Summary
Background
Given prior studies demonstrating the marked clinical activity of oral estrogens in prostate cancer, more recent data demonstrating the safety of transdermal estradiol, and the renewed interest in targeting testosterone metabolism and androgen receptor pathways, we report the results of a trial of transdermal estradiol in advanced heavily pre-treated castrate and chemotherapy refractory patients.
Material/Methods
Patients with prostate cancer progressing after androgen ablation therapy and chemotherapy were treated with transdermal estradiol patches (0.4 mg per 24 hours total) applied weekly and assessed for tolerability and biochemical activity.
Results
Twenty-two patients were treated on study with all patients evaluable for safety and 20 patients evaluable for response. All patients had aggressive and resistant disease, as demonstrated by a median PSA of 170 ng/mL (range 14 to 5030 ng/mL), with more than 60% having been treated with two or more prior chemotherapy regimens, and 20% with visceral disease. Nine patients had a decrease in PSA, of which two patients had a PSA response defined as a decline in PSA by 50%. Therapy was well tolerated and no thrombotic events were observed.
Conclusions
In heavily pre-treated patients with advanced castrate and chemotherapy refractory metastatic prostate cancer, transdermal estradiol was safe and had biochemical activity. These data support further studies to understand if transdermal estradiol can be useful following multiple standard therapies.
Keywords: estradiol, abiraterone, testosterone, prostate cancer
Background
The era of hormonal therapy for prostate cancer began 60 years ago, when Huggins et al. first described the beneficial effects of androgen ablation on locally advanced and metastatic prostatic carcinoma [1,2]. Landmark studies produced from this group led to the routine use of bilateral orchiectomy and/or estrogens as a means to deprive prostatic tumor cells of testosterone [2]. While orchiectomy was considered the gold standard of hormonal therapy, treatment with estrogens was also very effective [3–5]. In fact, the estrogen diethylstilbestrol was the non-surgical treatment of choice for first line therapy of metastatic hormone-sensitive prostate cancer and was shown to achieve castrate levels of testosterone [3,6,7]. Administration of estrogen suppresses gonadatropin secretion thereby decreasing testosterone produced by the Leydig cells of the testes and also reduces levels of adrenal androgens [8,9]. Currently, however, gonadotropin-releasing hormone (GnRH) agonists are the standard of care for achieving castrate levels of testosterone [10,11], because of concerns of increased cardiovascular toxicity including pulmonary emboli, myocardial infarction and cerebrovascular accidents associated with oral estrogens [3,5–7].
Although the use of estrogen as primary hormonal therapy for patients with castrate sensitive prostate cancer disease has been supplanted by GnRH agonists, a clinical benefit in patients with castrate resistant prostate cancer (CRPC) is possible and warrants further study. Maximal inhibition of the androgen receptor remains an important goal in men with CRPC [12,13], providing a rationale for further development of estrogen as a therapy for this population with limited therapeutic options beyond GnRH agonist therapy and chemotherapy.
There is also renewed interest in hormonal approaches to prostate cancer with the recent development of active agents that alter testosterone metabolism, such as abiraterone, or agents that target the androgen receptor pathway, such as MDV-3100, increasing the importance of studies on hormonally active agents, such as estrogen. Previously, Beer et al. demonstrated activity of transdermal estradiol in androgen-independent prostate cancer patients that had not received chemotherapy [14]. Given the lack of thromboembolic toxicity with transdermal estrogen in contrast to oral estrogen, and its potential benefit in men with CRPC, we evaluated transdermal estrogen 0.4 mg/day in men with CRPC who had progressed on chemotherapy. The results of our trial are important, as they provide preliminary evidence of activity of estradiol in a heavily pretreated population and support future studies of estrogen following newer agents in development, or recently approved options [15–17].
Material and Methods
Patients
Eligible subjects had metastatic prostate adenocarcinoma, with progressive disease while receiving treatment with castrating therapy (either GnRH angoist or orchiectomy), and had received at least one docetaxel based chemotherapy based regimen. Patients on antiandrogens must have had progression after withdrawal of the antiandrogen for 4 weeks (flutamide) or 6 weeks (bicalutamide). Other key inclusion criteria were a PSA ⩾10ng/ml, ECOG performance status ⩽2, serum creatinine ⩽2× ULN, total bilirubin <2× ULN, and AST/ALT <2× ULN. Subjects with a history of a pulmonary embolus or deep venous thrombosis (DVT) on anticoagulation for less than 6 months prior to enrollment, severe cardiovascular disease, known CNS metastasis, triglyceride >2× ULN or patients taking herbal supplements were excluded.
Treatment
Patients were instructed to apply 4 transdermal estradiol patches, each patch releasing 0.1 mg per 24 hours for a total of 0.4 mg/day, once weekly. The patch was applied to a clean, dry, intact area of the lower abdomen or the upper quadrant of the buttock. All 4 patches were changed every 7 days and the site of application was rotated. Generic transdermal estradiol patches were allowed. Each treatment cycle lasted 21 days. Treatment continued until there was disease progression, as measured by RECIST, or there was unacceptable toxicity.
Response criteria
Disease response and progression were defined by RECIST criteria. In patients with radiographically stable disease, PSA response and progression were defined by the PSA working group criteria, which defined PSA response as a decline from baseline value by ⩾50%, or normalization of PSA (defined as PSA less than 0.2 ng/ml), confirmed by a second measurement ⩾3 weeks later. PSA progression was defined as a ⩾25% rise and a value of at least 5 ng/ml in subjects without PSA response, or an increase of serum PSA above the nadir value by ⩾50%, provided that the increase is a minimum of 5 ng/mL, confirmed by a second PSA measurement ⩾3 weeks later. Patients were monitored with physical exam, toxicity assessment, PSA, and testosterone levels every 3 weeks. Bone scans (and CT scan if measurable disease is present a baseline) were performed every 12 weeks.
Statistical considerations
In this phase II trial of transdermal estradiol in patients with castrate and chemotherapy resistant prostate cancer, the primary endpoint was PSA response defined as a confirmed 50% decrease in PSA from baseline or normalization of PSA (less than 0.2 ng/ml), confirmed by a second measurement ⩾3 weeks later. Using a Simon’s two-stage design, the null hypothesis is that the response rate is below or equal 20%. To have an 80% power to reject the null hypothesis when the true response rate is 40% the trial recruited 18 patients evaluable for response. Enrollment was planned to terminate if 4 or fewer patients had a PSA response. Otherwise, an additional 15 patients were to be added.
Results
There were 23 registered patients and enrolled on study, with one patient withdrawing consent prior to initiating treatment on protocol. The characteristics of the 22 patients that were treated on study are shown in Table 1. The median age was 72 years (range 57–85). Patients had aggressive and resistant disease, as demonstrated by a median PSA of 170 ng/mL (range 14 to 5030 ng/mL), and more than 60% of patients enrolled had been treated with two or more chemotherapy regimens. Additionally, four patients had visceral disease defined as liver and/or lung metastasis and ECOG performance status was ⩾1 in 14 patients on study.
Table 1.
Patient demographics.
| Characteristics | Values |
|---|---|
| Number of patients treated | 22 |
| Median age (range) | 72 (57–85 years) |
| ECOG performance status | |
| 0 | 8 |
| 1 | 10 |
| 2 | 4 |
| Median PSA level (range) at start | 170 (13.8–5030 ng/ml) |
| Site of metastases | |
| Bone only | 10 |
| Lymph node only | 3 |
| Viscera (lung, liver) only | 3 |
| Bone and node | 5 |
| Bone and viscera | 1 |
| Prior therapy | |
| Nonsteroidal antiandrogen | 68% |
| Ketoconazole | 18% |
| One prior chemotherapy regimen | 36% |
| Two prior chemotherapy regimens | 32% |
| Three prior chemotherapy regimens | 27% |
| Four prior chemotherapy regimens | 5% |
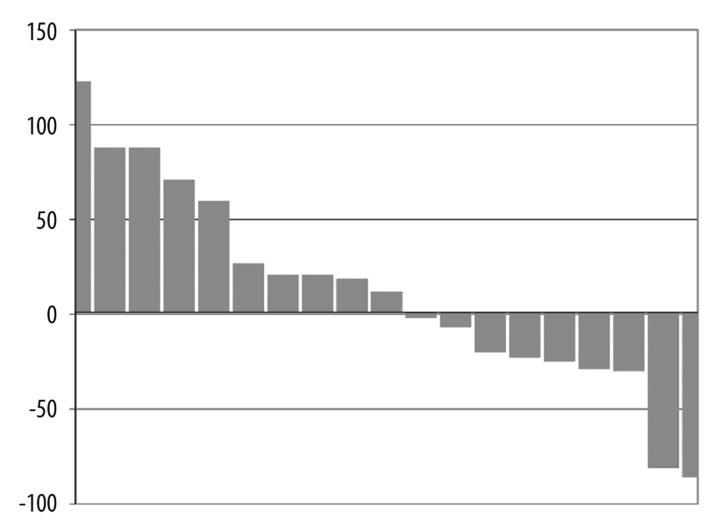
A PSA response defined as a decline in PSA by 50% was seen in two patients who remained on study for 9 weeks (3 cycles) and 45 weeks (15 cycles), respectively. An additional 7 patients had some decrease in PSA. Ten patients had stable disease and remained on study for 4 or more cycles. Maximal PSA response during treatment is depicted in the waterfall plot in Figure 1. As shown in this figure, 9 patients had a decrease in PSA during therapy. No objective disease responses by RECIST were noted. Serum testosterone levels at baseline and at cycle 3 are shown in Table 2. One of the two patients with >50% PSA decrease (patient #12 and #22) had a decrease in serum testosterone on therapy.
Figure 1.
Waterfall plot: The maximum percentage PSA response is shown for 19 of the 20 patients treated beyond cycle 1 (one of 20 patients with a 900% increase was excluded from the figure to better represent an appropriate scale). The y-axis represents the percentage change in PSA on study, with patients represented on the x-axis. As shown, 9 patients (including 2 patients with greater than a 50% decrease) had a decrease in PSA and 11 patients (including the patient not shown that had a 900% increase in PSA) had an increase.
Table 2.
Testosterone levels in patients at baseline and at cycle 3.
| Patient number | Pretreatment testosterone (ng/dL) | Cycle 3 testosterone (ng/dL) |
|---|---|---|
| 1 | 19 | 14 |
| 2 | 18 | 25 |
| 3 | <10 | <10 |
| 4 | <10 | <10 |
| 6 | 29.4 | 10.3 |
| 8 | 35 | 48 |
| 12 | <10 | <10 |
| 14 | 25 | 10 |
| 15 | 7 | 7 |
| 17 | 25 | 7 |
| 19 | 17 | 19 |
| 22 | 115 | 32 |
| 23 | 10 | 10 |
Treatment with transdermal estradiol was well tolerated with no grade 4 toxicities and no evidence of thrombotic events (Table 3). Only 2 of the 22 patients did not receive a second cycle of therapy, with one patient being unable to have the patches adhere to the skin, and one patient required radiation to the spine shortly after starting treatment for a pre-existing lesion. Two additional patients did not receive a third cycle of therapy. In the 20 patients treated beyond cycle 1, no one was removed from the study due to toxicity with the most common reason for discontinuing therapy was disease progression. The only grade 3 toxicities were transient increase in AST and increased alkaline phosphatase, likely due to bone progression.
Table 3.
Toxicity occurrence in the 22 patients treated with transdermal estradiol according to the NCI Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| % | ||||
| Fatigue | 9 | 0 | 0 | 0 |
| Diaphoresis | 5 | 0 | 0 | 0 |
| Weight loss | 5 | 9 | 0 | 0 |
| Rash | 5 | 0 | 0 | 0 |
| Pruritus/itching | 9 | 0 | 0 | 0 |
| Anorexia | 5 | 0 | 0 | 0 |
| Nausea | 14 | 0 | 0 | 0 |
| Edema: limb | 14 | 0 | 0 | 0 |
| AST increase | 0 | 0 | 5 | 0 |
| Alkaline phosphatase | 0 | 0 | 5 | 0 |
| Pain – Bone/muscle | 5 | 5 | 0 | 0 |
| Pain – Breast | 14 | 0 | 0 | 0 |
| Gynecomastia | 9 | 0 | 0 | 0 |
| Thrombosis | 0 | 0 | 0 | 0 |
Discussion
These data demonstrate the safety and activity of transdermal estradiol in heavily pretreated patients resistant to androgen ablation therapy and multiple chemotherapy regimens. This study is timely, as it is a proof-of-principle that supports further studies to understand the role of transdermal estradiol with recently approved and new agents in development, and agents that target the testosterone receptor pathway.
Our study results are consistent with prior studies assessing the activity of transdermal estradiol in patients with less advanced disease. For example, Beer et al. demonstrated that treatment with six 0.1 mg per 24 hour patches in patients with CRPC without prior treatment with chemotherapy, demonstrated three out of 24 patients with a PSA reduction of > 50% [14]. Baseline characteristics of the patients enrolled in the Beer study compared to our present study reveal a less aggressive disease compared to those enrolled in our study. The median PSA at the start of that study was 22.3 ng/mL, 25% of the patient did not have metastasis, and no patients were noted to have visceral disease compared to our study with a median starting PSA of 170 ng/mL, and all patients having metastatic chemorefractory disease, including 20% of patients with visceral disease. Although we treated patients with clearly more advanced disease with only four patches, we noted biochemical activity as shown in the waterfall plot in Figure 1. Further studies would be needed to understand the role of transdermal estradiol compared to other hormonal manipulations, especially newer agents such as abiraterone, which were not assessed in this study as they were not approved and/or generally available during our study period.
The mechanism of estradiol activity in CRPC is unclear. Prior studies have considered any activity to be due to an effect on testosterone, as well as a direct cytotoxic effect on the tumor. Transdermal estradiol clearly lowers testosterone in patients with androgen sensitive disease. Ockrim et al. treated 20 men with locally advanced or metastatic prostate cancer for eight weeks with 0.6 mg/24 hours of 17b-estradiol, reduced the number of patches to maintain castrate levels of testosterone, and found that an average of two patches per day was sufficient to maintain androgen ablation [18]. In our study of castrate and chemotherapy resistant prostate cancer patients, although one patient with a decrease of >50% in PSA had an associated decrease in testosterone, few patients overall with a decrease in PSA had a concurrent decrease in testosterone (Table 2). Other potential mechanisms of estradiol activity in CRPC include direct effects on the estrogen receptor. Pravettoni et al. demonstrated a direct anti-proliferative effect of estrogen receptor beta activation in the androgen independent prostate cancer DU145 cell line [19]. Additionally, estrogenic compounds may cause androgen receptor down regulation by direct repression of androgen receptor gene transcription [20,21]. Clearly, further studies would be needed to understand the mechanism of activity in this patient population.
Similar to the experience by Beer et al., our study supports the safety of transdermal estradiol for future studies. Both in the Beer study and our study, therapy was well tolerated without any thrombotic events. In fact, prior studies have supported that estrogen administrated via intramuscular or a transdermal route, as opposed to orally, is less thrombogenic by avoiding exposure of the liver to high estrogen concentrations from the hepatic circulation, which results in increased synthesis of thrombophilic coagulation factors [22–25]. Specifically, oral estrogen leads to increased factor VIII activity, increased factor VII and increased resistance to activated protein C among other changes [26]. Other toxicities were also uncommon including grade 1 gynecomastia reported in only 9% of patients enrolled on the present trial.
Conclusions
In summary, our study of transdermal estradiol in heavily pre-treated castrate and chemotherapy refractory patients with metastatic disease supports the safety and potential clinical activity of this approach for future studies. Given that this study was conducted during a period in which newer hormonal agents such as abiraterone were not approved or available, further studies could be considered along with, or following, newly approved agents and active hormonal agents under development that either modulate testosterone metabolic pathways or androgen receptor pathways [16,17].
Acknowledgement
The Cancer Institute of New Jersey Oncology Group investigators including Bonnie Guerin, MD, Daniel Moriarity, MD, Michael Nissenblatt, MD, and Steven Stanzione, MD.
Footnotes
Source of support: P30CA072720
References
- 1.Huggins C, Hodges CV. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. CA Cancer J Clin. 1972;22(4):232–40. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168(1):9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 3.Byar DP. Proceedings: The Veterans Administration Cooperative Urological Research Group’s studies of cancer of the prostate. Cancer. 1973;32:1126. doi: 10.1002/1097-0142(197311)32:5<1126::aid-cncr2820320518>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Cassileth BR, Soloway MS, Vogelzang NJ, et al. Patients’ choice of treatment in stage D prostate cancer. Urology. 1989;33:57. doi: 10.1016/0090-4295(89)90108-8. [DOI] [PubMed] [Google Scholar]
- 5.Loblaw DA, Mendelson DS, Talcott JA, et al. American Society of Clinical Oncology Recommendations for the Initial Hormonal Management of Androgen-Sensitive Metastatic, Recurrent, or Progressive Prostate Cancer. J Clin Oncol. 2004;22:2927. doi: 10.1200/JCO.2004.04.579. [DOI] [PubMed] [Google Scholar]
- 6.Malkowicz SB. The role of diethylstilbestrol in the treatment of prostate cancer. Urology. 2001;58:108. doi: 10.1016/s0090-4295(01)01252-3. [DOI] [PubMed] [Google Scholar]
- 7.Seidenfeld J, Samson DJ, Hasselblad V, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med. 2000;132:566. doi: 10.7326/0003-4819-132-7-200004040-00009. [DOI] [PubMed] [Google Scholar]
- 8.Kitahara S, Umeda H, Yano M, et al. Effects of intravenous administration of high dose-diethylstilbestrol diphosphate on serum hormonal levels in patients with hormone-refractory prostate cancer. Endocr J. 1999;46:659. doi: 10.1507/endocrj.46.659. [DOI] [PubMed] [Google Scholar]
- 9.Kitahara S, Yoshida K, Ishizaka K, et al. Stronger suppression of serum testosterone and FSH levels by a synthetic estrogen than by castration or an LH-RH agonist. Endocr J. 1997;44:527. doi: 10.1507/endocrj.44.527. [DOI] [PubMed] [Google Scholar]
- 10.Shahinian VB, Kuo YF, Freeman JL, et al. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 11.Weight CJ, Klein EA, Jones JS. Androgen deprivation falls as orchiectomy rates rise after changes in reimbursement in the U.S. Medicare population. Cancer. 2008;112:2195. doi: 10.1002/cncr.23421. [DOI] [PubMed] [Google Scholar]
- 12.Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell. 2009;16:458. doi: 10.1016/j.ccr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Harris WP, Mostaghel EA, Nelson PS, et al. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bland LB, Garzotto M, DeLoughery TG, et al. Phase II study of transdermal estradiol in androgen-independent prostate carcinoma. Cancer. 2005;103:717. doi: 10.1002/cncr.20857. [DOI] [PubMed] [Google Scholar]
- 15.Crawford ED, Flaig TW. Optimizing outcomes of advanced prostate cancer: drug sequencing and novel therapeutic approaches. Oncology. 2012;26:70. [PubMed] [Google Scholar]
- 16.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ockrim JL, Lalani EN, Laniado ME, et al. Transdermal estradiol therapy for advanced prostate cancer – forward to the past? J Urol. 2003;169:1735. doi: 10.1097/01.ju.0000061024.75334.40. [DOI] [PubMed] [Google Scholar]
- 19.Pravettoni A, Mornati O, Martini PG, et al. Estrogen receptor beta (ERbeta) and inhibition of prostate cancer cell proliferation: studies on the possible mechanism of action in DU145 cells. Mol Cell Endocrinol. 2007;263:46. doi: 10.1016/j.mce.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharyya RS, Krishnan AV, Swami S, et al. Fulvestrant (ICI 182,780) down-regulates androgen receptor expression and diminishes androgenic responses in LNCaP human prostate cancer cells. Mol Cancer Ther. 2006;5:1539. doi: 10.1158/1535-7163.MCT-06-0065. [DOI] [PubMed] [Google Scholar]
- 21.Bonkhoff H, Berges R. The Evolving Role of Oestrogens and Their Receptors in the Development and Progression of Prostate Cancer. European Urology. 2009;55:533. doi: 10.1016/j.eururo.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Aro J, Haapiainen R, Rasi V, et al. The effect of parenteral estrogen versus orchiectomy on blood coagulation and fibrinolysis in prostatic cancer patients. Eur Urol. 1990;17:161. doi: 10.1159/000464026. [DOI] [PubMed] [Google Scholar]
- 23.Hedlund PO, Ala-Opas M, Brekkan E, et al. Parenteral estrogen versus combined androgen deprivation in the treatment of metastatic prostatic cancer – Scandinavian Prostatic Cancer Group (SPCG) Study No. 5. Scand J Urol Nephrol. 2002;36:405. doi: 10.1080/003655902762467549. [DOI] [PubMed] [Google Scholar]
- 24.von Schoultz B, Carlstrom K, Collste L, et al. Estrogen therapy and liver function – metabolic effects of oral and parenteral administration. Prostate. 1989;14:389. doi: 10.1002/pros.2990140410. [DOI] [PubMed] [Google Scholar]
- 25.Kroon UB, Silfverstolpe G, Tengborn L. The effects of transdermal estradiol and oral conjugated estrogens on haemostasis variables. Thromb Haemost. 1994;71:420. [PubMed] [Google Scholar]
- 26.Lycette JL, Bland LB, Garzotto M, et al. Parenteral estrogens for prostate cancer: can a new route of administration overcome old toxicities? Clin Genitourin Cancer. 2006;5:198. doi: 10.3816/CGC.2006.n.037. [DOI] [PubMed] [Google Scholar]