Summary
Background
Our aim was to assess the differences in intraregional prevalence of asthma in adolescents in Split-Dalmatia County to determine asthma risk factors in our population and estimate the specificity and sensitivity of the questionnaire used.
Material/Methods
We conducted the study using the European Community Respiratory Health Survey II short questionnaire supplemented by some questions from the International Study of Asthma in Childhood questionnaire. The participants suspected to have asthma were invited for examination by an asthma specialist who established the final diagnosis of asthma according to the medical history, physical examination, skin-prick tests, and peak flow measurements.
Results
A total of 4027 students (51.2% male) participated in the study. According to the prevalence of wheezing during the last 12 months, asthma prevalence was estimated at 9.7%. The total prevalence of asthma confirmed by an asthma specialist in the selected population was 5.60% (95% CI, 4.93–6.36%); 6.18% in Split (95% CI, 5.37–7.09), 5.63% in Imotski (95% CI, 3.48–8.58), and 2.90% in Sinj (95% CI, 1.67–4.68) (P=0.0028). We found sensitization to aeroallergens and peanuts, and active smoking to be independent risk factors for asthma.
Conclusions
Split-Dalmatia County has moderate asthma prevalence, with a significant intraregional difference. Asthma prevalence estimated by a questionnaire (9.7%) overestimates the prevalence of asthma confirmed by an asthma specialist (5.6%) in adolescents in Croatia. Our data confirmed the need of a more complex questionnaire to evaluate the accurate prevalence of current asthma or the need for subsequent clinical evaluation of the questionnaire obtained data. Allergic sensitization to aeroallergens and active smoking were important risk factors for asthma.
Keywords: asthma, prevalence, ISAAC, ECRHS, Croatia, intraregional, risk factors
Background
Asthma is a complex syndrome with many clinical phenotypes. Its development is determined mostly by interplay of inherited propensity for atopy and various environmental factors. Major characteristics of asthma include variable degree of airflow obstruction, bronchial hyper-responsiveness and airway inflammation [1].
The prevalence of asthma has been stabilizing in western European countries and increasing in regions with previously low prevalence, showing global prevalence differences diminishing, but the burden of asthma still continuing to rise [2]. Despite the large number of epidemiologic studies, the clear-cut reason for the observed increase of asthma prevalence still remains unknown. Although many generated hypotheses await confirmation, the multifactorial cause could be proposed [3].
The International Study of Asthma in Childhood (ISAAC) was the initial study developing standardized tools and methodology for measuring the prevalence of allergic diseases in children suitable for international comparison. It is developed as a three-phase study. The goal of Phase One was to obtain baseline descriptive epidemiological data, the prevalence and severity of the allergic diseases, including asthma [4]. The European Community Respiratory Health Survey (ECRHS) was the first such study in adults that was primarily designed to cover all areas of the European Community in adults between 20–44 years of age [5]. Detailed descriptions of the ISAAC and ECRHS study protocols were published previously [5,6]. Using the ISAAC methodology, 4 studies were conducted in Croatia to determine the regional prevalence of asthma and allergic diseases in school children [7–10].
The aim of our study, however, was to assess the intraregional differences in asthma prevalence in adolescents in Split-Dalmatia County, to determine risk factors for asthma in our population and to estimate the specificity and sensitivity of the questionnaire used.
Material and Methods
The study, approved by the local Ethics Review Committee, was conducted in Split-Dalmatia County, Croatia. This county has an ethnically very uniform population of >99% Caucasians (96.3% Croatians), and has a Mediterranean climate characterized by hot summers and mild winters. Geographically, this county may be divided into 3 main subregions: the Dalmatian interior, an elevated hinterland with numerous karst fields; a densely populated narrow coastal strip; and the Dalmatian islands (specific isolated small populations). The study population comes from 2 different subregions, or more accurately, from 3 towns and their surrounding areas in these subregions that are all less than 50 km apart. Split is the economic and administrative center of the region, located on the Adriatic coast, with nearly 200,000 inhabitants. In the hinterland there are 2 major cities – Sinj and Imotski (population 25,373 and 10,213, respectively).
The target population was first-year high-school students (average age 15.6 years, range 14–16 years) representing 62.7% of total population of that age in the county. This population was chosen because 95% of cumulative asthma incidence becomes apparent by 15 years of age, almost all the population of that age from these subregions is concentrated in local high schools in larger centers (towns) and because adolescents as a population represent the most difficult population in which to diagnose and treat asthma and other chronic diseases. All high schools in the selected area were approached and included in the study. All high school students attending the first grade of high school for the first time, present in a school at the time of study, were included. The whole population taken into consideration for the inclusion into the study was 4086 adolescents registered in first grade of included high schools. The expected response rate of at least 80% would give us at least 3268 participants, producing a representative sample according to ECRHS [5]. Finally, 4027 adolescents were included, giving an initial response rate of 98.56%. The study protocol was implemented in all schools by the same investigational team, which was trained and supervised by the coordinator for ISAAC in Croatia. Initial data were collected using the ECRHS II short questionnaire, supplemented by questions from the ISAAC questionnaire regarding asthma risk factors (Table 1). Participants completed the questionnaires in classrooms under team supervision so that any unclear phrase could be explained. Any inconsistencies found were eliminated in a phone conversation with the parents [4]. Participants suspected of having asthma based on replies to initial questionnaire (positive answers to at least 1 of the first 9 questions in Table 1) were invited to participate in the second phase. They underwent medical examination by a single asthma specialist, who made the final diagnosis of asthma after a diagnostic workup including medical history, physical examination, allergy skin-prick tests and peak flow measurements (peak flow was used because diagnostic workup was done in the local facility to facilitate better compliance).
Table 1.
Main and additional questions used in a study screening questionnaire.
Questionnaire
|
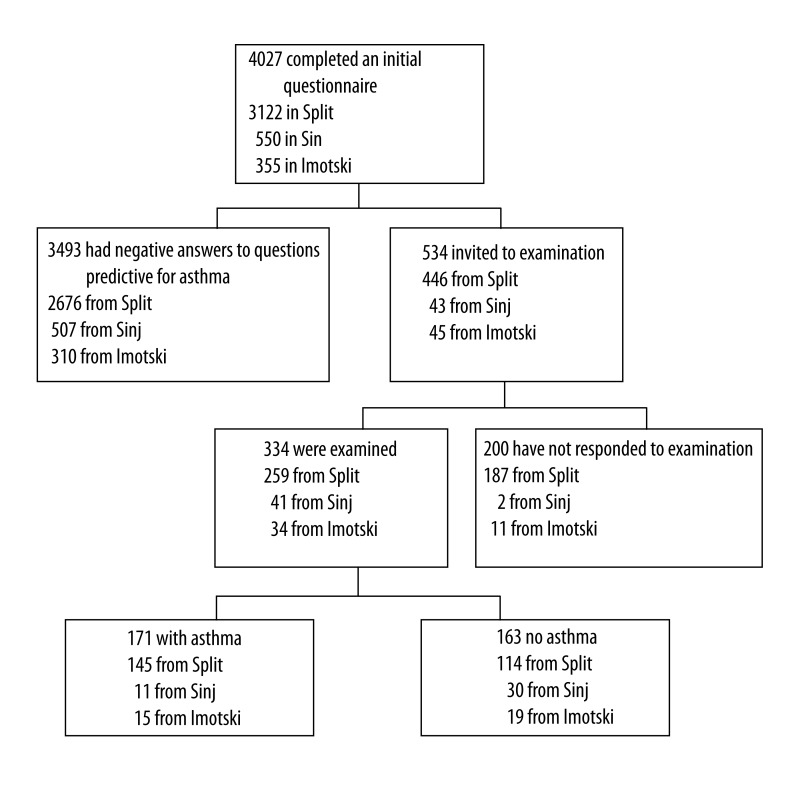
Skin-prick tests were performed according to the EAACI recommendations (11) using common aero and food allergens (ALK-Abelló, Hørsholm, Denmark): Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat and dog dander, moulds, pollens (trees, grasses, and weeds) with peanut and hazelnut. Pollen allergen mixtures were chosen according to the local pollen calendar. A test was read as positive (SPT+) if there was a wheal of 3 mm diameter or larger, and flare appeared. Peak expiratory flow rate (PEFR) measurements were performed using a Vitalograph Peak flow meter (Vitalograph Ireland Ltd., Ennis, Co., Clare, Ireland) with results in L/min and as percentage (%) of expected (12). The flow of participants through the study is shown in Figure 1.
Figure 1.
The flow of participants through the study.
Statistical analysis
Statistical analysis was performed using STATISTICA for Windows, version 6.0 (StatSoft, Inc. Tulsa, OK, USA). Basic descriptive summaries of data were obtained, and differences between investigated groups were calculated with cross-tabulation and the chi-square test or Fisher exact test for qualitative and ANOVA for quantitative variables. Univariate and multivariate logistic regression analyses were used to depict risk factors for asthma and asthma diagnosis. Goodness of fit for each predictor was assessed using Wald test and chi-square statistics and Hosmer-Lemeshow test were used for multivariate models. As participants with a positive answer to at least 1 question were evaluated by an asthma specialist for a diagnosis of asthma, and as there was a very little chance that participants that answered negatively to all questions would have asthma, we tried to estimate the sensitivity and specificity of the questionnaire using available data, appreciating all the restrictions coming from such an approach. P<0.05 was considered as statistically significant for all analyses.
Results
The total of 4027 students (51.2% male) (out of 4086 eligible; response rate 98.56%) were recruited in the study: 3122 (77.5%) in Split, 550 (13.7%) in Sinj, and 355 (8.8%) in Imotski. Their characteristics are shown in Table 2.
Table 2.
Characteristics of the study participants.
| Characteristics | Split (n=3122) | Sinj (n=550) | Imotski (n=355) | Statistics | P |
|---|---|---|---|---|---|
| Sex, female (%) | 1495 (47.9) | 283 (51.5) | 189 (53.2) | χ2=5.37 | 0.068 |
|
| |||||
| Age, average (SD) | 15.6 (0.5) | 15.6 (0.4) | 15.6 (0.4) | F=1.11 | 0.331 |
|
| |||||
| Number of household members, average (SD) | 4.5 (1.1) | 5.3 (1.5) | 5.6 (1.5) | F=188.93 | <0.0001 |
|
| |||||
| Crowdedness, average (SD) | 1.39 (0.59) | 1.29 (0.47) | 1.35 (0.57) | F=8.56 | 0.0002 |
|
| |||||
| Type of fuel used for heating, number (%) | Fisher exact | <0.001 | |||
| Wood/coal | 748 (24.4) | 372 (68.8) | 225 (64.5) | ||
| Oil | 53 (1.7) | 9 (1.7) | 8 (2.3) | ||
| Electricity | 1846 (60.1) | 91 (16.8) | 56 (16.1) | ||
| Central heating | 395 (12.9) | 69 (12.8) | 60 (17.2) | ||
| Gas | 29 (0.9) | 0 | 0 | ||
|
| |||||
| Gas used for cooking, number (%) | 2408 (77.7) | 424 (77.1) | 286 (81.7) | χ2=3.16 | 0.206 |
|
| |||||
| Pets, number (%) | Fisher exact | <0.001 | |||
| Dog | 386 (37.4) | 73 (29.8) | 61 (51.7) | ||
| Cat | 294 (28.5) | 98 (40.0) | 49 (41.5) | ||
| Bird | 275 (26.7) | 64 (26.1) | 8 (6.8) | ||
| Other | 76 (7.4) | 10 (4.1) | 0 | ||
|
| |||||
| Active smokers, number (%) | 323 (10.4) | 33 (6.0) | 37 (10.4) | χ2=10.24 | 0.006 |
|
| |||||
| Passive smokers, number (%) | 1998 (64.3) | 354 (64.5) | 225 (63.4) | χ2=0.14 | 0.934 |
The asthma symptoms-related answers are shown in Table 3. According to the prevalence of wheeze during the last 12 months, we estimated the prevalence of asthma to be 9.7% (95% CI, 8.8–10.7%). We found no significant sex difference, although the incidence was higher in girls in all 3 cities: 9.8% vs. 11.3%; 5.62% vs. 6.4%; and 4.8% vs. 8.5% (boys vs. girls; Split, Sinj, Imotski, respectively). The prevalence of breathlessness when wheezing was significantly higher in Split compared to Sinj (Split 4.4%, Sinj 1.3%; Chi2=13.15; P=0.0014), and in girls compared to boys (5.8% vs. 3.2%, Chi2=13.28, P=0.0003; RR 1.86; 95% CI 1.32–2.60). The prevalence of awakenings due to attack of coughing was also significantly higher in girls in all 3 cities (27.3% vs. 14.7%, Chi2=73.86, P<0.001; 20.5% vs. 13.5%, Chi2=4.77, P=0.029; 30.2% vs. 16.9%, Chi2=8.57, P=0.0034; Split, Sinj, Imotski, respectively). Other gender comparisons were not statistically significant.
Table 3.
The asthma symptoms-related answers.
| Question | Split (n=3122) | Sinj (n=550) | Imotski (n=355) | Statistics | P |
|---|---|---|---|---|---|
| Have you had wheezing or whistling in your chest at any time in the last 12 months? Yes (%) | 330 (10.5) | 35 (6.0) | 26 (6.8) | χ2=14.53 | 0.0007 |
| Have you been at all breathless when wheezing noise was present? Yes (%) | 140 (4.4) | 8 (1.3) | 12 (3.1) | χ2=13.15 | 0.0014 |
| Have you had this wheezing or whistling when you did not have a cold? Yes (%) | 118 (3.7) | 10 (1.8) | 11 (3.1) | χ2=5.29 | 0.0711 |
| Have you been woken up with a feeling of tightness in your chest at any time in the last 12 months? Yes (%) | 154 (4.9) | 7 (1.3) | 10 (2.8) | χ2=16.78 | 0.0002 |
| Have you been woken by an attack of shortness of breath at any time in the last months? Yes (%) | 216 (6.9) | 16 (2.9) | 17 (4.8) | χ2=14.21 | 0.0008 |
| Have you been woken by an attack of coughing at any time in the last 12 months? Yes (%) | 650 (20.7) | 94 (17.1) | 85 (23.9) | χ2=6.60 | 0.0368 |
| Have you had an attack of asthma in the last 12 months? Yes (%) | 46 (1.3) | 2 (0.4) | 3 (0.9) | χ2=4.51 | 0.1049 |
| Are you currently taking any medicine (including inhalers, aerosols, or tablets) for asthma? Yes (%) | 85 (2.6) | 6 (1.1) | 6 (1.6) | χ2=7.35 | 0.0254 |
| Have you ever been admitted to hospital because of asthma? Yes (%) | 29 (0.9) | 4 (0.7) | 4 (1.1) | χ2=0.395 | 0.8208 |
| In the past 12 months, have you missed a school because of asthma? Yes (%) | 43 (1.3) | 1 (0.2) | 2 (0.6) | χ2=6.59 | 0.0370 |
| In the past 12 months, how many days of a school have you missed because of asthma? average mean time (SD) | 15.4 (11.2) | 10.0 (0) | 3.0 (1.4) | F=1.28 | 0.2880 |
Based on answers to questions on asthma symptoms, 534 participants were identified as suspected of having asthma and were invited for additional examination (446 from Split, 43 from Sinj, and 45 from Imotski). The response rate was 62.5% – 259 (58.1%) of invited students in Split, 41 (95.45%) in Sinj, and 34 (75.6%) in Imotski (chi2=26.81, P<0.0001) (Figure 1).
The final diagnosis of asthma, confirmed by an asthma specialist, was established in a total of 171 students (4.24%; 95% CI, 3.64–4.92%), and was more frequent in Split (4.64%) than in Imotski (4.23%), and Sinj (2.00%). In the majority of cases (73.7%) asthma was newly diagnosed. Concerning the fact that 200 adolescents (47.9% from Split, 4.6% from Sinj and 24.4% from Imotski) did not respond to the invitation to participate in the second phase of the study, we tried to estimate the number of asthmatic adolescents among them. For this purpose, we separately evaluated all answers to questions of the initial phase questionnaire. The rates of positive answers obtained from adolescents who were additionally examined were compared to rates from adolescents who were not examined. We found lower rates of all positive answers in adolescents who did not respond to invitation for further study. The multivariate logistic regression model that included these variables and gender showed an odds ratio (OR) of 7.9, with sensitivity of 70.8% and specificity of 76.5% in establishing the clinical diagnosis of asthma in adolescents who were examined. By using the described model we estimated that an additional 57 adolescents from the non-respondent group could have asthma based on the model. The total estimated prevalence of asthma in the selected population was than 5.60% (95% CI, 4.93–6.36%); with 6.18% in Split (95% CI, 5.37–7.09), 5.63% in Imotski (95% CI, 3.48–8.58), and 2.90% in Sinj (95% CI, 1.67–4.68), showing a significant intraregional difference (P=0.0028).
According to area of residence, we found a difference in number of household members (minimum in Split, maximum in Imotski) (F=188.93, P<0.001), crowdedness (number of household members per number of rooms) (minimum in Sinj, maximum in Split) (F=8.56, P=0.0002), type of fuel used for heating (electricity in Split, wood/coal in Sinj and Imotski) (Fisher exact, P<0.001), pet owning households (dog, cat, bird) (Fisher exact, P<0.001), and incidence of active smoking (minimum in Sinj, equally in Split and Imotski) (chi2=10.24, P<0.001).
Based on univariate analysis, the significant risk factors for current asthma included living in Imotski and Split compared to Sinj, having allergies, being an active or passive smoker (P<0.05 for all) (Table 4). After applying the multivariate analysis, only living in Split, having allergies and being an active smoker were found to be significant (P<0.05 for all, P=0.0000 for the model) (Table 4).
Table 4.
Risk factors for current asthma.
| Risk factor | Univariate logistic regression | Multivariate logistic regression | ||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | P | OR (95% CI) | P | |
| Area of residence | ||||
| Sinj | 1.000 | Referent | ||
| Imotski | 2.162 (1.001–4.732) | 0.0252 | ||
| Split | 2.387 (1.285–4.437) | 0.0023 | 2.016 (1.121–3.624) | 0.0192 |
|
| ||||
| Sex, female | 1.085 (0.801–1.469) | 0.5988 | – | – |
|
| ||||
| Allergy | 25.300 (17.89–35.78) | <0.0001 | 24.580 (17.33–34.87) | <0.0001 |
|
| ||||
| Household members, each | 0.960 (0.861–1.071) | 0.4652 | – | – |
|
| ||||
| Crowdedness | 1.063 (0.895–1.264) | 0.4854 | – | – |
|
| ||||
| Type of fuel used for heating, Central/classic | 1.204 (0.865–1.677) | 0.2708 | – | – |
|
| ||||
| Gas used for cooking | 1.149 (0.784–1.684) | 0.4749 | – | – |
|
| ||||
| Pets, dog or cat ownership over 12 months | 1.036 (0.635–1.689) | 0.8868 | – | – |
|
| ||||
| Active smoking | 2.698 (1.828–3.981) | <0.0001 | 2.373 (1.508–3.734) | 0.0002 |
|
| ||||
| Passive smoking | 1.651 (1.164–2.342) | 0.0049 | 1.406 (0.958–2.062) | 0.0816 |
The characteristics of participants with clinically diagnosed asthma are shown in Table 5. Data from Table 5 show that more than a third of asthmatics had relatives with asthma, that their asthma was mostly (98.8%) intermittent or mild persistent, and mostly (81.9%) allergic in nature, with more than a half of patients allergic to mites. According to the low severity of asthma in our patients, a low rate of lung function impairment (9%) was expected. Using multivariate logistic regression analysis the positive skin prick test to mites, weeds and peanuts were found to be significant risk factors for asthma (p<0.05 for all, p=0.0000 for the model).
Table 5.
Characteristics of participants with clinically diagnosed asthma.
| Characteristics | Number (%) | Statistics, P value |
|---|---|---|
| Sex, male | 85 (49.7) | Z=0.0765, P>0.9999 |
|
| ||
| Asthma in the family | ||
| Father | 16 (9.4) | |
| Mother | 10 (5.8) | |
| Sibling | 19 (11.1) | |
| Grandfather/grandmother | 20 (11.7) | |
| Others | 1 (0.6) | |
| Total | 65 (38.0) | |
|
| ||
| Asthma severity | ||
| Intermittent | 123 (71.9) | |
| Mild persistent | 46 (26.9) | |
| Moderate persistent | 2 (1.1) | |
| Severe persistent | 0 | |
|
| ||
| Type of asthma | ||
| Allergic | 140 (81.9) | |
| Non-allergic | 27 (15.8) | |
| Exercise-induced | 4 (2.3) | |
|
| ||
| Positive prick test to: | ||
| Dermatophagoides pt. | 90 (54) | |
| Dermatophagoides far. | 78 (46) | |
| Cat dander | 27 (16) | |
| Dog dander | 4 (2) | |
| Moulds | 9 (5) | |
| Mixed tree pollen | 10 (6) | |
| Mixed grass pollen | 45 (27) | |
| Mixed weed polen | 9 (5) | |
| Peanut | 18 (11) | |
| Hazelnut | 21 (12) | |
|
| ||
| PEFR <80% expected | 15 (9) | |
| PEFR, average (SD), L/min | 457 (64) | |
| PEFR, average (SD), % of expected value | 95 (12.1) | |
Finally, we tried to estimate the specificity and sensitivity of each question of the asthma questionnaire completed by adolescents in whom the clinical diagnosis of asthma was objectively established. The majority of symptoms (wheezing during the past 12 months, breathless when wheezing, wheezing without cold, feeling of chest tightness, awaking due to shortness of breath or coughing, having an asthma attack, currently taking asthma medicine, being admitted to hospital because of asthma, and missing school because of asthma) were estimated to have significant univariate positive predictive value for asthma (P<0.001 for all). In a multivariate model, we estimated that all items, except asthma attacks and school absenteeism, were statistically significant as predictive variables with OR for this model of 142.03, with sensitivity of 78.9% and specificity of 96.4% for clinical diagnosis of asthma (P=0.0000 for the model). Adding the information on allergies, this model had a sensitivity of 85.4% with a specificity of 96.4% (P=0.0000 for the model).
Discussion
The present study showed, like previous studies in Croatia using ISAAC and ECRHS methodologies, an overall moderate prevalence of asthma [7–10]. The highest asthma prevalence was found in Split, the urban center of Split-Dalmatia County (6.18%), with highly significant intraregional differences in a very uniform population, and thus most probably associated with environmental factors. In comparison to asthma prevalence determined objectively by clinical evaluation, the prevalence assumed by used questionnaire (ECRHS II) was significantly overestimated (5.6% vs. 9.7%) in the adolescent population. A positive answer to the question on wheezing in the past 12 months showed the sensitivity comparable to clinical evaluation, but with very low specificity (<50%). The model we used (questionnaire followed by clinical evaluation) has been previously shown to be appropriate for epidemiologic evaluation of asthma prevalence [13]. The important disadvantage of this model is the difficulty in organizing clinical evaluations. Many invited students from Split did not attend clinical evaluation, although, based on evaluation of the non-respondents, they were significantly less likely to have asthma. In Sinj and Imotski, due to easier contact, this problem was significantly less apparent, with a response rate in Sinj of 95.4%.
One of the factors influencing the difference between Split and Sinj/Imotski could be the climate, which is slightly different between these subregions. It has been proven earlier that asthma prevalence is significantly affected by climatic and geographic differences within the same country, with increasing prevalence rates at higher annual temperatures, at decreasing latitude, and at decreasing distance from the sea [14]. Since Split and Dalmatia County have an average of 2600 sunny hours per year without significant intraregional difference, we considered that all adolescents have similar sun exposure, and minor risk for development of vitamin D deficiency, suggesting that vitamin D deficiency most probably is not a risk factor for development of asthma in Croatian adolescents in Split and Dalmatia County. If attributed to the moderate-to-low asthma prevalence among adolescents in Split and Dalmatia County, sun exposure and vitamin D level could also be a protective factor for asthma development, as published in some previous studies [15]. Differences in intraregional asthma prevalence demonstrated by our study could also be influenced by differences in diet, especially fresh fruit and raw vegetables, which are more prevalent in the diet of rural populations of Sinj and Imotski, as shown in a study done in Greece [16]. Humidity and warmth contributes to home dampness and higher levels of dust mites and molds in households [17,18]; early sensitization to perennial aeroallergens negatively affects lung function at school age [19]. Recent studies have shown that house dust mites, besides sensitization, have a propensity of inducing a non-allergic inflammation and asthma pathology [20], making them an even more important environmental risk factor. They also can act in synergy with mold infection in inducing allergic airway inflammation [21]. There is also the difference in exposure to air pollutants, since Split is the administrative and commercial center, with much higher traffic density [22]. The protective “farming effect”, supported by numerous previous studies [23,24], could also partially explain the difference, as Sinj and Imotski are primarily rural populations with increased microbial exposure that can influence innate and adaptive immune responses [24,25]. Drinking unpasteurized milk and exposure to livestock, more common in the Dalmatian hinterland, have also been found by different studies to be protective factors for atopic disease development [26]. The difference in asthma prevalence between Sinj and Imotski could come from the difference in the prevalence of active smokers, pet ownership, and crowdedness, all favoring allergy and asthma in Imotski [22,27–29].
Our study also confirmed allergic sensitization as one of the most important risk factors for asthma [30]. Among aeroallergens, we found sensitization to house dust mite to be an independent risk factor for asthma in schoolchildren. Previously, Sears et al. published similar results [26]. We also found sensitization to peanuts to be an independent risk factor for asthma. This observation in our population deserves further investigation.
Among pollutants, active smoking was found to be a risk factor for asthma, with an OR of 2.7 for active smokers. It has been shown that remodeling in asthmatics can be induced by TGF-beta [31], enhanced by cigarette smoke [32]. That also could contribute to higher prevalence of asthma in Split and Imotski compared to Sinj, which had a lower rate of active smokers in the investigated age group, but it does not clarify the difference between Split and Imotski, where the rates of active smokers were comparable. Passive smoking was equally distributed among all 3 cities, with a significant effect in univariate analysis but a marginal effect on the risk for asthma in the multivariate model.
Limitations of our study stem from several methodological conditions. The ECRHS II short questionnaire was probably not the best choice for adolescents because it was developed for young adults [5]. The additional questionnaire was incomplete as it does not include many environmental factors such as air pollution exposure (traffic, etc.), genetic background, respiratory infectious diseases, nutrition, and exposure to animals. The cross-sectional design of the study per se has some inherent limitations in ability to establish cause-effect relationships. In addition, the study design with 2 phases in which not all participants were compared to the gold standard (clinical diagnosis) also has important limitations for estimation of the sensitivity and specificity of the questionnaire, although; there was a very little chance that participants that answered negatively to all questions would have asthma.
Conclusions
Although almost all asthma prevalence rates for children in Croatia show a moderate prevalence, they also show significant intra- and inter-regional differences that deserve further detailed study of environmental factors in this relatively ethnically and genetically uniform population. The results of our study indicate that the ECRHS questionnaire estimating prevalence of current asthma significantly overestimated asthma diagnosis made by an asthma specialist when used with adolescents in Croatia (9.7% vs. 5.6%). These data confirm the need of a more complex questionnaire to accurately evaluate the prevalence of current asthma or the need for subsequent clinical evaluation of the questionnaire-obtained data. As expected, allergic sensitization to aeroallergens (eg, mites, weed pollen) was an important risk factor for current asthma, together with active smoking, suggesting that additional progress on smoking prevention is needed. As most of the cases of current asthma in our study were newly diagnosed, additional effort is needed concerning public health programs for early diagnosis of allergic and respiratory disorders in our population.
The local Ethics Review Committee approved this study.
Footnotes
Source of support: Departmental sources
References
- 1.Busse WW, Lemanske RF. Asthma. N Engl L Med. 2001;344:350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Pearce N, Aït-Khaled N, Beasley R, et al. Worlwide trends in the prevalence of asthma symptoms: Phase III of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2007;62:758–66. doi: 10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce N, Sunyer J, Cheng S, et al. Comparison of asthma prevalence in the ISAAC and the ECRHS. Eur Respir J. 2000;16:420–26. doi: 10.1183/9031936.00.16337700. [DOI] [PubMed] [Google Scholar]
- 4.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variations in the prevalence of asthma symptoms: International Study of Asthma and Allergies in Childhood (ISAAC) Eur Respir J. 1998;12:315–35. doi: 10.1183/09031936.98.12020315. [DOI] [PubMed] [Google Scholar]
- 5.Burney PGJ, Luczynska C, Chinn S, et al. The European Community Respiratory Health Survey. Eur Respir J. 1994;7:954–60. doi: 10.1183/09031936.94.07050954. [DOI] [PubMed] [Google Scholar]
- 6.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variations in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225–32. [PubMed] [Google Scholar]
- 7.Stipić-Marković A, Pevec B, Pevec MR, et al. Prevalence of symptoms of asthma, allergic rhinitis, conjunctivitis and atopic eczema: ISAAC (International Study of Asthma and Allergies in Childhood) in a population of schoolchildren in Zagreb. Acta Med Croatica. 2003;57(4):281–85. [PubMed] [Google Scholar]
- 8.Banac S, Tomulić KL, Ahel V, et al. Prevalence of asthma and allergic diseases in Croatian children is increasing: survey study. Croat Med J. 2004;45(6):721–26. [PubMed] [Google Scholar]
- 9.Munivrana H, Vorko-Jovic A, Munivrana S, et al. The prevalence of allergic diseases among Croatian school children according to the ISAAC Phase One questionnaire. Med Sci Monit. 2007;13(11):CR505–9. [PubMed] [Google Scholar]
- 10.Drkulec V, Navratil M, Maloča I, et al. The prevalence of allergic disorders among Croatian school children according to socioeconomic status. Pediatr Allergy Immunol. 2009;20(Suppl 20):61. [Google Scholar]
- 11.Dreborg S, Frew AJ. Allergen sensitization and skin tests. Allergy. 1993;48:49–54. [Google Scholar]
- 12.Miller MR, Hankinson J, Brusaco V, et al. Standardization of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 13.Remes ST, Pekkanen J, Remes K, et al. In search of childhood asthma: questionnaire, tests of bronchial hyperresponsiveness, and clinical evaluation. Thorax. 2002;57(2):120–26. doi: 10.1136/thorax.57.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanolin ME, Pattaro C, Corsico A, et al. for the ISAYA Study Group. The role of climate on the geographic variability of asthma, allergic rhinitis and respiratory symptoms: results from the Italian study of asthma in young adults. Allergy. 2004;59:306–14. doi: 10.1046/j.1398-9995.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 15.Arnedo-Pena A, Garcia-Marcos L, Fernandez-Espinar JF, et al. Sunny hours and variations in the prevalence of asthma in schoolchildren according to the International Study of Asthma and Allergies (ISAAC) Phase III in Spain. Int J Biometeorol. 2011;55:423–34. doi: 10.1007/s00484-010-0353-x. [DOI] [PubMed] [Google Scholar]
- 16.Haidopoulou K, Hatzistilianou M, Petridou A, et al. Plasma fatty acids in asthmatic children of Northern Greece. Arch Med Sci. 2009;5:51–56. [Google Scholar]
- 17.Brunekreef B. Associations between questionnaire reports of home dampness and childhood respiratory symptoms. Sci Total Environ. 1992;127:79–89. doi: 10.1016/0048-9697(92)90471-4. [DOI] [PubMed] [Google Scholar]
- 18.Nikolai T, Illi S, von Mutius E. Effect of dampness at home in childhood on bronchial hyper reactivity in adolescence. Thorax. 1998;53:1035–40. doi: 10.1136/thx.53.12.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Illi S, von Mutius E, Lau S, et al. Perennial allergen sensitization early in life and chronic asthma in children; a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 20.Lan F, Liu K, Zhang J, et al. Th17 response is augmented in OVA-induced asthmatic mice exposed to. HDM Med Sci Monit. 2011;17(5):BR132–38. doi: 10.12659/MSM.881759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukushima C, Matsuse H, Fukahori S, et al. Aspergillus fumigatus synergistically enhances mite-induced allergic airway inflammation. Med Sci Monit. 2010;16(7):BR197–202. [PubMed] [Google Scholar]
- 22.Leonard SA, Sicherer SH. Less Air Pollution Leads to Rapid Reduction of Airway Inflammation and Improved Airway Function in Asthmatic Children. Pediatrics. 2009;124:S117–18. doi: 10.1542/peds.2008-1153. [DOI] [PubMed] [Google Scholar]
- 23.Von Mutius E. Asthma and Allergies in Rural Areas of Europe. Proc Am Thorac Soc. 2007;4:212–16. doi: 10.1513/pats.200701-028AW. [DOI] [PubMed] [Google Scholar]
- 24.Ege MJ, Mayer M, Normand AC, et al. for GABRIELA Transregio study group. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;24; 364(8):701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 25.Burrows B, Martinez FD, Holonen M, et al. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–77. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 26.Sears M, Green J, Willan A, et al. A longitudinal, population based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 27.Simpson A, Ćustović A. Pets and the development of allergic sensitization. Curr Allergy Asthma Rep. 2005;5(3):212–20. doi: 10.1007/s11882-005-0040-x. [DOI] [PubMed] [Google Scholar]
- 28.McLeish AC, Zvolensky MJ. Asthma and cigarette smoking: a review of the empirical literature. J Asthma. 2010;5(3):212–20. doi: 10.3109/02770900903556413. [DOI] [PubMed] [Google Scholar]
- 29.Antova T, Pattenden S, Brunekreef B, et al. Research report; Exposure to indoor mould and children’s respiratory health in the PATY study. J Epidemiol Community Health. 2008;62:708–14. doi: 10.1136/jech.2007.065896. [DOI] [PubMed] [Google Scholar]
- 30.Stipić-Marković A, Čvoriščec B, Pevec B, et al. Increasing incidence of allergy in Croatia, Rad Medical Sciences. Zagreb. 2008:105–16. [Google Scholar]
- 31.Michalik M, Pierzchalska M, Legutko A, et al. Asthmatic bronchial fibroblasts demonstrate enhanced potential to differentiate into myofibroblasts in culture. Med Sci Monit. 2009;15(7):BR194–201. [PubMed] [Google Scholar]
- 32.Kim DY, Kwon EY, Hong GU, et al. Cigarette smoke exacerbates mouse allergic asthma through Smad proteins expressed in mast cells. Respir Res. 2011;12:49. doi: 10.1186/1465-9921-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]