Summary
Background
Imatinib is a highly effective drug in up-front treatment of chronic myeloid leukemia (CML). In children impaired longitudinal growth has been reported as side effect exerted by this drug under prolonged therapy. We therefore prospectively evaluated alterations of bone biochemical markers in pediatric patients with CML under ongoing imatinib exposure.
Material/Methods
Bone metabolic markers (calcium, phosphate, magnesium, parathyroid hormone, vitamin D, procollagen type l N propeptide [PINP], and C-terminal cross-linking telopeptide of collagen [CTX-I], osteocalcin [OC]; pyridinoline [PYD], and desoxypyridinoline [DPD]) were determined in 17 patients with CML aged 4–17 years under imatinib treatment in three-month intervals over a 2.5 year period.
Results
Hyperparathyroidism developed in 8/17 patients and low 25-hydroxyvitamin-D3 levels were found in 15/17 patients. Increased OC levels were detected in 58% of all specimen showing a linear significant decline of −0.30 μg OC per l per week (p=0.04). Serum PINP was lowered in 25% and serum CTX-I was above the normal range in 57% of the specimen originating exclusively from prepupertal patients. Urine PYD and Urine DPD levels were above the normal range in 10% and 9%, respectively, of all specimen collected and a statistically significant linear decline of −0.16 nmol DPD/mg creatinine/week was calculated (p=0.01).
Conclusions
Bone remodeling may be dysregulated by imatinib. Data suggest that impaired bone formation exceeds that of decreased bone resorption. Regular evaluation of the skeletal actions during long-term imatinib treatment in childhood CML is warranted.
Keywords: chronic myeloid leukemia, children, imatinib, bone metabolism
Background
Chronic myeloid leukemia (CML) represents the first human malignancy in which molecular targeted therapy by imatinib mesylate leads to a dramatic clinical response. Building on evidence that the BCR-ABL oncoprotein resulting from the chromosomal 9;22 translocation (Philadelphia chromosome; Ph+) expresses an increased tyrosine kinase activity, investigators rationally designed imatinib as a selective inhibitor (TKI). The drug attaches to the ATP-pocket of the BCR-ABL protein tyrosine kinase, thereby precluding ATP-binding and inhibiting phosphorylation of substrates. [1] Because CML in the pediatric age group is rare, imatinib has been evaluated only in small patient numbers studied prospectively in trials with Ph+ leukemias. The drug induced sustained response rates comparable with those highly impressive results reported in adults. [2–6] In all studies imatinib was tolerated well by children and adolescents with only mild treatment-related symptoms. However, the durability of CML responses as well as long-term side effects of this drug in pediatric patients especially with respect to different age groups still remain to be determined [7,8].
Little is known yet regarding changes in cell metabolism during exposure to imatinib. Although imatinib was designed to specifically target BCR-ABL, also besides ABL many off-target kinases like c-kit (receptor of SCF), c-fms (receptor of M-CSF), platelet-derived growth factor receptors (PDGFR-α, -β), the ABL-related gene product (ARG), NAD(P)H: quinone oxidoreductase (NQO2), Collagen-induced discoiddin domain receptor (DDR1), human carbonic anhydrases (hCA)II, and h(CA)XIV, and collagen-induced discoiddin domain receptor (DDR2) are affected by the drug at plasma concentrations achievable clinically at standard dosages [9]. Therefore metabolic effects on glucose, lipid, and bone metabolism have been reported as a consequence of imatinib treatment in adult patients. [8–10] The unanticipated side-effect of imatinib targeting cells of the skeleton which may result in dysregulation of bone remodeling (for a comprehensive review see [9]) is of special concern in a not yet outgrown pediatric cohort. Retrospective analysis of data published recently from a French cohort of 44 children with CML, from a Japanese study (n=48), and India (n=20) point to a substantial longitudinal growth retardation in prepubertal patients with CML under imatinib treatment. [11–13] We here report on alterations of bone biochemical markers in the plasma and urine of children and adolescents with CML under ongoing treatment with imatinib over a two-year period.
Material and Methods
Seventeen patients (age 4 4/12–17 5/12 years, median: 10 7/12 years; 13 males, 4 females) with newly diagnosed chronic phase Ph+ CML were enrolled into trial CML-paed II (ClinicalTrials.gov Identifier: NCT00445822). The protocol was approved by the Dresden Medical Faculty Ethical Review Board and all patients’ parents provided informed written consent. Up-front treatment consisted of 260–300 mg/sqm imatinib orally once daily [2,7,8]. Data concerning clinical characteristics of the patients were collected on standardized forms at fixed intervals and sent to the study chair. Over a period of 2.5 years (range 0–138 weeks; median: 68 weeks) therapy was monitored for therapeutic response (clinical examination, blood cell count, bone marrow cytogenetics, ratio BCR-ABL/ABL by quantitative RT-PCR) and side effects.
To investigate side effects on the skeleton biochemical measurements were undertaken by collection of specimen (plasma, serum, 2nd morning void urine) from the patients under appropriate circumstances (immediate processing, freezing at −20°C, light protection, dry ice shipment) in 3-month intervals. None of the patients received additional medication, especially no vitamin supplements. Comprehensive analysis included determination of calcium, phosphate, parathyroid hormone (PTH), 25-Hydroxyvitamin-D3 (25-vit-D3), 1,25-Dihydroxyvitamin-D3 (1,25-vit-D3), procollagen type l N propeptide (PINP), and C-terminal cross-linking telopeptide of collagen type I (CTX-I) in serum; determination of plasma levels of bone alkaline phosphatase (BAP) and osteocalcin; and assessment of calcium, phosphate, creatinine, pyridinium crosslinks pyridinoline (PYD) and desoxypyridinoline (DPD) levels in urine specimen. [14] All assays were performed centrally in the Laboratory of Dr. Limbach and Partners (Heidelberg, Germany).
The biochemical parameters calcium and phosphate were measured by means of an automated clinical chemistry analyzer (Modular Analytics SWA; Roche Diagnostics, Mannheim, Germany) and had a variance of less than 3 percent. Osteocalcin levels in serum were measured by means of an electrochemiluminescence immunoassay (ELICA) for use on the Modular Analytics E170 immunoassay analyzer (Roche Diagnostics, Mannheim, Germany). The monoclonal antibodies used in the test react specifically with epitopes on the N-MID-fragments and the N-terminal fragment and detects the stable N-MID-fragment as well as the intact osteocalcin. Therefore smaller, instable fragmented osteocalcin degradation forms have no impact on the test results. PTH was measured in plasma with the use of an ELICA for use on the Modular Analytics E170 immunoassay analyzer. The osteocalcin assay had a total imprecision of <3.0 percent, and the PTH of <4.0 percent. The serum level of BAP was determined by means of a paramagnetic particle chemiluminescent immunoassay (Access OSTASE; Access Immunoassay System, Beckman Coulter GmbH, Krefeld, Germany); the total imprecision was <8.0 percent. Serum levels of 25-Vit-D3 were determined with an ELICA for use on the Modular Analytics E170 immunoassay analyzer. The between-assay variance from the mean 25-vit-D3 level of 33 μg per liter (82 nmol per liter) in healthy controls was 10.0 percent. 1,25-Vit-D3 was quantitated in serum with a 125I-labeled coated tube radioimmunoassay (RIA) (DIAsource ImmunoAssays S.A., Nivelles, Belgium) after micro-column purification of the sample. Serum levels of CTX were measured by means of an ELICA for use on the Modular Analytics E170 immunoassay analyzer. The total imprecision was <7.0 percent. Pyridinium crosslinks PYD and DPD were determined with a laboratory “in-house‟-method. Results correspond to methods applied with commercial assays using HPLC and were expressed in relation to urine creatinine excretion [15].
Statistical analysis
Differences between means of biochemical markers were evaluated by analyses of variance including imatinib treatment and age groups as fixed factors and covariables like age and weeks on imatinib treatment. Multiple comparisons between means of a factor were performed using Tukey’s honestly significant difference. Multinomial Logistic Regression procedure was used to model the dependence of the nominal categorical response (25-vit-D3, 1,25-vit-D3) on season as a discrete predictor variable. P-values less than α=0.05 were considered significant. All analyses were performed with SPSS 16.0 (SPSS Inc., Chicago IL, USA).
Results
Serum calcium and phosphate levels were analyzed in 57 specimen. Hypocalcemia was detected in 6 specimen from 4 patients presenting at week 1, 4, 10, 13, 88 and 135, respectively, while hypercalcemia was found in 3 patients at week 23, 29, and 90. Serum phosphate levels ranged from 1.00–1.81 mmol per l and were found within the age-matched normal range in all but 3 patients expressing hypophosphatemia at week 10, 88 and 137 [16]. Serum PTH levels ranged from 14–71 ng per l and were found normal (Table 1) only in 8 out of the 17 patients at all time points of analysis while the rest of the cohort (55%) expressed hyperparathyroidism.
Table 1.
Normal ranges, medians and standard deviations of bone metabolic markers adjusted to age and sex.
| Biochemical Marker | Specimen | Sex | Age [years] | Normal range [median] | Ref. |
|---|---|---|---|---|---|
| PTH | Serum | ♀ & ♂ | – | 11–43 [27] ng per L | [28] |
| 25-vit-D3 | Serum | ♀ & ♂ | – | >29 μg per L | [16] |
| 1,25-vit-D3 | Serum | ♀ & ♂ | – | 35–90 [62.5] ng per L | [16] |
| Osteocalcin | Plasma | ♂ | Tanner I | 13.4–36.1 [24.1] μg per L | [16] |
| Tanner II – IV | 18.5–51.2 [28.5] μg per L | ||||
| Tanner V | 6.1–29.5 [10.5] μg per L | ||||
| ♀ | Tanner I | 15.9–37.1 [25.3] μg per L | |||
| Tanner II – IV | 5.6–42.4 [24.3] μg per L | ||||
| Tanner V | 3.4–11.8 [6.3] μg per L | ||||
| PINP | Serum | ♀ & ♂ | 1–10 | 277–824 [550.5] μg per L | [31] |
| ♂ | 12–15 | 282–1604 [943] μg per L | |||
| 17–19 | 81–476 [278.5] μg per L | ||||
| ♀ | 17–19 | 41–133 [87] μg per L | |||
| CTX-I | Serum | ♀ & ♂ | 1–9 | 146–818 [462] ng per L | [35] |
| ♂ | 10–14 | 213–1238 [725.5] ng per L | |||
| 15–17 | 240–1734 [987] ng per L | ||||
| ♀ | 15–19 | 48–579 ng [313.5] per L | |||
| PYD | Urine | ♂ | Tanner I | [383±16] nmol PYD/mmol Crea | [40] |
| Tanner II – IV | [402±63] nmol PYD/mmol Crea | ||||
| Tanner V | [126±15] nmol PYD/mmol Crea | ||||
| ♀ | Tanner I | [440±35] nmol PYD/mmol Crea | |||
| Tanner II | [510±42] nmol PYD/mmol Crea | ||||
| Tanner III/IV | [320±105] nmol PYD/mmol Crea | ||||
| Tanner V | [150±21] nmol PYD/mmol Crea | ||||
| DPD | Urine | ♂ | Tanner I | [66±3] nmol DPD/mmol Crea | [40] |
| Tanner II – IV | [61±9] nmol DPD/mmol Crea | ||||
| Tanner V | [20±3] nmol DPD/mmol Crea | ||||
| ♀ | Tanner I | [75±7] nmol DPD/mmol Crea | |||
| Tanner II | [82±7] nmol DPD/mmol Crea | ||||
| Tanner III/IV | [51±18] nmol DPD/mmol Crea | ||||
| Tanner V | [15±6] nmol DPD/mmol Crea |
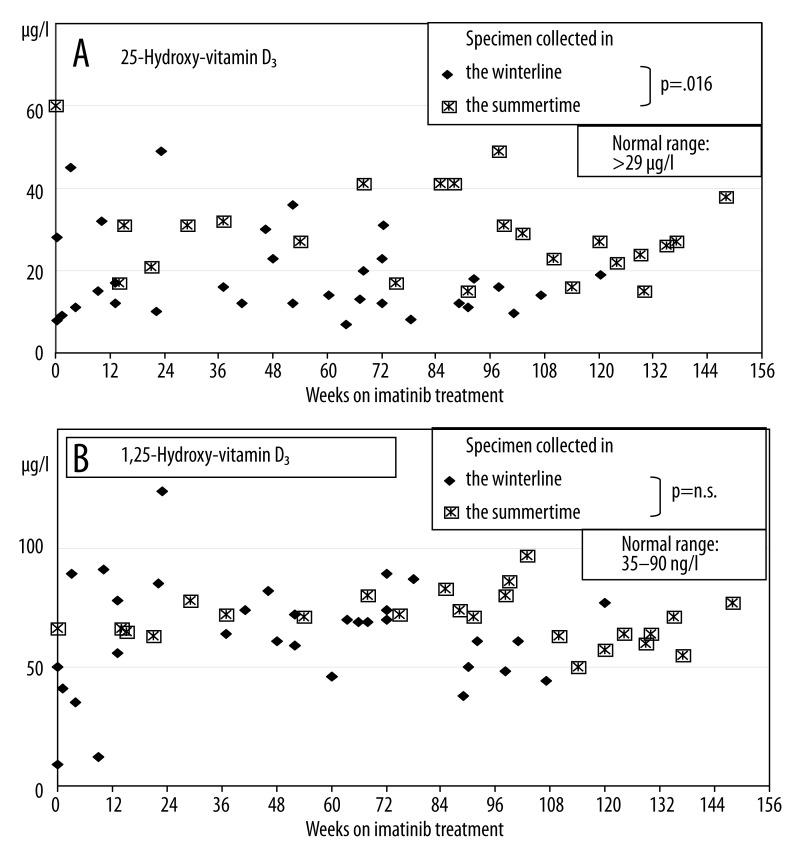
Serum 25-vit-D3 levels ranged from 6.9–60.0 μg per l (Figure 1A). Only 15 out of 56 specimen were found within the normal range (Table 1) and these were collected from only 2 out of the total cohort of 17 patients. In addition, values were analyzed with respect to the season when specimen was collected. During months with reduced sunshine exposure (November to April) 81% of the measurements (26/32) were found below 30 ng per l while during May to September only 58% of the measurements (14/24) were found below this lower threshold level. Comparison of these periods showed that the probability to exhibit normal measurements was 5.86-fold higher than in the summertime than in the wintertime (p=0.016). Over the time while on imatinib treatment a linear non-significant decline of −0.03 μg 25-vit-D3 per l per week could be calculated (p=0.55).
Figure 1.
(A) 25-vit-D3 (major circulating vitamin D metabolite) serum levels during imatinib treatment. Compared to months with higher sunlight exposure measurements were significantly lower in the winter time. The probability to exhibit normal measurements was calculated to be 5.86-fold higher in the summertime than in the wintertime (p=0.016). (B) 1,25-vit-D3 serum levels during imatinib treatment. No effect of exposure to sunlight could be detected.
Serum 1,25-vit-D3 levels ranged from 10–124 ng per l and were found below the normal range in 2 out of 56 measurements and above the normal range (Table 2) in 3 out of 56 measurements (Figure 1B). When measurements were analyzed with respect to the season when specimen was collected, 12% of the measurements (4/32) were found outside the normal range during wintertime (each 2 below and above normal) while 5% of the measurements (1/24) were found above the upper threshold level during summertime. Compared measurements in months with higher sunlight exposure were statistically not significantly lower to measurements in the wintertime. Over the time while on imatinib treatment a linear non-significant decline of −0.04 ng 1,25-vit-D3 per l per week could be calculated (p=0.54).
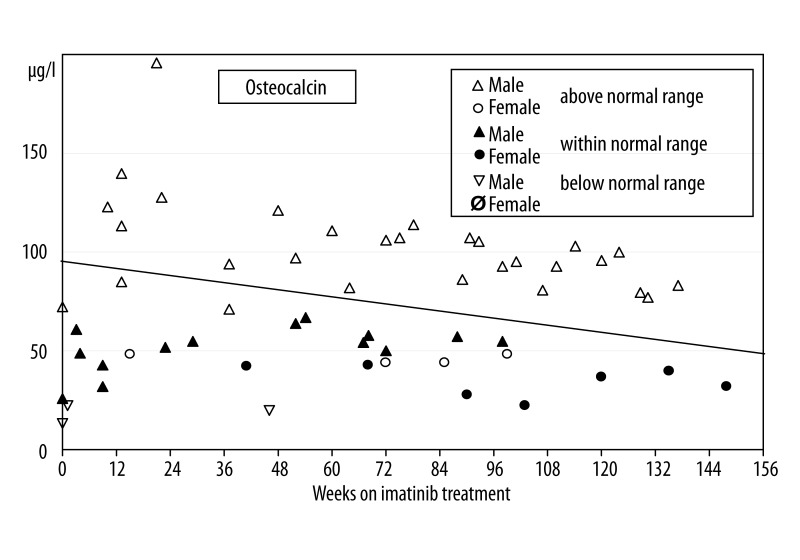
Plasma osteocalcin levels ranged from 13–196 μg per l. Twenty-one out of 57 measurements (37%) were distributed within the normal age- and sex-matched ranges (Table 1) while 3 measurements (5%) from 3 boys were below the normal range and 33 measurements (58%) showed increased osteocalcin levels. Figure 2 shows the osteocalcin measurements plotted against the time on imatinib treatment demonstrating a linear significant decline of −0.30 μg osteocalcin per l per week (p=0.04).
Figure 2.
Osteocalcin (marker of bone formation) plasma levels during imatinib treatment. Normal values are age and sex dependent and measurements were classified as above, within, or below the normal range in correlation to Tanner stages as indicated in reference [20]. Thirty-three out of 57 measurements (58%) were above the normal range. A significant decline over time of 0.03 mg osteocalcin per l per week could be calculated.
Serum PINP measurements were performed in 41 specimens from 14 patients (11 boys, 3 girls) and showed levels from 47–825 μg per l. No measurements were found above the normal sex and age dependent range (Table 1), while 31 out of 41 (75%) measurements were in the normal range and 10 out of 41 (25%) below the normal range. All measurements exhibiting values below the normal range originated from prepubertal patients younger than 11 years. No significant trend could be detected when measurements were analyzed with respect to the duration of the imatinib treatment.
Plasma BAP levels ranged from 5–89 μg per l and were within the normal range (age and sex-dependent reference values were depicted from Ref. [17,18]) with the exception of 4 specimen exhibiting values below the lower normal range. These 4 measurements (out of a total of 10 measurements performed within the age group 15–17 years old) were presented by 2 male adolescent patients aged 15 and 17 years at single time points after 1, 3, and 18 weeks of imatinib treatment and from an 17 year old female at week 46 of imatinib treatment.
Serum CTX-I measurements could not be performed in the total cohort due to shortage of the volume of blood collected. Measurements were performed in 30 specimens from 15 patients (11 male, 4 female) and ranged from 410–2600 ng per l. Seventeen out of 30 measurements (57%) were above the normal range (Table 1) while the remaining 13 measurements showed normal values. All elevated measurements were found in patients younger than 14 years old. A linear non-significant decline of −3.35 ng CTX-I per l per week over the treatment time could be calculated (p=0.65).
Urine PYD levels were analyzed in 57 specimens and ranged from 60–399 nmol PYD per mmol creatinine. Ten percent (6/57) of the measurements were above the normal range (Table 1) while only 1 measurement from a 9 year old boy was below the normal range after 9 weeks of imatinib treatment. No statistically significant changes could be shown in correlation to the duration of the treatment.
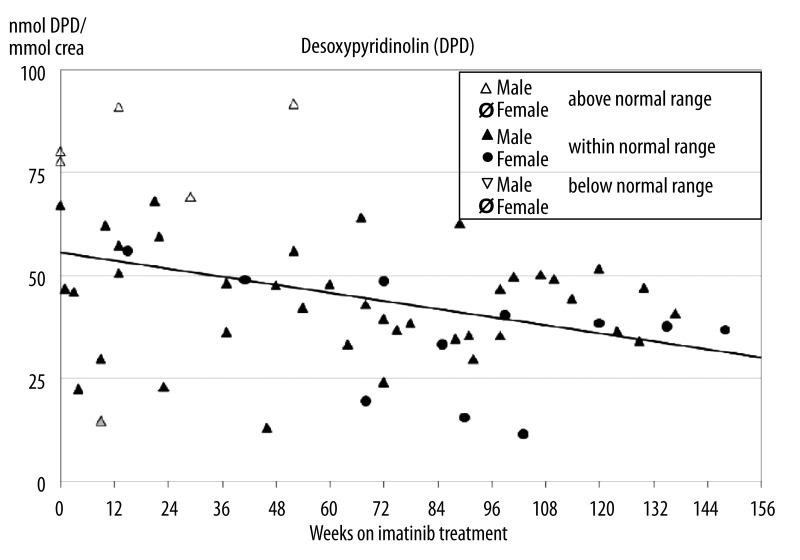
Urine DPD levels of 57 measurements ranged from 15–92 nmol DPD per mmol creatinine (Figure 3) whereby 9% (5/57) were above the normal range. In these 5 specimens also elevated PYD levels had been measured while the sixth specimen exhibiting elevated PYD concentration was measured with high-normal concentration of DPD. Only one measurement was below the normal range, this specimen was identical with the one exhibiting reduced PYD concentration. Although 50 out of 57 measurements were found within the normal range a statistically significant linear decline of −0.16 nmol DPD per mg creatinine per week was calculated during imatinib treatment (p=0.01).
Figure 3.
Desoxypyridinoline (DPD, marker of bone resorption) concentration in second void urine specimen during imatinib treatment. Although 50 out of 57 measurements were found within the normal range a significant linear decline of −0.16 nmol DPD per mg creatinine per week could be demonstrated.
Discussion
This study is the first report on changes in biochemical skeletal metabolic markers in children under ongoing imatinib therapy. In adults Berman et al. described for the first time 16 patients who experienced hypophosphatemia and a compensatory increase of parathyroid hormone during treatment with imatinib [19]. These findings were confirmed in conjunction with variable changes in markers of bone turnover in cross-sectional and short-term prospective studies in small adult cohorts [20]. As changes occurred in the early stages of imatinib therapy a shift in bone remodeling to increased formation was suggested due to direct stimulation of osteoblasts with restrained resorption. Sullivan et al. demonstrated that these biochemical changes persist but do not progress during a two year therapy with imatinib [21]. In a biphasic fashion bone formation is initially increased while in the later course bone turnover is affected by impairment of both: decreased resorption by osteoclasts and decreased formation by osteoblasts. This is due to the inhibition of osteoclasts by blocking CAII as well as c-kit, c-fms, and PDGFR signalling, and activation of osteoblasts’ activity through the inhibition of PDGFR by imatinib. The resulting positive effects on bone mineral density and trabecular bone volume may suggest increased bone strength in adult patients with CML [22].
In childhood a growing skeleton evidently is increasing its mass, however, more cellular activity is committed to removing and replacing existing skeletal structures (i.e., remodeling) than to forming new bone at sites where none previously existed (i.e., modeling) [23]. As imatinib impairs the function of osteoclasts as well as osteoblasts a resulting negative impact on modelling is highly probable. It also can be expected that the changes in the profile of bone metabolic markers differ in childhood under imatinib challenge as bone mass is increasing while in adults the bone mass is constant or reduced because of developing osteoporosis. As imatinib inhibits the differentiation of osteoblasts from precursor cells and also the activity of mature osteoclasts, the net results of this inhibition is hard to predict with respect to the dynamic state of modeling and remodeling in the growing skeleton [9].
Decreased calcium levels resulting from hypophosphatemia have been reported in at least 50% of adult patients under imatinib treatment [19–21]. In this study only 25% children exhibited low Ca-serum levels and 3 out of these also had low serum phosphate with increased PTH. Due to the small number of pediatric pts in this study it is impossible to judge whether hypophosphatemia under imatinib is less frequent in children than in adults. In addition, our study was limited by the timing of the blood and urine sampling because calcium, phosphate, and PTH were not measured in a fasting state. PTH also causes phosphaturia, resulting in a low-normal or low serum phosphorus level. Imatinib is typically taken after breakfast and dietary phosphate intake (sausages, soft drinks) might have contributed to increase lowered serum phosphate levels in this cohort, contrasting reports from adults [19]. PTH activates osteoblasts, which stimulate the transformation of preosteoclasts into mature osteoclasts. In adults imatinib therapy is associated with the development of secondary hyperparathyroidism in a high proportion of patients. Also in the presented pediatric cohort 50% of the patients showed hyperparathyroidism. With respect to collection time of the specimen in the later morning it must be stressed that serum levels of PTH are typically low at this time as PTH levels show diurnal variation, with a peak in the early morning, between 3–5 hrs and a second peak at 17 hrs [24–26].
25-vit-D3 is the major circulating vitamin D metabolite and is used to determine a patient’s vitamin D status. 25-vit-D3 levels are inversely associated with PTH levels [27,28]. PTH increases the metabolism of 25-vit-D3 to 1,25-vit-D3, which further exacerbates the vitamin D deficiency. Contrasting data from adults, the majority of children under imatinib treatment in this cohort experienced vitamin 25-vit-D3 insufficiency (21 to 29 ng per ml) or deficiency (<21 ng per ml) being more pronounced in the wintertime (Figure 1A) which is in line with the finding of hyperparathyroidism in half of the patients. [21] As the renal production of 1,25-vit-D3 is tightly regulated by plasma parathyroid hormone levels, hyperparathyroidism may result in normal 1,25-vit-D3 levels as observed in this pediatric cohort (Figure 1B).
Analysis of specific bone formation markers (osteocalcin, PINP, BAP) exhibited divergent results. Osteocalcin levels were found elevated in most patients, however, a statistically significant decline per week during the treatment time could be demonstrated. PINP levels were in the normal range in 75% of all patients but found reduced in the rest of the cohort. BAP levels also were found normal with the exception of 4 decreased measurements. These findings contrast other investigations where significant correlations between osteocalcin and BAP have been found in childhood, particularly in patients with high rates of bone turnover like during puberty [29]. Osteocalcin is regarded a more specific marker than BAP and PINP because it is only present in bone tissue [30]. Therefore the elevated osteocalcin levels point towards a moderate increased bone formation rate, which however decreases significantly over time. This is in line with reports from adults showing that bone formation markers were higher than baseline values during the first 6 months of imatinib treatment and also declined subsequently [21].
Bone resorption marker CTX-I serum levels are associated with the physiological bone resorption cathepsin-K-dependent pathway. The variation with age and sex is similar to patterns previously observed for other markers of collagen formation and breakdown in children and reflects the pediatric growth curve [31–33]. The timing of peak concentrations of serum bone resorption markers in relation to chronological age coincided with the timing of peak height velocity in each sex on a population basis. Highest concentrations of serum markers of bone collagen turnover occur overnight, decreasing to relatively constant concentrations between 10 to 15 hrs [34]. The timing of collection of the blood samples in our study almost coincided with this optimal period and is appropriate to most routine clinical samples [33]. In contrast to data from adults showing that imatinib exerted a mild antiresorptive effect we found increased osteoclasts’ activity as indicated by increased CTX-I levels. However, markers PYD and DPD determined in second void urine specimen were found -with few exceptions- mostly in the normal range whereby DPD showed a significant decline during ongoing imatinib treatment. It has been demonstrated that excretion of PYD and DPD is significantly correlated to growth velocity [29,35]. PYD is present in several types of tissues, including bone, cartilage, ligaments, and vessels while DPD, in contrast, is found only in the bone and dentin. Therefore DPD is considered more specific as a bone resorption marker [30,36]. In line with data from children exhibiting growth retardation under prolonged imatinib treatment [11,12,37,38] the normal but declining levels of DPD in our cohort point towards ongoing impairment of osteoclasts’ function while the elevated CTX-I levels do not support this conclusion. However, in general only weak correlations between markers are detected, as these reflect different biological processes during skeletal growth, which itself is nonlinear [18]. Markers are also released during different stages of bone formation, bone resorption or growth processes, and may have different elimination pathways and serum half-lives, affecting their relation at distinct time points during growth. In a small cohort of 38 adults patients the authors also did not find any correlation between imatinib plasma levels and total serum calcium, phosphorus, 25-vit-D3, 1,25-vit-D3, PTH and the degradation products of CTX-I [39]. Two case reports on children experiencing massive growth retardation under imatinib treatment described that bone formation markers (osteocalcin and BAP) as well as urinary DPD (bone resorption marker) were lower than those of age matched controls also suggesting that the effect of decreased bone formation exceeds that of decreased bone resorption [37,38].
Conclusions
In conclusion, there is sound evidence in adults and children that dysregulated bone remodeling may be a side effect of imatinib treatment. This highly probably results from off-target effects of imatinib on osteoclasts and osteoblasts. Bone is constantly remodeled in children as well as in adults, but children also grow, whereby bone modeling is achieved by appositional growth along periostal surfaces and by calcification of cartilage adjacent to the growth plate. While biochemical markers of bone turnover are not specific for either bone modeling or remodeling changes in biochemical markers of bone turnover will also reflect changes in growth velocity [15,19]. In addition, data from human and animal models under long-term imatinib treatment have shown that bone mass density increases, but changes may not be identical at different sites of the skeleton [9,19,20,37]. So, in children with CML regular evaluation of the skeletal actions and safety of imatinib during longer-term therapy is warranted.
Abbreviations
- ABL
Abelson leukemia virus
- ATP
adenosine triphosphate
- BAP
bone alkaline phosphatase
- BCR-ABL
breakpoint cluster region – Abelson leukemia virus
- CAII
carbonic anhydrase II
- c-fms
receptor of macrophage-colony-stimulating factor (M-CSF)
- c-kit
receptor of stem cell factor (SCF)
- CML
chronic myeloid leukemia
- CTX-I
C-terminal cross-linking telopeptide of collagen type I
- DPD
desoxypyridinoline
- ELICA
electrochemiluminescence immunoassay
- OC
osteocalcin
- PDGFR
Platelet-derived growth factor receptor
- Ph+
Philadelphia-Chromosome positive
- PINP
prokollagen type l N propeptide
- PTH
parathyroid hormone
- PYD
pyridinoline
- RIA
radioimmunoassay
- RT-PCT
reverse transkriptase polymerase chain reaction
- TKI
tyrosine kinase inhibitor
- 1,25-vit-D3
1,25-dihydroxyvitamin D3
- 25-vit-D3
25-hydroxyvitamin D3
Footnotes
Clinical trial registration number
The trial “CML-paed II” is listed and registered in: ClinicalTrials.gov Identifier: NCT00445822.
Source of support: The work was in part funded by the German Research Foundation (Grant No: DFG SU 122/3-1)
Financial disclosures and conflict of interests
M Suttorp has received research funding from Novartis. HJ Roth is employed by Limbach Laboratory, Heidelberg, Germany.
References
- 1.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 2.Champagne MA, Capdeville R, Krailo M, et al. Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: results from a Children’s Oncology Group phase 1 study. Blood. 2004;104:2655–60. doi: 10.1182/blood-2003-09-3032. [DOI] [PubMed] [Google Scholar]
- 3.Millot F, Guilhot J, Nelken B, et al. Imatinib mesylate is effective in children with chronic myelogenous leukemia in late chronic and advanced phase and in relapse after stem cell transplantation. Leukemia. 2006;20:187–92. doi: 10.1038/sj.leu.2404051. [DOI] [PubMed] [Google Scholar]
- 4.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27:5175–81. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millot F, Baruchel A, Guilhot J, et al. Imatinib is effective in children with previously untreated chronic myelogenous leukemia in early chronic phase: results of the French national phase IV trial. J Clin Oncol. 2011;29:2827–32. doi: 10.1200/JCO.2010.32.7114. [DOI] [PubMed] [Google Scholar]
- 6.Champagne MA, Fu CH, Chang M, et al. Higher dose imatinib for children with de novo chronic phase chronic myelogenous leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;57:56–62. doi: 10.1002/pbc.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barr RD. Imatinib mesylate in children and adolescents with cancer. Pediatr Blood Cancer. 2010;55:18–25. doi: 10.1002/pbc.22484. [DOI] [PubMed] [Google Scholar]
- 8.Suttorp M, Millot F. Treatment of pediatric chronic myeloid leukemia in the year 2010: use of tyrosine kinase inhibitors and stem-cell transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:368–76. doi: 10.1182/asheducation-2010.1.368. [DOI] [PubMed] [Google Scholar]
- 9.Vandyke K, Fitter S, Dewar AL, et al. Dysregulation of bone remodeling by imatinib mesylate. Blood. 2010;115:766–74. doi: 10.1182/blood-2009-08-237404. [DOI] [PubMed] [Google Scholar]
- 10.Breccia M, Alimena G. The metabolic consequences of imatinib mesylate: Changes on glucose, lypidic and bone metabolism. Leuk Res. 2009;33:871–75. doi: 10.1016/j.leukres.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Millot F, Baruchel A, Guilhot J, et al. Imatinib Is Efficient but Has a Negative Impact On Growth in Children with Previously Untreated chronic Myelogenous Leukaemia (CML) in Early Chronic Phase (CP): Results of the French National Phase IV Trial (Abstract) Blood. 2009;110:863. [Google Scholar]
- 12.Shima H, Tokuyama M, Tanizawa A, et al. Distinct impact of imatinib on growth at prepubertal and pubertal ages of children with chronic myeloid leukemia. J Pediatr. 2011;159:676–81. doi: 10.1016/j.jpeds.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 13.Bansal D, Shava U, Varma N, et al. Imatinib has adverse effect on growth in children with chronic myeloid leukemia. Pediatr Blood Cancer. 2012;59(3):481–84. doi: 10.1002/pbc.23389. [DOI] [PubMed] [Google Scholar]
- 14.Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45:153–59. doi: 10.1258/acb.2007.007091. [DOI] [PubMed] [Google Scholar]
- 15.Black D, Duncan A, Robins SP. Quantitative analysis of the pyridinium crosslinks of collagen in urine using ion-paired reversed-phase high-performance liquid chromatography. Anal Biochem. 1988;169:197–203. doi: 10.1016/0003-2697(88)90274-6. [DOI] [PubMed] [Google Scholar]
- 16.van der Sluis IM, Hop WC, van Leeuwen JP, et al. A cross-sectional study on biochemical parameters of bone turnover and vitamin d metabolites in healthy dutch children and young adults. Horm Res. 2002;57:170–79. doi: 10.1159/000058378. [DOI] [PubMed] [Google Scholar]
- 17.Reiss I, Interrieden D, Kruse K. Bestimmung der knochenspezifischen alkalischen phosphatase bei Störungen des Kalziumstoffwechsels im Kindesalter. Monatsschr Kinderheilkd. 1996;1 44:885–90. [in German] [Google Scholar]
- 18.Rauchenzauner M, Schmid A, Heinz-Erian P, et al. Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007;92:443–49. doi: 10.1210/jc.2006-1706. [DOI] [PubMed] [Google Scholar]
- 19.Berman E, Nicolaides M, Maki RG, et al. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med. 2006;354(19):2006–13. doi: 10.1056/NEJMoa051140. [DOI] [PubMed] [Google Scholar]
- 20.Osorio S, Noblejas AG, Durán A, Steegmann JL. Imatinib mesylate induces hypophosphatemia in patients with chronic myeloid leukemia in late chronic phase, and this effect is associated with response. Am J Hematol. 2007;82:394–95. doi: 10.1002/ajh.20778. [DOI] [PubMed] [Google Scholar]
- 21.O’Sullivan S, Horne A, Wattie D, et al. Decreased bone turnover despite persistent secondary hyperparathyroidism during prolonged treatment with imatinib. J Clin Endocrinol Metab. 2009;94:1131–36. doi: 10.1210/jc.2008-2324. [DOI] [PubMed] [Google Scholar]
- 22.Jönsson S, Olsson B, Ohlsson C, et al. Increased cortical bone mineralization in imatinib treated patients with chronic myelogenous leukemia. Haematologica. 2008;93:1101–3. doi: 10.3324/haematol.12373. [DOI] [PubMed] [Google Scholar]
- 23.Seeman E, Delmas PD. Bone quality – the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–61. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 24.el-Hajj Fuleihan G, Klerman EB, Brown EN, et al. The parathyroid hormone circadian rhythm is truly endogenous--a general clinical research center study. J Clin Endocrinol Metab. 1997;82:281–86. doi: 10.1210/jcem.82.1.3683. [DOI] [PubMed] [Google Scholar]
- 25.Gertz BJ, Clemens JD, Holland SD, et al. Application of a new serum assay for type I collagen cross-linked N-telopeptides: assessment of diurnal changes in bone turnover with and without alendronate treatment. Calcif Tissue Int. 1998;63:102–6. doi: 10.1007/s002239900497. [DOI] [PubMed] [Google Scholar]
- 26.Hannon R, Eastell R. Preanalytical variability of biochemical markers of bone turnover (Review) Osteoporos Int. 2000;11(Suppl 6):30–44. doi: 10.1007/s001980070004. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 28.Marx SJ. Hyperparathyroid and hypoparathyroid disorders. N Engl J Med. 2000;343:1863–75. doi: 10.1056/NEJM200012213432508. [DOI] [PubMed] [Google Scholar]
- 29.Rauch F, Schönau E, Woitge H, Remer T, Seibel M. Urinary excretion of hydroxy- pyridinium cross-links of collagen reflects skeletal growth velocity in normal children. Exp Clin Endocrinol. 1994;102:94–97. doi: 10.1055/s-0029-1211269. [DOI] [PubMed] [Google Scholar]
- 30.Szulc P, Seeman E, Delmas PD. Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int. 2000;11:281–94. doi: 10.1007/s001980070116. [DOI] [PubMed] [Google Scholar]
- 31.Crofton PM, Evans N, Taylor MR, Holland CV. Procollagen type I amino-terminal propeptide: pediatric reference data and relationship with procollagen type I carboxyl- terminal propeptide. Clin Chem. 2004;50:2173–76. doi: 10.1373/clinchem.2004.039958. [DOI] [PubMed] [Google Scholar]
- 32.Rauch F, Schoenau E. Markers of bone metabolism – use in pediatrics (Review) Clin Lab. 1997;43:743–52. [Google Scholar]
- 33.Crofton PM, Evans N, Taylor MR, Holland CV. Serum CrossLaps: pediatric reference intervals from birth to 19 years of age. Clin Chem. 2002;48:671–73. [PubMed] [Google Scholar]
- 34.Christgau S, Bitsch-Jensen O, Hanover Bjarnason N, et al. Serum CrossLaps for monitoring the response in individuals undergoing antiresorptive therapy. Bone. 2000;26:505–11. doi: 10.1016/S8756-3282(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 35.Marowska J, Kobylińska M, Lukaszkiewicz J, et al. Pyridinium crosslinks of collagen as a marker of bone resorption rates in children and adolescents: normal values and clinical application. Bone. 1996;19:669–77. doi: 10.1016/s8756-3282(96)00284-0. [DOI] [PubMed] [Google Scholar]
- 36.Watts NB. Clinical utility of biochemical markers of bone remodeling. Clin Chem. 1999;45:1359–68. [PubMed] [Google Scholar]
- 37.Mariani S, Giona F, Basciani S, et al. Low bone density and decreased inhibin-B/FSH ratio in a boy treated with imatinib during puberty. Lancet. 2008;372:111–12. doi: 10.1016/S0140-6736(08)61023-5. [DOI] [PubMed] [Google Scholar]
- 38.Schmid H, Jaeger BA, Lohse J, Suttorp M. Longitudinal growth retardation in a prepuberal girl with chronic myeloid leukemia on long-term treatment with imatinib. Haematologica. 2009;94:1177–79. doi: 10.3324/haematol.2009.008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legros L, Breuil V, Ferrari P, et al. Hypophosphatemia Observed in Chronic Myeloid Leukemia Patients Treated with Imatinib Mesylate (Gleevec(R)) Is Related to Digestive Side-Effects (Abstract) Blood. 2006;108:4765. [Google Scholar]
- 40.Yang L, Grey V. Pediatric reference intervals for bone markers. Clin Biochem. 2006;39:561–68. doi: 10.1016/j.clinbiochem.2005.11.015. [DOI] [PubMed] [Google Scholar]