Summary
Background
The receptor-binding cancer antigen expressed on SiSo cells (RCAS1) is a human tumor-associated antigen that contributes to tumor progression by enabling cancer cells to evade immune surveillance. The present study aimed to evaluate the clinical significance of RCAS1 expression in human benign and malignant thyroid lesions.
Material/Methods
RCAS1 protein expression was assessed immunohistochemically on paraffin-embedded thyroid tissues from 121 patients with benign and malignant lesions and was associated with type of thyroid histopathology and tumor stage parameters such as tumor size, lymph node metastases, capsular, lymphatic and vascular invasion.
Results
RCAS1 positivity, overexpression and staining intensity provided a distinct discrimination between benign and malignant thyroid cases (p=0.0006, p=0.0001 and p=0.0001, respectively), as well as between hyperplastic nodule and papillary carcinoma cases (p=0.0229, p=0.0001 and p=0.0001, respectively). RCAS1 positivity, overexpression and staining intensity also provided distinct discrimination between cases with Hashimoto thyroiditis and those with hyperplastic nodule (p=0.0221, p=0.0001 and p=0.0019, respectively). In the subgroup of malignant thyroid lesions, RCAS1 overexpression was significantly associated with large tumor size (p=0.0246), the presence of lymph node metastases (p=0.0351) and capsular invasion (p=0.0397).
Conclusions
RCAS1 protein may participate in thyroid neoplastic transformation and could be considered as a useful biomarker to improve diagnostic scrutiny.
Keywords: RCAS1, thyroid malignancy, immunohistochemistry, clinicopathological parameters, diagnosis, biomarker
Background
Receptor-binding cancer antigen expressed on SiSo cells (RCAS1) was initially recognized by the mouse monoclonal antibody 22-1-1, which was raised by immunization of mice with the human uterine cervical adenocarcinoma cell line SiSo [1]. It is a 40-kd type II membrane protein that forms homo-oligomers through its C-terminal coiled-coil structures, whereas it also exists in soluble form, probably by alternative splicing [2]. RCAS1 has currently been considered as a novel tumor-associated antigen that induces cell-cycle arrest and/or apoptosis in RCAS1 receptor-bearing human cells [3]. Substantial evidence also supports that RCAS1 can function as a ligand for a putative receptor present on various human cell lines such as erythroid leukemia, K562 cells, and normal peripheral lymphocytes such as T, B and NK cells, inhibiting cell growth and inducing apoptosis [4]. Notably, several studies documented that tumor cells may develop the ability to evade immune surveillance by several mechanisms, including RCAS1 up-regulation, and that RCAS1 positive tumor cells may induce apoptosis to their surrounding tumor infiltrating lymphocytes (TILs) [5,6]. Thus, it is speculated that RCAS1 can exert oncogenic properties, leading to immune cell depletion in several types of neoplasia [7].
Benign and malignant thyroid lesions constitute the most common neoplasia of endocrine glands, with growing rates during the last 2 decades [8,9]. The rapidly increasing incidence of thyroid cancer has mainly been attributed to the improvement in detection of small papillary tumors [10–13]. Most thyroid tumors can be readily diagnosed using histopathological criteria, allowing the pathologist to distinguish malignant from benign lesions and guarantying accurate classification for the majority of thyroid carcinoma variants [14]. However, in many cases the distinction between benign and malignant is quite subtle and the decision favoring one or another has clinical consequences and implies different treatment modalities [14]. Currently, there has been considerable progress in identifying biomarkers in thyroid tumors that improve the accuracy of fine-needle aspiration biopsy and contribute to the accurate estimation of tumor aggressiveness or behavior [15–17].
In the last few years, RCAS1 protein expression has been described in a variety of human malignancies, being correlated with demographic and clinicopathological characteristics of therapeutic and prognostic significance [7]. Notably, RCAS1 has been reported to be expressed in several head and neck neoplasms, including oral, esophageal and thyroid carcinoma [18–25]. RCAS1 overexpression was significantly associated with the dedifferentiation of thyroid carcinoma, being more frequently observed in anaplastic compared to papillary and follicular variants [25]. However, there are no available studies evaluating whether RCAS1 protein detection by immunohistochemistry can distinguish benign from malignant thyroid lesions and whether it correlates with tumor characteristics. In view of above considerations, the present study aimed to assess the immunohistochemical RCAS1 expression in a large series of benign and malignant thyroid lesions and to evaluate its diagnostic utility. The association of RCAS1 immunostaining with tumor stage parameters, such as tumor size, lymph node metastases, and capsular, lymphatic and vascular invasion was also examined.
Material and Methods
Patients
One hundred twenty-one formalin-fixed, paraffin-embedded thyroid tissues from an equal number of patients who had undergone thyroid surgery for benign or malignant disease were included in this study. Informed consent was obtained from all patients. None of the patients received any kind of anti-cancer treatment prior to surgery. Each case was classified according to the WHO histological classification of thyroid tumors [26]. The clinical material consisted of 68 benign (59 hyperplastic nodules and 9 Hashimoto thyroiditis) and 53 malignant (41 papillary, 5 medullary, 5 follicular and 2 anaplastic thyroid carcinomas) cases. The characteristics of the population under study categorized into benign and malignant thyroid lesions are summarized in Table 1.
Table 1.
Study population characteristics.
| Clinicopathological parameters | Benign | Malignant |
|---|---|---|
| N=121 | 68 (56%) | 53 (44%) |
|
| ||
| Age (mean ±SD; ys) | 48.95±14.21 | 50.09±15.37 |
| 49.38±15.11 | ||
|
| ||
| Gender | ||
|
| ||
| Male | 15 (12%) | 11 (9%) |
|
| ||
| Female | 53 (44%) | 42 (35%) |
|
| ||
| Histopathology | Hyperplastic nodule | Papillary |
| 59 (49%) | 41 (34%) | |
|
| ||
| Hashimoto thyroiditis | Medullary | |
| 9 (7%) | 5 (4%) | |
|
| ||
| Follicular | ||
| 5 (4%) | ||
|
| ||
| Anaplastic | ||
| 2 (2%) | ||
|
| ||
| Tumor size (T) | N/A* | |
|
| ||
| T1 | 36 (68%)** | |
|
| ||
| T2 | 13 (24%) | |
|
| ||
| T3 | 2 (4%)** | |
|
| ||
| T4 | 2 (4%)** | |
|
| ||
| Lymph node metastases (N) | N/A | |
|
| ||
| N0 | 47 (89%)** | |
|
| ||
| N1 | 6 (11%)** | |
|
| ||
| Distant metastases (M) | N/A | |
|
| ||
| M0 | 53 (100%) | |
|
| ||
| Capsular invasion | N/A | |
|
| ||
| No | 40 (75%)** | |
|
| ||
| Yes | 13 (25%)** | |
|
| ||
| Lymphatic invasion | N/A | |
|
| ||
| No | 44 (83%)** | |
|
| ||
| Yes | 9 (17%)** | |
|
| ||
| Vessel invasion | N/A | |
|
| ||
| No | 50 (94%)** | |
|
| ||
| Yes | 3 (6)** | |
|
| ||
| Ki-67 protein statement | p-value=0.0019 | |
|
| ||
| < mean value | 56 (46%) | 30 (25%) |
|
| ||
| ≥ mean value | 12 (10%) | 23 (19%) |
N/A – not applicable;
percentages in parentheses correspond to the number of malignant thyroid cases.
Immunohistochemistry
RCAS1 immunostaining was performed on formalin-fixed, paraffin-embedded thyroid tissue sections using a mouse monoclonal anti-RCAS1 antibody (MBL International Co, Nagoya, Japan) as previously described [27]. Appropriate negative controls were performed by omitting the primary antibody and/or substituting it with an irrelevant anti-serum. Pancreatic cancer tissue sections with known increased RCAS1 immunoreactivity were used as positive control [27]. Follicular cells’ proliferative capacity was assessed immunohistochemically using a mouse anti-human Ki-67 antigen; IgG1k antibody (clone MIB-1, Dakopatts, Glostrup, Denmark), as previously described (28, 29).
Evaluation of immunohistochemistry
Stained sections were independently assessed by S.T. and P.A., who were blinded to the clinical data, with complete observers’ agreement. Specimens were considered “positive” for RCAS1 when more than 5% of follicular cells within the section were positively stained (28, 29). The extent of RCAS1 expression was calculated by the percentage of the RCAS1-positive follicular cells in the total number of follicular cells within each specimen. The specimens were characterized to present overexpression for RCAS1 when the percentage of the positively stained follicular cells exceeded the mean percentage value [27]. In RCAS1-positive cases, the intensity of immunostaining was also estimated and graded in a 3 step scale as weak (+), moderate (++) and strong (+++) [27]. Ki-67 labeling index was classified into 2 categories, below and over mean value, as previously described (Table 2) [28,29].
Table 2.
Associations of RCAS1 positivity with patients’ clinicopathological characteristics.
| Clinicopathological characteristics | RCAS1 positivity | p-value | |
|---|---|---|---|
| Negative (%) | Positive (%) | ||
| All cases, N=121 | 53 (44) | 68 (56) | |
| Age (mean ±SD; ys) | 49.87±12.76 | 51.09±18.12 | 0.5097 |
| Gender | |||
| Female | 38 (31) | 57 (47) | 0.1071 |
| Male | 15 (12) | 11 (9) | |
| Histopathology | |||
| Benign | 39 (32) | 29 (24) | 0.0006 |
| Malignant | 14 (12) | 39 (32) | |
| Ki-67 protein statement | |||
| < mean value | 46 (38) | 40 (33) | 0.0007 |
| ≥ mean value | 7 (6) | 28 (23) | |
| Malignant cases, N=53 | 14 (26) | 39 (74) | |
| Tumor size (T) | 0.0964 | ||
| T1 | 12 (23) | 24 (45) | |
| T2-4 | 2 (4) | 15 (28) | |
| Lymph node metastases (N) | 0.1191 | ||
| N0 | 14 (26) | 33 (62) | |
| N1 | 0 (0) | 6 (11) | |
| Capsular invasion | 0.0779 | ||
| No | 13 (25) | 27 (51) | |
| Yes | 1 (2) | 12 (23) | |
| Lymphatic invasion | 0.2530 | ||
| No | 13 (25) | 31 (58) | |
| Yes | 1 (2) | 8 (15) | |
| Vascular invasion | |||
| No | 14 (26) | 36 (68) | 0.2853 |
| Yes | 0 (0) | 3 (6) | |
Statistical analysis
Chi-square tests were used to assess the difference of RCAS1 positivity, overexpression and staining intensity between malignant and benign thyroid lesions, as well as between papillary carcinoma and hyperplastic nodules, which comprise the most numerous histopathological entities of malignant and benign lesions, respectively. Chi-square tests were also performed to assess the association of RCAS1 positivity, overexpression and staining intensity with clinicopathological characteristics in the subgroup of malignant thyroid cases. Mann-Whitney U test and Kolmogorov-Smirnov test were further applied to evaluate the difference in the percentage of RCAS1-positive stained follicular cells between malignant and benign thyroid lesions, as well as between papillary carcinoma and hyperplastic nodules. A 2-tailed p<0.05 was considered statistically significant. Statistical analyses were performed using the software package SPSS for Windows (version 11.0; SPSS Inc., Chicago, IL, USA).
Results
RCAS1 positivity was noted in 68 out of 121 cases (56%) with thyroid lesions. The pattern of RCAS1 distribution was both cytoplasmic and membranous in all positive cases. Normal thyroid tissue was found negative for RCAS1. Representative RCAS1 immunostainings in cases with papillary and follicular carcinoma, as well as hyperplastic nodule and Hashimoto thyroiditis, are depicted in Figure 1. Forty-two out of 121 cases (35%) showed overexpression for RCAS1, while 50 cases (41%) presented moderate/strong RCAS1 staining intensity (Tables 3, 4).
Figure 1.
Representative immunostainings of RCAS1 protein expression in malignant thyroid cases with (A). Papillary carcinoma and (B). Follicular carcinoma and in benign thyroid cases with (C). Hyperplastic nodules and (D). Hashimoto thyroiditis (Original magnification ×400, Streptavidin-biotin-peroxidase, DAB chromogen, Harris hematoxylin counterstain).
Table 3.
Associations of RCAS1 overexpression with patients’ clinicopathological characteristics.
| Clinicopathological characteristics | RCAS1 overexpression | p-value | |
|---|---|---|---|
| < mean value (%) | ≥ mean value (%) | ||
| All cases, N=121 | 79 (65) | 42 (35) | |
| Age (mean ±SD; ys) | 49.31±12.11 | 51.82±18.33 | 0.5908 |
| Gender | |||
| Female | 61 (50) | 34 (28) | 0.6337 |
| Male | 18 (15) | 8 (7) | |
| Histopathology | |||
| Benign | 58 (48) | 10 (8) | 0.0001 |
| Malignant | 21 (17) | 32 (26) | |
| Ki-67 protein statement | |||
| < mean value | 63 (52) | 23 (19) | 0.0039 |
| ≥ mean value | 16 (13) | 19 (16) | |
| Malignant cases, N=53 | 21 (40) | 32 (60) | |
| Tumor size (T) | 0.0246 | ||
| T1 | 18 (34) | 18 (34) | |
| T2-4 | 3 (6) | 14 (26) | |
| Lymph node metastases (N) | 0.0351 | ||
| N0 | 21 (40) | 26 (49) | |
| N1 | 0 (0) | 6 (11) | |
| Capsular invasion | 0.0397 | ||
| No | 19 (36) | 21 (40) | |
| Yes | 2 (4) | 11 (21) | |
| Lymphatic invasion | 0.0549 | ||
| No | 20 (38) | 24 (45) | |
| Yes | 1 (2) | 8 (15) | |
| Vascular invasion | |||
| No | 21 (40) | 29 (55) | 0.1485 |
| Yes | 0 (0) | 3 (6) | |
Table 4.
Associations of RCAS1 staining intensity with patients’ clinicopathological characteristics.
| Clinicopathological characteristics | RCAS1 staining intensity | p-value | |
|---|---|---|---|
| Negative/weak (%) | Moderate/strong (%) | ||
| All cases, N=121 | 71 (59) | 50 (41) | |
| Age (mean ±SD; ys) | 49.02±12.45 | 51.44±18.56 | 0.7374 |
| Gender | |||
| Female | 52 (43) | 43 (36) | 0.0924 |
| Male | 19 (16) | 7 (6) | |
| Histopathology | |||
| Benign | 51 (42) | 17 (14) | 0.0001 |
| Malignant | 20 (17) | 33 (27) | |
| Ki-67 protein statement | |||
| < mean value | 57 (47) | 29 (24) | 0.0077 |
| ≥ mean value | 14 (12) | 21 (17) | |
| Malignant cases, N=53 | 20 (38) | 33 (62) | |
| Tumor size (T) | 0.8010 | ||
| T1 | 14 (26) | 22 (42) | |
| T2-4 | 6 (11) | 11 (21) | |
| Lymph node metastases (N) | 0.8132 | ||
| N0 | 18 (34) | 29 (55) | |
| N1 | 2 (4) | 4 (8) | |
| Capsular invasion | 0.5508 | ||
| No | 16 (30) | 24 (45) | |
| Yes | 4 (8) | 9 (17) | |
| Lymphatic invasion | 0.7649 | ||
| No | 17 (32) | 27 (51) | |
| Yes | 3 (6) | 6 (11) | |
| Vascular invasion | |||
| No | 20 (38) | 30 (57) | 0.1650 |
| Yes | 0 (0) | 3 (6) | |
RCAS1 positivity, overexpression and staining intensity provided statistically significant discriminations between cases with malignant and benign thyroid lesions (p=0.0006, p=0.0001 and p=0.0001, respectively), as well as between cases with hyperplastic nodule and papillary carcinoma (p=0.0229, p=0.0001 and p=0.0001, respectively). RCAS1 positivity, overexpression and staining intensity also provided a distinct discrimination between cases with Hashimoto thyroiditis and those with hyperplastic nodules (p=0.0221, p=0.0001 and p=0.0019, respectively). RCAS1 positivity, overexpression and staining intensity was positively associated with follicular cells proliferative capacity, reflected by Ki-67 labelling index (p=0.0007, p=0.0039 and p=0.0077, respectively). An increased incidence of RCAS1 positivity and moderate/strong RCAS1 staining intensity in female compared to male patients was noted, without reaching statistical significance (p=0.1071 and p=0.0924, respectively).
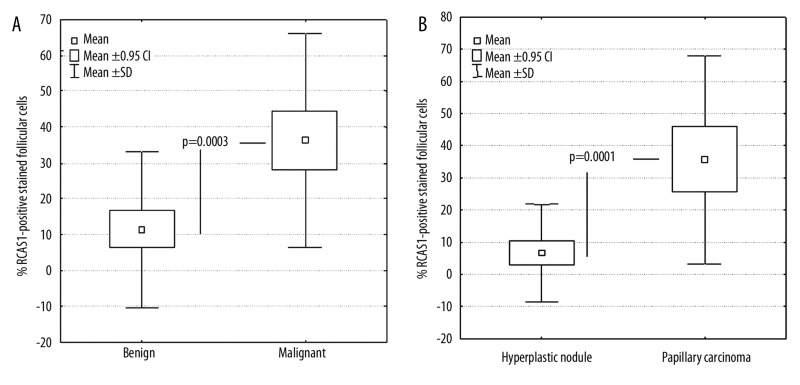
Mann-Whitney U tests and Kolmogorov-Smirnov tests were further performed to assess whether the percentage of RCAS1-positive stained follicular cells could provide discrimination among the different histopathological entities of thyroid malignancy. The percentage of RCAS1-positive stained follicular cells was significantly increased in cases with malignant compared to those with benign thyroid lesions (Figure 2A, mean percent RCAS1 positive staining: 35.51±29.91% vs. 11.43±21.87%, p=0.0001). Papillary carcinoma cases showed significantly increased percentage of RCAS1-positive stained follicular cells compared to those with hyperplastic nodules (Figure 2B, mean percent RCAS1-positive staining: 35.73±32.28% vs. 6.64±15.08%, p=0.0003). The percentage of RCAS1-positive stained follicular cells was also significantly increased in cases with Hashimoto thyroiditis compared to those with hyperplastic nodules (mean percent RCAS1-positive staining: 42.7±832.89% vs. 6.64±15.08%, p=0.0015).
Figure 2.
Box-whisker plots of% RCAS1 positive stained follicular cells: (A) Benign and Malignant thyroid lesions and (B). Hyperplastic nodules and Papillary carcinomas.
We also assessed whether RCAS1 positivity, overexpression and staining intensity bears a pronounced diagnostic effect on patients with malignant thyroid lesions. We stratified by pT stage (small vs. large tumor size, pT1 vs. pT2-4), lymph nodal status (absence vs. presence of lymph node metastases, pN0 vs. pN1), and capsular, lymphatic and vascular invasion (absence vs. presence of invasion). In cross-tables, the incidence of RCAS1-positivity was borderline increased in patients with tumors exhibiting large size (pT, p=0.0964) and capsular invasion (p=0.0779). RCAS1 overexpression was significantly more frequently observed in patients having tumors with large size (pT, p=0.0246), presence of lymph node metastases (p=0.0351) and capsular invasion (p=0.0397), while a correlation trend with vascular invasion (p=0.0549) was also noted. RCAS1 staining intensity was not associated with any demographic and clinicopathological parameters in the subgroup of patients with malignant thyroid lesions.
Discussion
It is well-established that RCAS1 is overexpressed in various tumors and seems to affect many aspects of cancer biology such as differentiation, proliferation, invasion and angiogenesis [6,7]. Elevated RCAS1 expression has been associated with tumor malignancy in several tissue types, being considered to exert a crucial role in tumor progression by enabling cancer cells to evade immune surveillance [6,7]. However, assessment of the clinical significance of RCAS1 expression in thyroid neoplasia remains scarce [15]. In this context, the present study aimed to assess the diagnostic utility of RCAS1 expression in thyroid neoplasia and its association with clinicopathological parameters crucial for patients’ management and prognosis.
We showed that RCAS1 immunoreactivity (positivity, overexpression and staining intensity) was significantly increased in cases with malignant compared to those with benign thyroid lesions. Such a distinct discrimination was also obtained between cases with papillary carcinoma and those with hyperplastic nodule, which comprise the most common histopathological entities of malignant and benign thyroid lesions. Moreover, in the subgroup of malignant thyroid lesions, RCAS1 overexpression was significantly associated with large tumor size, the presence of lymph node metastases and capsular invasion. These findings suggest that RCAS1 protein may participate in thyroid neoplastic transformation and could be considered as a potential marker to improve diagnostic scrutiny. In line with the present study, Ito et al showed that normal epithelium and follicular adenoma did not express or only faintly expressed RCAS1 [25]. Remarkably, in the subgroup of thyroid carcinomas, the same study clearly indicated that RCAS1 overexpression was significantly associated with tumor dedifferentiation, being more frequently observed in anaplastic compared to papillary and follicular carcinomas [25]. We further showed that cases with Hashimoto thyroiditis presented significantly increased RCAS1 immunoreactivity compared to those with hyperplastic nodules, with a mean percentage value of RCAS1-positive stained follicular cells slightly higher that that observed in papillary carcinomas. In this aspect, it should be noted that although Hashimoto thyroiditis is considered as a benign condition, it almost always harbors a genetic rearrangement that is strongly associated with and is highly specific for papillary thyroid carcinoma [30].
RCAS1 expression has been well-described in several types of head and neck neoplasia. More to the point, in oral squamous cell carcinoma (SCC), enhanced RCAS1 expression was significantly associated with large tumor size, presence of lymph node metastases, advanced disease stage and poor prognosis [18,20]. Notably, enhanced RCAS1 expression was significantly associated with the presence of apoptotic TILs in this type of malignancy [18,19]. Moreover, in esophageal SCC, increased RCAS1 expression was significantly associated with advanced disease stage, presence of lymph node metastasis and poor patient survival [21–24]. Preliminary studies by our group also showed that RCAS1 expression was significantly associated with muscular invasion, depth of invasion, mitotic index and stromal inflammatory reaction in mobile tongue SCC (Theocharis et al, unpublished data). Data from different studies points towards combined functional properties of RCAS1. The expression of RCAS1 putative receptor was found to be enhanced in lymphocytes activated by IL-2 [3–6]. Secreted RCAS1 also inhibited the growth of such activated cells in vitro by inducing their apoptotic cell death [3–6]. In this aspect, it was suggested that tumor cells may evade immune surveillance by expressing RCAS1 [31–33]. Moreover, RCAS1 was shown to facilitate tumor cell invasion of connective tissue in uterine cervical cancer by induction of stromal tissue remodeling, as well as through evasion of antitumor immune surveillance by an apoptotic counter-attack mechanism against lymphocytes [6,32,33].
Conclusions
RCAS1 protein expression detected by immunohistochemistry proved to be a useful diagnostic marker for discriminating malignant from benign thyroid cases and papillary carcinoma from hyperplastic nodules. The association of RCAS1 protein expression with tumor stage provided evidence for its possible implication in the development and progression of thyroid carcinoma. Further studies applying Western blot analysis and fluorescent techniques, as well as quantitative microscopy and correlation with additional markers for cell differentiation and behavior, should be performed to elucidate the underlining pathogenetic mechanisms. As RCAS1 is easily detectable in biofluids (34), further research effort should focus on the determination of RCAS1 levels in serum or plasma before and after treatment, with the aim of recognizing recurrence.
Footnotes
Source of support: Departmental sources
References
- 1.Sonoda K, Nakashima M, Kaku T, et al. A novel tumor-associated antigen expressed in human uterine and ovarian carcinomas. Cancer. 1996;77:1501–9. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1501::AID-CNCR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi K, Enjoji M, Nakashima M, et al. Novel serum tumor marker, RCAS1, in pancreatic diseases. World J Gastroenterol. 2005;11:5199–202. doi: 10.3748/wjg.v11.i33.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakashima M, Sonoda K, Watanabe T. Inhibition of cell growth and induction of apoptotic cell death by the human tumor-associated antigen RCAS1. Nat Med. 1999;5:938–42. doi: 10.1038/11383. [DOI] [PubMed] [Google Scholar]
- 4.Matsushima T, Nakashima M, Oshima K, et al. Receptor binding cancer antigen expressed on SiSo cells, a novel regulator of apoptosis of erythroid progenitor cells. Blood. 2001;98:313–21. doi: 10.1182/blood.v98.2.313. [DOI] [PubMed] [Google Scholar]
- 5.Sonoda K, Miyamoto S, Hirakawa T, et al. Association between RCAS1 expression and microenvironmental immune cell death in uterine cervical cancer. Gynecol Oncol. 2005;97:772–79. doi: 10.1016/j.ygyno.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Sonoda K, Miyamoto S, Nakashima M, Wake N. The biological role of the unique molecule RCAS1: a bioactive marker that induces connective tissue remodeling and lymphocyte apoptosis. Front Biosci. 2008;13:1106–16. doi: 10.2741/2748. [DOI] [PubMed] [Google Scholar]
- 7.Giaginis C, Giagini A, Theocharis S. Receptor-binding Cancer Antigen expressed on SiSo cells (RCAS1): a novel biomarker in the diagnosis and prognosis of human neoplasia. Histol Histopathol. 2009;24:761–76. doi: 10.14670/HH-24.761. [DOI] [PubMed] [Google Scholar]
- 8.Leenhardt L, Grosclaude P, Cherie-Challine L. Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14:1056–60. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 10.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–67. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 11.Ward EM, Jemal A, Chen A. Increasing incidence of thyroid cancer: is diagnostic scrutiny the sole explanation? Future Oncol. 2010;6:185–88. doi: 10.2217/fon.09.161. [DOI] [PubMed] [Google Scholar]
- 12.Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007;36:707–35. doi: 10.1016/j.ecl.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Stang MT, Carty SE. Recent developments in predicting thyroid malignancy. Curr Opin Oncol. 2009;21:11–17. doi: 10.1097/CCO.0b013e32831db2af. [DOI] [PubMed] [Google Scholar]
- 14.Fischer S, Asa L. Application of immunohistochemistry to thyroid neoplasms. Arch Pathol Lab Med. 2008;132:359–72. doi: 10.5858/2008-132-359-AOITTN. [DOI] [PubMed] [Google Scholar]
- 15.Vriens MR, Schreinemakers JM, Suh I, et al. Diagnostic markers and prognostic factors in thyroid cancer. Future Oncol. 2009;5:1283–93. doi: 10.2217/fon.09.85. [DOI] [PubMed] [Google Scholar]
- 16.Besic N, Sesek M, Peric B, et al. Predictive factors of carcinoma in 327 patients with follicular neoplasm of the thyroid. Med Sci Monit. 2008;14(9):CR459–67. [PubMed] [Google Scholar]
- 17.Golbidi S, Laher I. Antioxidant therapy in human endocrine disorders. Med Sci Monit. 2010;16(1):RA9–24. [PubMed] [Google Scholar]
- 18.Fukuda M, Tanaka A, Hamao A, et al. Expression of RCAS1 and its function in human squamous cell carcinoma of the oral cavity. Oncol Rep. 2004;12:259–67. [PubMed] [Google Scholar]
- 19.Toyoshima T, Nakamura S, Kumamaru W, et al. Expression of tumor-associated antigen RCAS1 and its possible involvement in immune evasion in oral squamous cell carcinoma. J Oral Pathol Med. 2006;35:361–68. doi: 10.1111/j.1600-0714.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsai TC, Yu CH, Cheng SJ, et al. Expression of RCAS1 is significantly associated with the progression and prognosis of oral squamous cell carcinomas in Taiwan. Oral Oncol. 2008;44:759–66. doi: 10.1016/j.oraloncology.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Nakakubo Y, Hida Y, Miyamoto M, et al. The prognostic significance of RCAS1 expression in squamous cell carcinoma of the oesophagus. Cancer Lett. 2002;177:101–5. doi: 10.1016/s0304-3835(01)00773-x. [DOI] [PubMed] [Google Scholar]
- 22.Ikeguchi M, Ohoro S, Maeda Y, et al. Protein and gene expression of tumor-associated antigen RCAS1 in esophageal squamous cell carcinoma. Oncol Rep. 2003;10:1891–94. [PubMed] [Google Scholar]
- 23.Kato H, Nakajima M, Masuda N, et al. Expression of RCAS1 in esophageal squamous cell carcinoma is associated with a poor prognosis. J Surg Oncol. 2005;90:89–94. doi: 10.1002/jso.20249. [DOI] [PubMed] [Google Scholar]
- 24.Tsujitani S, Saito H, Oka S, et al. Prognostic significance of RCAS1 expression in relation to the infiltration of dendritic cells and lymphocytes in patients with esophageal carcinoma. Dig Dis Sci. 2007;52:549–54. doi: 10.1007/s10620-006-9408-6. [DOI] [PubMed] [Google Scholar]
- 25.Ito Y, Yoshida H, Nakano K, et al. Overexpression of human tumor-associated antigen, RCAS1, is significantly linked to dedifferentiation of thyroid carcinoma. Oncology. 2003;64:83–89. doi: 10.1159/000066517. [DOI] [PubMed] [Google Scholar]
- 26.Rosai J. Appendix C: Staging of cancer. In: Houston M, editor. Rosai and Ackerman’s Surgical Pathology. 9th ed. London: Mosby; 2004. pp. 2809–10. [Google Scholar]
- 27.Giaginis C, Davides D, Zarros A, et al. Clinical significance of tumor-associated antigen RCAS1 expression in human pancreatic ductal adenocarcinoma. Dig Dis Sci. 2008;53:1728–34. doi: 10.1007/s10620-007-0035-7. [DOI] [PubMed] [Google Scholar]
- 28.Giaginis C, Zarros A, Alexandrou P, et al. Evaluation of coxsackievirus and adenovirus receptor expression in human benign and malignant thyroid lesions. APMIS. 2010;118:210–21. doi: 10.1111/j.1600-0463.2009.02582.x. [DOI] [PubMed] [Google Scholar]
- 29.Giaginis C, Michailidi C, Stolakis V, et al. Expression of DNA repair proteins, MSH2, MLH1 and MGMT in human benign and malignant thyroid lesions: an immunohistochemical study. Med Sci Monit. 2011;17(3):BR81–90. doi: 10.12659/MSM.881444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arif S, Blanes A, Diaz-Cano SJ. Hashimoto’s thyroiditis shares features with early papillary thyroid carcinoma. Histopathology. 2002;41:357–62. doi: 10.1046/j.1365-2559.2002.01467.x. [DOI] [PubMed] [Google Scholar]
- 31.Akashi T, Oimomi H, Nishiyama K, et al. Expression and diagnostic evaluation of the human tumor-associated antigen RCAS1 in pancreatic cancer. Pancreas. 2003;26:49–55. doi: 10.1097/00006676-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Sonoda K, Miyamoto S, Nakashima M, Wake N. Receptor-binding cancer antigen expressed on SiSo cells induces apoptosis via ectodomain shedding. Exp Cell Res. 2010;316:1795–803. doi: 10.1016/j.yexcr.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Engelsberg A, Hermosilla R, Karsten U, et al. The Golgi protein RCAS1 controls cell surface expression of tumor-associated O-linked glycan antigens. J Biol Chem. 2003;278:22998–3007. doi: 10.1074/jbc.M301361200. [DOI] [PubMed] [Google Scholar]
- 34.Giaginis C, Margeli A, Kouraklis G, et al. Diagnostic and prognostic utility of serum Receptor-binding Cancer Antigen expressed on SiSo cells (RCAS1) levels in colon cancer patients. Int J Biol Markers. 2009;24:70–76. doi: 10.5301/jbm.2009.5545. [DOI] [PubMed] [Google Scholar]