Abstract
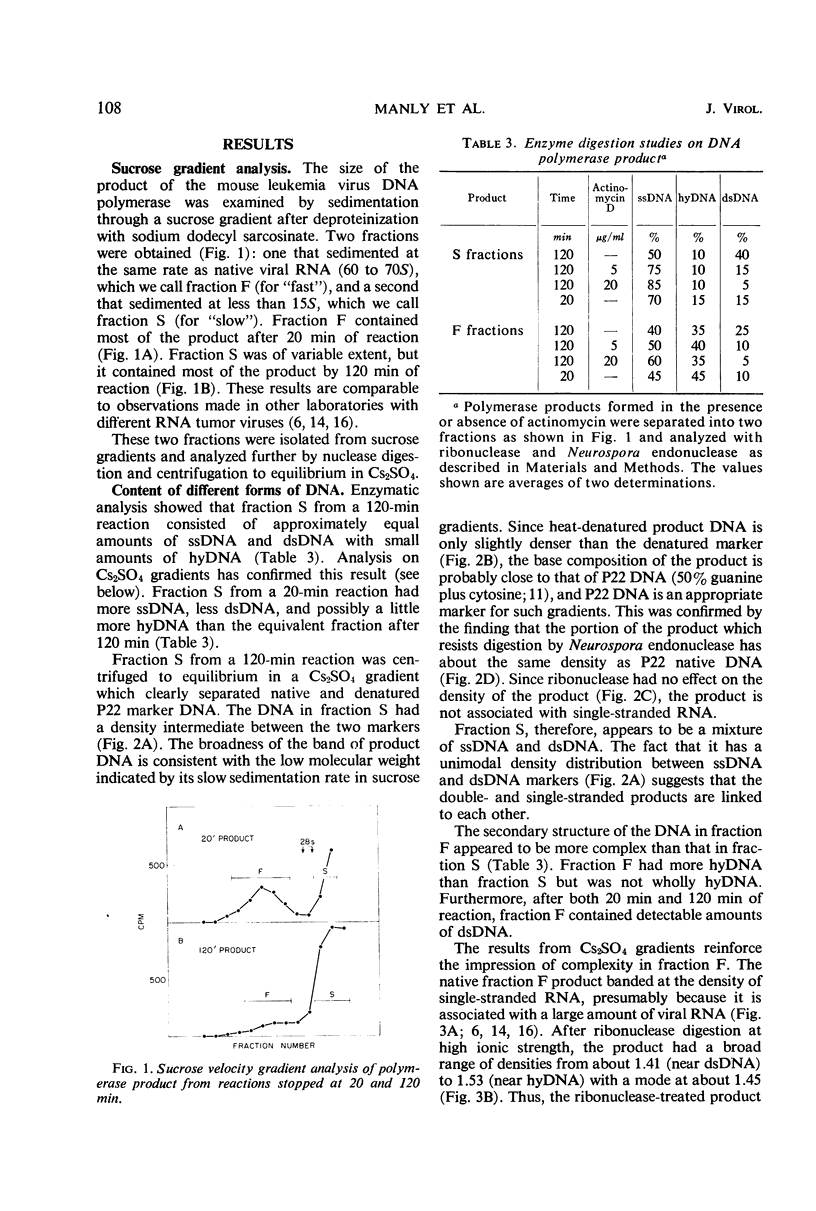
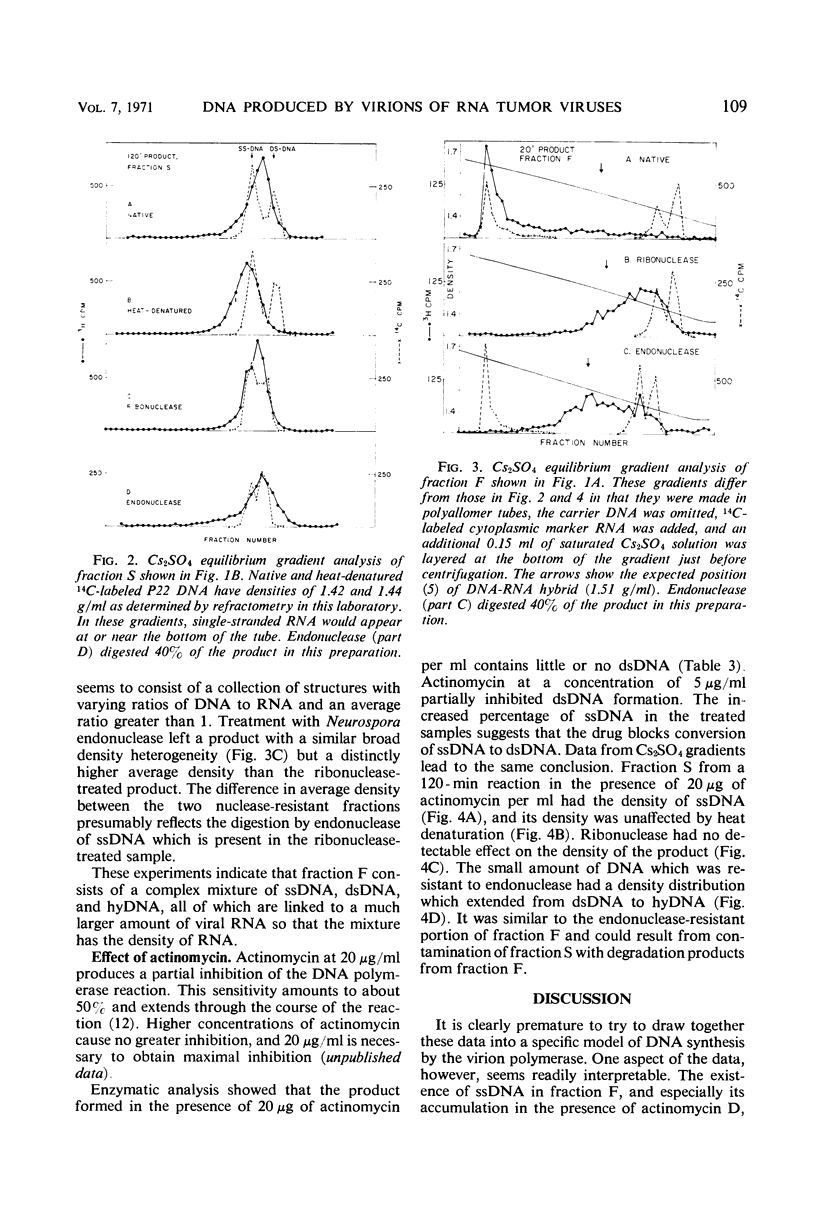
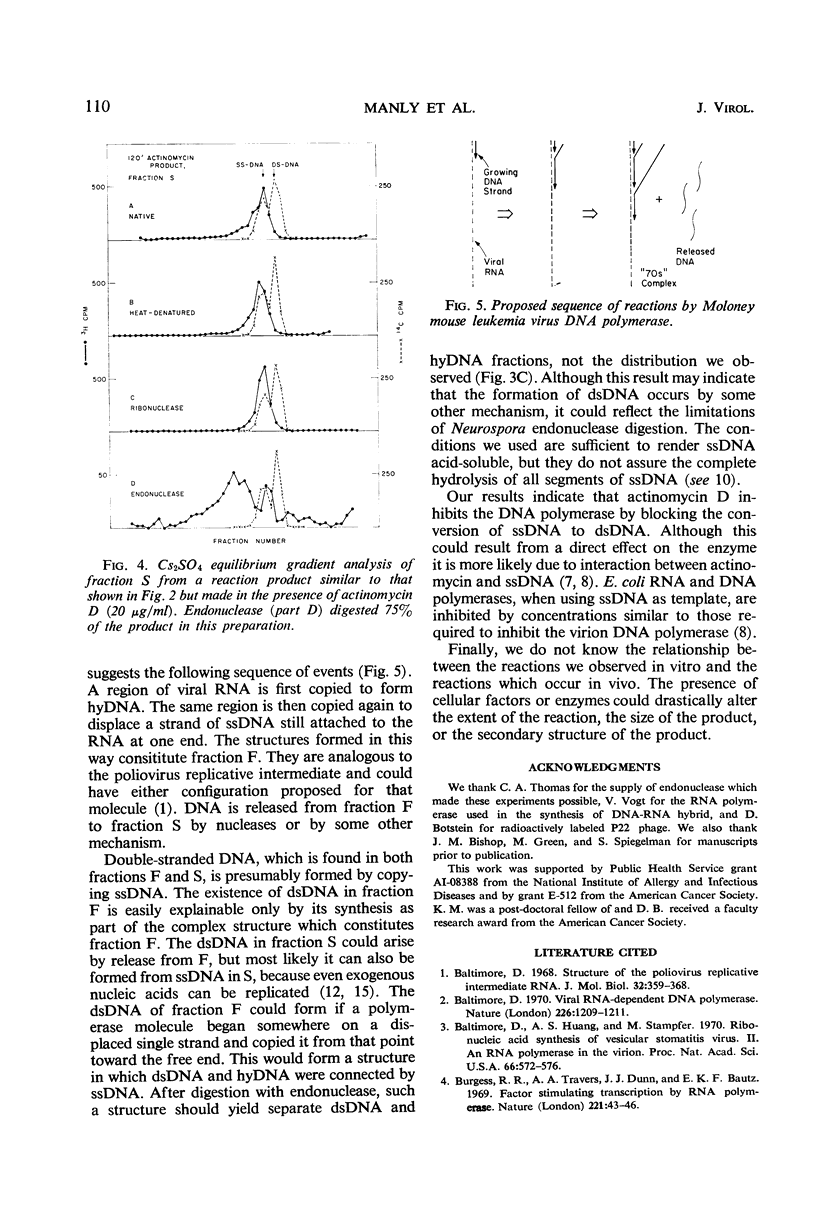
The in vitro product of mouse leukemia virus deoxyribonucleic acid (DNA) polymerase can be separated into two fractions by sedimentation in sucrose gradients. These two fractions were analyzed for their content of single-stranded DNA, double-stranded DNA, and DNA-ribonucleic acid (RNA) hybrid by (i) digestion with enzymes of known specificity and (ii) equilibrium centrifugation in Cs2SO4 gradients. The major fraction early in the reaction contained equal amounts of single-stranded DNA and DNA-RNA hybrid and little double-stranded DNA. The major fraction after extensive synthesis contained equal amounts of single-and double-stranded DNA and little hybrid. In the presence of actinomycin D, the predominant product was single-stranded DNA. To account for these various forms of DNA, we postulate the following model: the first DNA synthesis occurs in a replicative complex containing growing DNA molecules attached to an RNA molecule. Each DNA molecule is displaced as single-stranded DNA by the synthesis of the following DNA strand, and the single-stranded DNA is copied to form double-stranded DNA either before or after release of the single strand from the RNA. Actinomycin blocks this conversion of single-to double-stranded DNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Structure of the poliovirus replicative intermediate RNA. J Mol Biol. 1968 Mar 14;32(2):359–368. doi: 10.1016/0022-2836(68)90015-6. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. MECHANISM OF RNA POLYMERASE ACTION: FORMATION OF DNA-RNA HYBRIDS WITH SINGLE-STRANDED TEMPLATES. J Mol Biol. 1964 Feb;8:297–313. doi: 10.1016/s0022-2836(64)80139-x. [DOI] [PubMed] [Google Scholar]
- GOLDBERG I. H., RABINOWITZ M., REICH E. Basis of actinomycin action. I. DNA binding and inhibition of RNA-polymerase synthetic reactions by actinomycin. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2094–2101. doi: 10.1073/pnas.48.12.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., McDonnell J. P., Levinson W., Quintrell N., Fanshier L., Bishop J. M. Deoxyribonucleic acid polymerase associated with Rous sarcoma virus and avian myeloblastosis virus: properties of the enzyme and its product. J Virol. 1970 Nov;6(5):589–598. doi: 10.1128/jvi.6.5.589-598.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINN S., LEHMAN I. R. AN ENDONUCLEASE FROM NEUROSPORA CRASSA SPECIFIC FOR POLYNUCLEOTIDES LACKING AN ORDERED STRUCTURE. I. PURIFICATION AND PROPERTIES OF THE ENZYME. J Biol Chem. 1965 Mar;240:1287–1293. [PubMed] [Google Scholar]
- LINN S., LEHMAN I. R. AN ENDONUCLEASE FROM NEUROSPORA CRASSA SPECIFIC FOR POLYNUCLEOTIDES LACKING AN ORDERED STRUCTURE. II. STUDIES OF ENZYME SPECIFICITY. J Biol Chem. 1965 Mar;240:1294–1304. [PubMed] [Google Scholar]
- McDonnell J. P., Garapin A. C., Levinson W. E., Quintrell N., Fanshier L., Bishop J. M. DNA polymerases of Rous sarcoma virus: delineation of two reactions with actinomycin. Nature. 1970 Oct 31;228(5270):433–435. doi: 10.1038/228433a0. [DOI] [PubMed] [Google Scholar]
- Rabin E. Z., Mustard M., Fraser M. J. Specific inhibition by ATP and other properites of an endonuclease of Neurospora crassa. Can J Biochem. 1968 Oct;46(10):1285–1291. doi: 10.1139/o68-193. [DOI] [PubMed] [Google Scholar]
- Rokutanda M., Rokutanda H., Green M., Fujinaga K., Ray R. K., Gurgo C. Formation of viral RNA-DNA hybrid molecules by the DNA polymerase of sarcoma-leukaemia viruses. Nature. 1970 Sep 5;227(5262):1026–1028. doi: 10.1038/2271026a0. [DOI] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. Characterization of the products of DNA-directed DNA polymerases in oncogenic RNA viruses. Nature. 1970 Aug 8;227(5258):563–567. doi: 10.1038/227563a0. [DOI] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. DNA-directed DNA polymerase activity in oncogenic RNA viruses. Nature. 1970 Sep 5;227(5262):1029–1031. doi: 10.1038/2271029a0. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]



