAH [13]. Basal cisternal, subarachnoid, arachnoid villi fibrosis, and the formation of arachnoid/pial adhesions may play a major role in developing chronic hydrocephalus after SAH [14]. Although the causal materials of post-SAH fibrosis are still unknown, fibrogenic growth factors, such as TGF-β, have been noted as major factors [15]. Thus, the pathophysiology of INPH should be different from that of SAH-induced hydrocephalus, even though both are chronic communicating hydrocephalus conditions. Most previous studies have only compared the CSF biomarkers between NPH and non-hydrocephalic controls [7,8]. In those studies, the NPH included both idiopathic and SAH-induced chronic hydrocephalus. Recently, only 1 study has compared the biomarkers in the CSF between secondary NPH and INPH [16]. However, in that study, the etiologies of secondary NPH included SAH, other cerebrovascular disorders, and trauma. Thus, the aim of this current study is to identify the pathophysiology of both communicating hydrocephalic conditions (SAH-induced hydrocephalus and INPH) as reflected by the CSF biomarkers compared to obstructive hydrocephalus.
Material and Methods
We obtained CSF from 8 INPH patients and 10 SAH-induced hydrocephalus patients during performance of ventriculoperitoneal (V-P) shunt operations from 2007 to 2009. Control CSF samples were also collected in the same manner from 6 unmatched patients with non-hemorrhagic secondary chronic obstructive hydrocephalus. All the participants signed an informed consent form with regards to the aims of the study, and the study was approved by the Ethics Committee of Korea University Anam Hospital. INPH was confirmed according to radiological studies, the triad of hydrocephalus-induced symptoms, and the response after external lumbar drainage. SAH-induced hydrocephalus was defined as clinical and radiological hydrocephalus that required treatment, and the hydrocephalus was demonstrable at least 2 weeks after SAH as either the progression of acute hydrocephalus or the development of chronic hydrocephalus de novo. The control group included chronic hydrocephalus patients who required treatment secondary to benign tumors, or previous shunt failure from unknown causes in congenital aqueductal stenosis.
Cerebrospinal fluid samples
All the samples were taken during V-P shunt operations and through intraventricular catheterizations that were fitted for treating patients with hydrocephalus. Approximately 10 mL CSF was collected from the proximal intraventricular catheter before connecting the proximal catheter and shunt valve; the CSF was centrifuged at 800 g for 10 min to sediment both the hematogenous cells and other cells contaminating the sample, and the supernatant was aliquoted and stored at -80°C until analysis.
VEGF, TGF-β1, TNF-α, and total tau assays
We detected and analyzed specific biomarkers in the CSF by enzyme-linked immunosorbent assay (ELISA). The biomarkers were TGF-β1 for fibrosis and tissue repair, TNF-α for inflammation, VEGF for angiogenesis, and total tau protein for neurodegeneration. VEGF, TGF-β1, TNF-α, and total tau concentrations were determined using commercially available sandwich ELISAs (Bender MedSystems GmbH, Vienna Austria, and Invitrogen, Carlsbad, California, USA) according to the manufacturers’ instructions. The sensitivity of the assays were 7.9 pg/mL for VEGF, 9.0 pg/mL for TGF-β1, 1.65 pg/mL for TNF-α, and 12.0 pg/mL for total tau. The intra-assay coefficient of variation was 6.2% (mean: 571 pg/mL, n=8) for VEGF; for TGF-β1, it was 5.1% (mean: 14,346 pg/mL, n=8); for TNF-α, it was 6.0% (mean: 295 pg/mL, n=7); and for total tau, it was 4.4% (mean: 578 pg/mL, n=3). All assays were carried out in duplicate. All ELISA 96-well microtiter plates were analyzed using a microplate photometer with a maximum absorbance set at 470 nm (SpectraMax® M2e Microplate Reader, California, USA).
Statistical analysis
The significance of differences of each biomarker level among the SAH-induced hydrocephalus, INPH, and control groups was established by the Kruskal-Wallis test with asymptotic Sig. (p) values, as the distribution of the values was non-Gaussian. The significance of differences of each biomarker level between the SAH-induced hydrocephalus and INPH groups, the SAH-induced hydrocephalus and control groups, and the INPH and control groups was established by the Mann-Whitney U test with two-sided p values, as the distributions of the values were non-Gaussian. Statistical analyses were performed using the statistical software package SPSS 12.0 for Windows. A probability value of p<0.01 was set as statistical significance. The values throughout the study are expressed as means ± standard deviation (SD).
Results
Patient clinical profiles
Two of the eight INPH patients were male, and the rest were female. Their mean age was 67 years (range, 59–80 years). Their mean symptom duration was 17 months (range, 5 months-3 years). Two of the 10 SAH-induced hydrocephalus patients were male, and the rest were female. Their mean age was 66.2 years (range, 46–79 years). Their mean duration from SAH to V-P shunt was 84.2 days (range, 26–138 days). Seven and two patients underwent surgical aneurysm clipping and coil embolization, respectively, before the V-P shunt. One patient did not undergo any intervention for the aneurysm except a V-P shunt due to the patient’s old age (79 years old) and generally poor condition. Three patients underwent extraventricular CSF drainage between the times of aneurysm surgery and V-P shunt for treating their acute hydrocephalus. The non-hemorrhagic secondary obstructive chronic hydrocephalus patients in the control group comprised 3 males and 3 females. Their mean age was 43.3 years (range, 4–63 years). Four patients suffered from chronic hydrocephalus secondary to benign tumors, and two patients had chronic hydrocephalus secondary to the malfunctioning of shunt devices which had been implanted to treat a congenital aqueductal stenosis. Tables 1 and 2 summarize the characteristics of the patients with SAH-induced hydrocephalus and NPH and the patients in the control group, respectively.
Table 1.
Characteristics of patients with SAH-induced hydrocephalus and INPH.
| Case | Gender | Age | Cause | Symptom duration | Intervention before shunt |
|---|---|---|---|---|---|
| 1 | F | 70 | SAH | 72 days | Clipping |
| 2 | F | 67 | SAH | 50 days | Clipping |
| 3 | F | 70 | SAH | 138 days | Clipping |
| 4 | F | 63 | SAH | 90 days | Clipping |
| 5 | M | 69 | SAH | 120 days | Clipping |
| 6 | F | 63 | SAH | 95 days | Clipping |
| 7 | M | 46 | SAH | 50 days | Clipping |
| 8 | F | 79 | SAH | 128 days | None |
| 9 | F | 63 | SAH | 73 days | Coiling |
| 10 | F | 72 | SAH | 26 days | Coiling |
| 11 | F | 59 | Idiopathic | 3 years | None |
| 12 | M | 80 | Idiopathic | 3 years | None |
| 13 | F | 71 | Idiopathic | 1 year | None |
| 14 | F | 63 | Idiopathic | 6 months | None |
| 15 | F | 61 | Idiopathic | 5 months | None |
| 16 | F | 54 | Idiopathic | 2 years | None |
| 17 | M | 74 | Idiopathic | 1 year | None |
| 18 | F | 74 | Idiopathic | 6 months | None |
SAH – subarachnoid hemorrhage.
Table 2.
Characteristics of control patients with established non-hemorrhagic secondary chronic hydrocephalus requiring ventriculoperitoneal shunting.
| Case | Gender | Age | Diagnosis |
|---|---|---|---|
| 1 | M | 55 | Chronic obstructive hydrocephalus secondary to trigeminal schwannoma |
| 2 | F | 61 | Chronic obstructive hydrocephalus secondary to vestibular schwannoma |
| 3 | M | 19 | Chronic obstructive hydrocephalus secondary to aqueductal stenosis |
| 4 | F | 58 | Chronic obstructive hydrocephalus secondary to meningioma |
| 5 | M | 4 | Chronic obstructive hydrocephalus secondary to aqueductal stenosis |
| 6 | F | 63 | Chronic obstructive hydrocephalus secondary to meningioma |
Comparison of CSF biomarkers
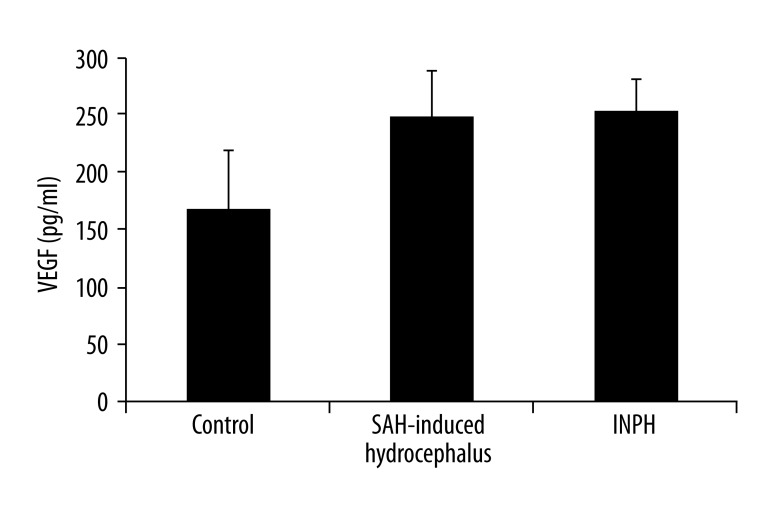
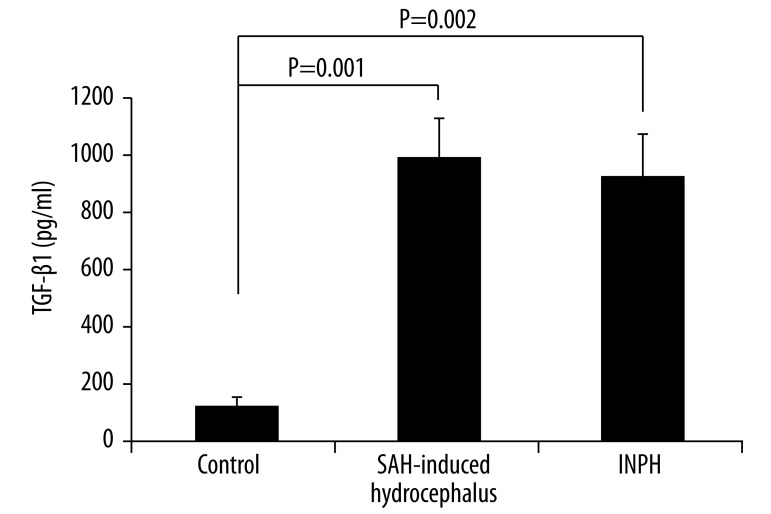
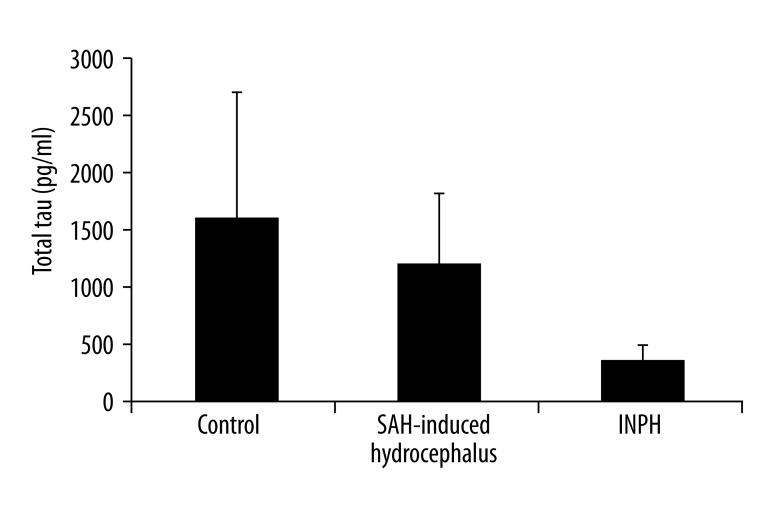
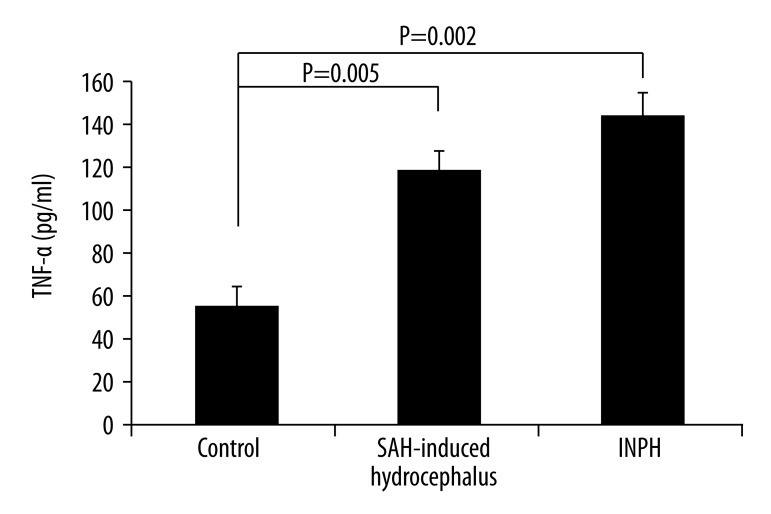
The mean VEGF concentrations (mean ±SD) in the CSF of the SAH-induced hydrocephalus, INPH, and control patients were 239±131 (median: 212), 239±75 (median: 224), and 163±122 (median: 146.5) pg/mL, respectively. No significant differences were observed among them. The mean total tau concentrations in the CSF of the SAH-induced hydrocephalus, INPH, and control patients were 1139±1900 (median: 332), 325±325 (median: 223), and 1550±2886 (median: 331) pg/mL, respectively. No significant differences were observed among them. The mean TNF-α concentrations in the CSF of the SAH-induced hydrocephalus, INPH, and control patients were 114±34 (median: 122), 134±38 (median: 123), and 55±16 (median: 53) pg/mL, respectively. A significant difference was seen among these concentration values (p<0.01). The mean TGF-β1 concentrations in the CSF of the SAH-induced hydrocephalus, INPH, and control patients were 953 ± 430 (median: 893), 869±447 (median: 763), and 136±63 (median: 149.5) pg/mL, respectively. Significant differences were observed among them (p<0.01).
We statistically analyzed the CSF biomarkers between each set of two groups by the Mann-Whitney U test. A significant difference was observed between the SAH-induced hydrocephalus and control groups, and between the INPH and control groups in the levels of only TNF-α and TGF-β1, respectively (p<0.01), whereas no significant differences were observed between the SAH-induced hydrocephalus and INPH groups for all examined biomarkers.
Figures 1–4 demonstrate the comparison of the concentrations of these CSF biomarkers among the three groups, and Table 3 shows each level of the CSF biomarkers of the three groups.
Figure 1.
VEGF levels in the CSF of patients with SAH-induced hydrocephalus, patients with INPH, and patients with chronic obstructive hydrocephalus (control; mean ±SEM). No significant differences were observed among the three groups (Kruskal-Wallis test).
Figure 4.
TGF-β1 levels in the CSF of patients with SAH-induced hydrocephalus, patients with INPH, and patients with chronic obstructive hydrocephalus (control; mean ±SEM). A significant difference was observed among the three groups (p=0.001, Kruskal-Wallis test). No significant difference was seen between SAH-induced hydrocephalus and INPH (Mann-Whitney U test), whereas a significant difference was observed between the SAH-induced hydrocephalus and control groups (p=0.001, Mann-Whitney U test) and between the INPH and control groups (p=0.002, Mann-Whitney U test).
Table 3.
ELISA results for VEGF, total tau, TNF-α, and TGF-β1 CSF levels in SAH-induced hydrocephalus patients, INPH, and control patients.
| SAH hydrocephalus (N=10) | INPH (N=8) | Control (N=6) | |
|---|---|---|---|
| VEGF | 239±131 (54–550, 212) | 239±75 (140–374, 224) | 163±122 (27–312, 146.5) |
| Tau | 1139±1900 (95–5696, 332) | 325±325 (52–1045, 223) | 1550±2886 (172–7422, 331) |
| TNF-α | 114±34 (49–170, 122) | 134±38 (102–214, 132) | 55±16 (33–77, 53) |
| TGF-β1 | 953±430 (347–1843, 893) | 869±447 (492–1912, 763) | 136±63 (44–226, 149.5) |
Values are means ± SDs and ranges with median. SAH – subarachnoid hemorrhage; INPH – idiopathic normal pressure hydrocephalus; VEGF – vascular endothelial growth factor; TNF – tumor necrosis factor; TGF – transforming growth factor.
Discussion
We failed to find significant differences of the levels of these four CSF biomarkers between the INPH and SAH-induced hydrocephalus groups. Our study demonstrated that no statistical difference in the above-mentioned biomarker levels was noted between the 2 types of NPH. This partially accorded with the previous study that compared CSF biomarkers, including tau, between secondary NPH and INPH [16]. Instead, when control CSF samples were obtained from patients with non-hemorrhagic secondary chronic obstructive hydrocephalus, the levels of TNF-α and TGF-β1 of both NPH types appeared to be elevated compared to this control group in our study.
The origin of tau is probably damaged or degenerative neuronal cells in the subependymal region of the dilated cerebral ventricles [7], while TNF-α may result from activated microglial or inflammatory cells in the brain during the development of chronic hydrocephalus [8]. TGF-β1 is expressed from endothelial, hematopoietic, and connective tissue cells in response to tissue injury for wound healing or fibrosis [17], whereas following hemorrhage, it is released from astrocytes [18] and platelets [5,6] into the CSF. Meanwhile, under normal conditions, only a diffuse expression of VEGF is observed in the brain, with the exception of some specialized cells, such as those of the epithelium in the choroid plexus [19]. In contrast, under local or systemic hypoxia, neurons, astrocytes, and microglial cells all show enhanced VEGF expression [20–22].
Our results suggest that the expression of some biomarkers in the CSF may be dependent on the time that the CSF was taken. In particular, the TGF-β1 level in the CSF appeared to be more elevated in the acute phase of hemorrhage. Flood et al. [5] observed that the CSF TGF-β1 levels were elevated at 1–2 days and at 9–10 days post-hemorrhage compared to non-hemorrhagic hydrocephalic levels. Douglas et al. [15] reported that the total TGF-β1 levels in post SAH hydrocephalus over 1–5 days were significantly higher than the levels in non-hemorrhagic hydrocephalic patients, and these significantly high levels were sustained through 6–14 days. In contrast, Heep et al. [6] found that the TGF-β1 CSF concentrations of premature infants with post-hemorrhagic hydrocephalus did not differ from those of newborn infants with non-hemorrhagic congenital hydrocephalus. They collected CSF samples at least 14 days after intraventricular hemorrhage in premature infants with post-hemorrhagic hydrocephalus. We also collected CSF samples at least 26 days after the chronic phase of SAH. Interestingly, Li et al. [1] reported that the CSF TGF-β1 levels of INPH patients were increased compared to those of the patient control group, who had only tension headaches. Taken together, in our series no difference was observed in the CSF TGF-β1 levels between patients with chronic SAH-induced hydrocephalus and those with INPH, whereas CSF TGF-β1 levels in both groups were elevated compared to the control group. These findings suggest that CSF TGF-β1 in the chronic phase of SAH-induced hydrocephalus and INPH is more expressed through the wound healing process and fibrosis compared to chronic obstructive hydrocephalus secondary to an intracranial tumor and congenital origins.
Some previous studies also reported that VEGF was increased in the CSF of patients with post-hemorrhagic hydrocephalus [6] or chronic obstructive hydrocephalus [23]. Heep et al. [6] demonstrated that in neonates, the CSF VEGF levels of patients with post-hemorrhagic hydrocephalus were significantly elevated compared to those of patients with non-hemorrhagic hydrocephalus, even though the CSF VEGF levels of post-hemorrhagic and non-hemorrhagic hydrocephalus patients were increased compared to those of the non-hydrocephalic neonate controls. Koehne et al. [24] reported that VEGF concentrations in hydrocephalus CSF samples, including those samples from post-hemorrhagic operations secondary to an intracranial tumor and congenital origins, were significantly elevated compared with those from routine diagnostic lumbar punctures for unrelated reasons. Chronic hydrocephalus probably increases intracranial pressure, and increased intracranial pressure may decrease the cerebral blood flow and induce chronic tissue hypoxia. Finally, such conditions may induce VEGF secretion from the choroid plexus or migration of VEGF from the surrounding brain tissue [6,23]. In our series, no significant differences in CSF VEGF levels were seen among the SAH-induced hydrocephalus, INPH, or control patients. This finding suggests that VEGF is probably well expressed in the CSF of patients with chronic hydrocephalus whether it is idiopathic or secondary, and communicating or non-communicating.
Some previous studies have demonstrated that the expression levels of tau [7,25] and TNF-α [8] in the CSF of NPH patients, regardless of whether it was idiopathic or post-SAH hydrocephalus, were elevated compared to that of the controls. Tau protein is a microtubule-associated protein, and it has been found to be elevated in the CSF of patients suffering from neurodegenerative diseases such as Alzheimer’s disease [26], Lewy body dementia, corticobasal degeneration [27], and Creutzfeldt-Jakob disease [28], indicating that it is a marker of neuronal degeneration. TNF-α is a proinflammatory cytokine that mediates myelin damage, and it is also overexpressed in the CSF of INPH patients and in patients with NPH induced by various etiologies, including SAH [8] and other neural disorders that present with demyelination, such as vascular dementia [29] and stroke with white matter lesions. By contrast, some studies showed that lumbar CSF TNF-α concentration was low in INPH patients [30]. Our results demonstrated that there was no significant difference in CSF total tau levels among SAH-induced hydrocephalus, INPH, and control patients. Interestingly, while no difference in CSF TNF-α level was observed between patients with chronic SAH-induced hydrocephalus and those with INPH, the TNF-α level was increased compared to that of the control group. These findings suggest that total tau is also well expressed in the CSF of patients with chronic hydrocephalus regardless of the causes, whereas CSF TNF-α of the chronic phase of post-SAH hydrocephalus and INPH is more expressed through an inflammatory reaction after tissue injury from hemorrhage and ventriculomegaly compared to chronic obstructive hydrocephalus secondary to an intracranial tumor and congenital origins.
This study had some limitations. As mentioned above, because we did not obtain the CSF biomarkers of non-hydrocephalic controls, no reference values are yet available for the biochemicals in the CSF of healthy subjects and we do not know the age-matched reference values of these biomarkers among the study populations. However, it is practically difficult to obtain CSF samples through ventricular catheterization from healthy people due to ethical issues and technical limitations. Besides, because the aim of this study was to examine the difference of CSF biomarkers between SAH-induced hydrocephalus and INPH compared to obstructive hydrocephalus, we did not consider a normal control group. Another limitation was the small number of patients in each group because this was a pilot study. Finally, we obtained CSF samples only one time during ventricular catheterization for a shunt operation. It has been demonstrated that the levels of certain markers in the CSF might fluctuate over time, so a sample at one time point might be of limited use [31]. However, repeated ventricular punctures or CSF collection from the implanted shunt devices to monitor the CSF biomarkers are limited due to infection and technical problems even though CSF in the brain ventricles is theoretically more stable and the values in this CSF probably provide more reliable informations than those for the CSF obtained by lumbar puncture in the spinal canal [32]. Moreover, little fluctuation in the levels of some Alzheimer disease biomarkers in lumbar CSF has been reported [33].
Conclusions
In the present study, we could not demonstrate a significant difference in the expression levels of biological CSF markers between INPH and SAH-induced chronic hydrocephalus. This finding suggests that the pathophysiology of both types of NPH may be similar. Instead, we showed that CSF TNF-α and TGF-β1 levels in patients with SAH-induced hydrocephalus and INPH were increased compared to those in patients with chronic obstructive hydrocephalus. It implies that post-SAH hydrocephalus and INPH are probably more destructive to neural tissue and then stimulate the inflammatory reaction and healing process to a greater extent compared with obstructive hydrocephalus. Further investigation in a larger group is warranted to settle these issues in the future. Finally, more refined assay techniques will probably lead to tests of the CSF composition that will indeed be useful for the clinical management of hydrocephalic patients.
Figure 2.
Total tau levels in the CSF of patients with SAH-induced hydrocephalus, patients with INPH, and patients with chronic obstructive hydrocephalus (control; mean ±SEM). No significant differences were observed among the three groups (Kruskal-Wallis test).
Figure 3.
TNF-α levels in the CSF of patients with SAH-induced hydrocephalus, patients with INPH, and patients with chronic obstructive hydrocephalus (control; mean ±SEM). A significant difference among the three groups was observed (p=0.003; Kruskal-Wallis test). No significant difference was observed between SAH-induced hydrocephalus and INPH patients (Mann-Whitney U test), whereas a significant difference was seen between the SAH-induced hydrocephalus and control groups (p=0.005, Mann-Whitney U test) and between the INPH and control groups (p=0.002, Mann-Whitney U test).
Footnotes
Conflicts of interest
The authors have no financial conflicts of interest.
Source of support: This study was supported in part by a Korea University Grant (K1220471) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0013525)
References
- 1.Li X, Miyajima M, Jiang C, Arai H. Expression of TGF-betas and TGF-beta type II receptor in cerebrospinal fluid of patients with idiopathic normal pressure hydrocephalus. Neurosci Lett. 2007;413:141–44. doi: 10.1016/j.neulet.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 2.Dengler J, Schefold JC, Graetz D, et al. Point-of-care testing for interleukin-6 in cerebrospinal fluid (CSF) after subarachnoid haemorrhage. Med Sci Monit. 2008;14(12):BR265–68. [PubMed] [Google Scholar]
- 3.Manckoundia P, Mazen E, Coste AS, et al. A case of meningitis due to Achromobacter xylosoxidans denitrificans 60 years after a cranial trauma. Med Sci Monit. 2011;17(6):CS63–65. doi: 10.12659/MSM.881796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagdev KJ, Kashyap RS, Deshpande PS, et al. Comparative evaluation of a PCR assay with an in-house ELISA method for diagnosis of Tuberculous meningitis. Med Sci Monit. 2010;16(6):CR289–95. [PubMed] [Google Scholar]
- 5.Flood C, Akinwunmi J, Lagord C, et al. Transforming growth factor-beta1 in the cerebrospinal fluid of patients with subarachnoid hemorrhage: titers derived from exogenous and endogenous sources. J Cereb Blood Flow Metab. 2001;21:157–62. doi: 10.1097/00004647-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Heep A, Stoffel-Wagner B, Bartmann P, et al. Vascular endothelial growth factor and transforming growth factor-beta1 are highly expressed in the cerebrospinal fluid of premature infants with posthemorrhagic hydrocephalus. Pediatr Res. 2004;56:768–74. doi: 10.1203/01.PDR.0000141524.32142.53. [DOI] [PubMed] [Google Scholar]
- 7.Kudo T, Mima T, Hashimoto R, et al. Tau protein is a potential biological marker for normal pressure hydrocephalus. Psychiatry Clin Neurosci. 2000;54:199–202. doi: 10.1046/j.1440-1819.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 8.Tarkowski E, Tullberg M, Fredman P, Wikkelso C. Normal pressure hydrocephalus triggers intrathecal production of TNF-alpha. Neurobiol Aging. 2003;24:707–14. doi: 10.1016/s0197-4580(02)00187-2. [DOI] [PubMed] [Google Scholar]
- 9.Tarnaris A, Watkins LD, Kitchen ND. Biomarkers in chronic adult hydrocephalus. Cerebrospinal Fluid Res. 2006;3:11. doi: 10.1186/1743-8454-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams RD, Fisher CM, Hakim S, et al. Symptomatic Occult Hydrocephalus with “Normal” Cerebrospinal-Fluid Pressure. A Treatable Syndrome. N Engl J Med. 1965;273:117–26. doi: 10.1056/NEJM196507152730301. [DOI] [PubMed] [Google Scholar]
- 11.Deo-Narine V, Gomez DG, Vullo T, et al. Direct in vivo observation of transventricular absorption in the hydrocephalic dog using magnetic resonance imaging. Invest Radiol. 1994;29:287–93. doi: 10.1097/00004424-199403000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Milhorat TH. The third circulation revisited. J Neurosurg. 1975;42:628–45. doi: 10.3171/jns.1975.42.6.0628. [DOI] [PubMed] [Google Scholar]
- 13.Vale FL, Bradley EL, Fisher WS., III The relationship of subarachnoid hemorrhage and the need for postoperative shunting. J Neurosurg. 1997;86:462–66. doi: 10.3171/jns.1997.86.3.0462. [DOI] [PubMed] [Google Scholar]
- 14.Kibler RF, Couch RS, Crompton MR. Hydrocephalus in the adult following spontaneous subarachnoid haemorrhage. Brain. 1961;84:45–61. doi: 10.1093/brain/84.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Douglas MR, Daniel M, Lagord C, et al. High CSF transforming growth factor beta levels after subarachnoid haemorrhage: association with chronic communicating hydrocephalus. J Neurol Neurosurg Psychiatry. 2009;80:545–50. doi: 10.1136/jnnp.2008.155671. [DOI] [PubMed] [Google Scholar]
- 16.Tullberg M, Blennow K, Mansson JE, et al. Cerebrospinal fluid markers before and after shunting in patients with secondary and idiopathic normal pressure hydrocephalus. Cerebrospinal Fluid Res. 2008;5:9. doi: 10.1186/1743-8454-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MD, Gold LI, Moses HL. Evidence for transforming growth factor-beta expression in human leptomeningeal cells and transforming growth factor-beta-like activity in human cerebrospinal fluid. Lab Invest. 1992;67:360–68. [PubMed] [Google Scholar]
- 18.Krupinski J, Kumar P, Kumar S, Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27:852–57. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- 19.Marti HH, Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci USA. 1998;95:15809–14. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi T, Abe K, Suzuki H, Itoyama Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–44. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- 21.Ogunshola OO, Stewart WB, Mihalcik V, et al. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res Dev Brain Res. 2000;119:139–53. doi: 10.1016/s0165-3806(99)00125-x. [DOI] [PubMed] [Google Scholar]
- 22.Plate KH, Beck H, Danner S, et al. Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J Neuropathol Exp Neurol. 1999;58:654–66. doi: 10.1097/00005072-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Dombrowski SM, Deshpande A, et al. VEGF/VEGFR-2 changes in frontal cortex, choroid plexus, and CSF after chronic obstructive hydrocephalus. J Neurol Sci. 2010;296:39–46. doi: 10.1016/j.jns.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koehne P, Hochhaus F, Felderhoff-Mueser U, et al. Vascular endothelial growth factor and erythropoietin concentrations in cerebrospinal fluid of children with hydrocephalus. Childs Nerv Syst. 2002;18:137–41. doi: 10.1007/s00381-002-0567-2. [DOI] [PubMed] [Google Scholar]
- 25.Tarnaris A, Toma AK, Chapman MD, et al. Use of cerebrospinal fluid amyloid-β and total tau protein to predict favorable surgical outcomes in patients with idiopathic normal pressure hydrocephalus. J Neurosurg. 2011;115:145–50. doi: 10.3171/2011.2.JNS101316. [DOI] [PubMed] [Google Scholar]
- 26.Blennow K, Wallin A, Agren H, et al. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26:231–45. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 27.Newman J, Rissman RA, Sarsoza F, et al. Caspase-cleaved tau accumulation in neurodegenerative diseases associated with tau and alpha-synuclein pathology. Acta Neuropathol. 2005;110:135–44. doi: 10.1007/s00401-005-1027-3. [DOI] [PubMed] [Google Scholar]
- 28.Van Everbroeck B, Boons J, Cras P. Cerebrospinal fluid biomarkers in Creutzfeldt-Jakob disease. Clin Neurol Neurosurg. 2005;107:355–60. doi: 10.1016/j.clineuro.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Tarkowski E, Blennow K, Wallin A, Tarkowski A. Intracerebral production of tumor necrosis factor-alpha, a local neuroprotective agent, in Alzheimer disease and vascular dementia. J Clin Immunol. 1999;19:223–30. doi: 10.1023/a:1020568013953. [DOI] [PubMed] [Google Scholar]
- 30.Leinonen V, Menon LG, Carroll RS, et al. Cerebrospinal fluid biomarkers in idiopathic normal pressure hydrocephalus. Int J Alzheimers Dis. 2011;2011:312526. doi: 10.4061/2011/312526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geracioti TD, Jr, Orth DN, Ekhator NN, et al. Serial cerebrospinal fluid corticotropin-releasing hormone concentrations in healthy and depressed humans. J Clin Endocrinol Metab. 1992;74:1325–30. doi: 10.1210/jcem.74.6.1317385. [DOI] [PubMed] [Google Scholar]
- 32.Talab R, Valis M, Rehak S, Krejsek J. Abnormalities of tau-protein and beta-amyloid in brain ventricle cerebrospinal fluid. Neuro Endocrinol Lett. 2009;30:647–51. [PubMed] [Google Scholar]
- 33.Moghekar A, Goh J, Li M, et al. Cerebrospinal fluid Aβ and tau level fluctuation in an older clinical cohort. Arch Neurol. 2012;69:246–50. doi: 10.1001/archneurol.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]