Summary
Background
Microvessel density in angiogenesis is regarded as a prognostic factor of tumour invasiveness, independent of cell proliferation. In recent studies of pituitary tumours, correlation between the expression of cyclooxygenase-2 (COX-2) and micro-vascularization density and microvessel surface density has been established. We studied the expression of COX-2 in different types of pituitary adenomas to determine the usefulness of COX-2 expression as a prognostic factor of tumour progression or recurrence in patients with hypophyseal tumours.
Material/Methods
We retrospectively studied a group of 60 patients of mean age 46.7±17.6 (range, 18 to 85) years who underwent pituitary tumour surgery. Expression of COX-2, as determined by immunohistochemistry, was analyzed in relation to histopathology features of tumour, clinical symptoms, MR imaging and post-operative recurrence/progression of disease.
Results
COX-2 was expressed in adenomas of 87% of patients, with a median index value of 57.5% [IQR=60.5]. Highest COX-2 expression was observed in hormonally inactive adenomas and gonadotropinomas and lowest in prolactinomas. We found no differences in COX-2 expression with respect to patient age, gender, tumour size, degree of tumour invasiveness, or whether tumours were immunopositive or immunonegative for pituitary hormones, nor have we found any relation between COX-2 expression and recurrence or progression of tumour size.
Conclusions
COX-2 does not appear to be a predictive factor for recurrence or progression of tumour size. Nevertheless, due to the observed relatively high expression of COX-2 in pituitary adenomas, further studies with COX-2 inhibitors are justified in these tumours.
Keywords: COX-2, pituitary, adenoma, tumour markers, non-steroidal anti-inflammatory drugs (NSAIDs)
Background
Currently, pituitary tumours are classified as pituitary adenomas or carcinomas; pituitary adenomas being distinguished as either typical or atypical [1].
While pituitary cancer is rare, some pituitary adenomas exhibit malignant features only locally, with local invasion and infiltration of structures adjacent to sella turcica (ie, sinus cavernosus), as well as rapid post-surgery regrowth. Large tumour size with local invasion, post-operative regrowth of tumour mass, or persistence of hormonal function despite near total resection, are potential indicators of aggressiveness of atypical adenomas [2–5]. Therefore, after neurosurgery, atypical adenomas should be followed by carefully adjusted therapy.
Early selection of patients with aggressive pituitary adenomas, specific molecular markers, prior to later confirmation of tumour recurrence by MR imaging, would allow such patients to be treated most effectively.
COX-2 is an accepted marker of angiogenesis and cancerogenesis in many tumours [6], such as adenocarcinomas of the gastrointestinal tract [7] or carcinomas of the breast [8,9], pancreas [10], prostate [11] and bladder [12,13]. Among other effects, high cellular concentration of COX-2 inhibits apoptosis and enhances Bcl 2 protein concentration, thus stimulating cell proliferation [14]. Overexpression of COX-2 leads to activation of metalloproteinases, increases production of proangiogenic factors and decreases that of E-cadherin, thus enhancing tumour growth and invasiveness [15–17].
It has been generally accepted that application of non-steroidal anti-inflammatory drugs (NSAIDs) which are cyclooxygenase inhibitors, reduces the relative risk of cancers of the gastrointestinal tract, including colon cancer [18–21]. Therefore, NSAID may be useful in cancer treatment and prevention [22].
In the case of pituitary adenoma, the extent of angiogenesis is a prognostic predictor of invasiveness in some tumour types, such as macroprolactinomas, independent of cell proliferation [23,24]. Correlation between COX-2 expression and microvessel density in pituitary tumours has recently been established [25–27]. Interestingly, over-expression of COX-2 was found in some non-functioning pituitary tumours, the pharmacological treatment of which is currently quite limited.
The aim of our work was to evaluate the expression of COX-2 and to investigate the usefulness of COX-2 expression as a prognostic factor of tumour regrowth or recurrence of disease in the treatment of patients with pituitary adenoma.
Material and Methods
The clinical material consisted of a group of 60 patients diagnosed with pituitary adenoma and treated at our Department of Endocrinology, Jagiellonian University Medical College (UJCM) during the years 2003–2006. These patients underwent neurosurgery at the Department of Neurosurgery, UJCM. The tumours were surgically removed by transsphenoidal resection and complementary craniotomy was performed where required. Patients underwent surgery independently of their pre-operative pharmacological treatment. The final diagnosis of these patients was based on demographic and clinical data, results of histopathology of post-surgical specimens and evaluation of MR imaging retrieved retrospectively from the medical records of these patients. The study was approved by the Bioethics Committee of the Jagiellonian University Medical College.
Imaging
Tumour size, defined as its largest dimension, and tumour relation to adjacent bone structures (sella turcica destruction, penetration into sinus cavernous, optic chiasm compression, suprasellar propagation) were evaluated in MRI prior to, and 3–6, 12, 24, 36 and 48 months following surgery. Tumour invasiveness was defined by radiological criteria [28,29] and from surgeons’ descriptions found in the medical records of patients. All MR images were performed at one department of radiology, using routine T1-weighted spin-echo sequences, before and after administration of 0.1 mmol gadolinium chelate and evaluated by one experienced radiologist.
Pharmacotherapy
Patients with functioning pituitary tumours, who, due to prolactin hypersecretion, required pharmacological treatment, received dopamine agonists (bromocriptine, quinagolide, cabergoline). Patients diagnosed with acromegaly were treated with somatostatin analogues (octreotide) prior to surgery. Patients with pituitary insufficiency received hormone substitution: hydrocortisone, L-thyroxine, and sex hormones where applicable. None of the patients were treated with NSAIDs. Prior to surgery none of the patients were diagnosed with diabetes insipidus.
Histopathology
Pituitary adenoma specimens obtained via neurosurgery were classified according to WHO criteria [1]. For immunohistochemistry, specific primary antibodies against pituitary hormones (ACTH, GH, PRL, TSH, LH, FSH [Dako, Glostrup, Denmark]) were used. All specimens were evaluated by the same experienced pathologist. Type of hematoxylin and eosin staining (acidophilic, basophilic, chromophobic), polymorphism of cell nuclei, presence of mitotic figures, and presence of immunopositive staining for anterior pituitary hormones, were analyzed. Tumours with no expression of ACTH, GH, PRL, TSH, LH or FSH were classified as hormone immunonegative adenomas [1].
COX-2 expression as evaluated by immunohistochemistry
Standard diagnostics of pituitary adenoma was supplemented, accordingly, by immunohistochemical staining for cyclooxygenase-2 (COX-2), by monoclonal IgG-class antibodies synthesized on the basis of murine myeloma (p3-NS1-Ag4-1) directed against N-terminal domain (aminoacids 38 do 163) of human cyklooxygenase-2 (NCL-COX-2 by Leica Microsystems), in optimum working dilution (1: 100). Sections of 5 μm thickness were used. After routine deparaffinization, rehydration and blocking of endogenous peroxidase activity, sections were subjected to antigen retrieval by microwave treatment at 95°C in citrate buffer, pH=6.0. Subsequently, slides were incubated with primary antiserum (NCL-COX-2), followed by incubation with secondary anti-murine antibody IgG-poly-HRP (NovoLink Polymer RE7112). Peroxidase activity was developed with diaminobenzidine (DAB solution RE7105) serving as chromogen, in NovoLink™ DAB Substrate Buffer (Polymer) RE7143. Finally, slides were stained with hematoxylin (Hematoxylin RE7107).
To confirm the specificity of the primary antibody, positive and negative control tests were performed, following the manufacturer’s instructions. Sections of human colon carcinoma were included as positive control. The negative control test included substitution of primary antibody with phosphate buffered saline, pH=7.4.
COX-2-immuno-stained sections were evaluated by 1 experienced pathologist using an optical microscope (NIKON OPTISHOT-2). Cells were evaluated over 10 different fields of view at 400× magnification using a reference grid. The COX-2 index was evaluated as the percentage of positively stained cells with respect to the total number viewed. In each section, the number of cells scored was 2000 or more.
Statistical analysis
The dependence between COX-2 expression and morphological features of pituitary adenoma, results of imaging and hormonal function prior to and post-surgery and type of pharmacological treatment prior to surgery and patient demographic data, were analyzed. Basic statistics and comparative analysis appropriate to the distributions of data points were performed. In these analyses, Kolmogorov-Smirnov, Mann-Whitney U, Kruskal-Wallis and Fischer’s exact tests were applied. Wherever applicable, median values and inter-quartile ranges [IQR] were given. In Kaplan-Meier plots time-to-incidence were given, with 48 months as the last observation period. Statistic tests were generated using the GraphPad package (GraphPad Prism version 5.03 for Windows, GraphPad Software, San Diego, CA, USA).
Results
Results, based on retrospective analysis of this group, are shown in Table 1
Table 1.
Patient characteristics (n=60, mean value ±1 SD, where given).
| General data | Age, years | 46.7±17.6 |
| Gender: Female (%)/Male (%) | 37 (62%)/23 (38%) | |
| Overt symptoms (%)/Incidentaloma (%) | 43 (71.7%)/17 (28.3%) | |
| Imaging results | Largest dimension of tumour [mm] | 25.1±17.6 |
| Microadenoma (%)/Macroadenoma (%) | 14 (23.3%)/46 (76.7%) | |
| Largest dimension of microadenoma [mm] | 5.9±2.2 | |
| Largest dimension of macroadenoma [mm] | 31.0±16.0 | |
| Patients with destruction of sella turcica (%) | 16 (26.7%) | |
| Patients with cavernous sinus penetration (%) | 36 (60.0%) | |
| Patients with optic chiazm compression (%) | 36 (60.0%) | |
| Final classification of pituitary adenomas in patient group | Patients with clinically non-functioning adenoma (%) | 19 (31.7%) |
| Patients with prolactinoma (%) | 13 (21.7%) | |
| Patients with acromegaly (%) | 16 (26.7%) | |
| Patients with Cushing disease (%) | 4 (6.7%) | |
| Patients with ganadotropinoma (%) | 5 (8.3%) | |
| Patients with thyrotropinoma (%) | 1 (1.7%) | |
| Patients with silent-ACTH adenoma (%) | 2 (3.4%) |
In our group, 43 cases (71.7%) of adenoma chromophobum, 10 cases (16,7%) of adenoma acidophilicum and 7 cases (11,6%) of adenoma partim acidophilicum were found, as based on histopathology records. Polymorphism of cell nuclei and presence of mitotic figures suggestive of more “aggressive” behavior were found in 6 (10%) of the examined adenomas.
Basing on immunohistochemistry records, occurrence of hormone immunopositive staining (ACTH, PRL, GH, TSH, LH, FSH) was established in 41 cases (68.3%) (Table 1). In some adenomas, expression of more than one hormone was found.
Recurrence or progression of pituitary tumour, as diagnosed by MRI, was stated in 22 patients (36.7%), including 7 patients with negative staining for pituitary hormones, over a period of 3 to 48 months of observation (Figure 1).
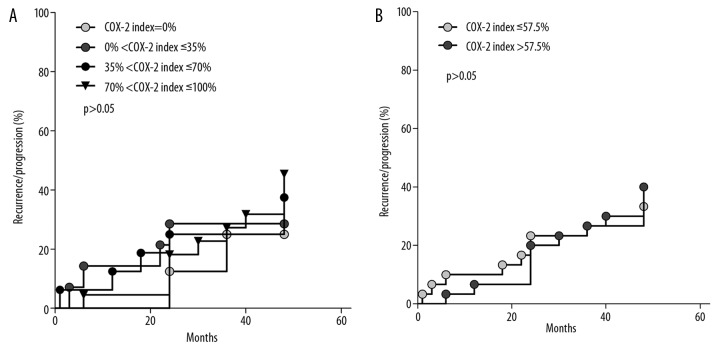
Figure 1.
Kaplan-Maier plots of MRI-observed recurrence/progression of pituitary adenoma against COX-2 index in patients of the studied group (n=60); (A) grouped with respect to values of COX-2 index: COX-2 index =0% (n=2/8 patients, 25.0%); 0% <COX-2 index ≤35% (n=4/14 patients, 28.6%); 35% <COX-2 index ≤70%(n=6/16 patients, 37.5%); 70% <COX-2 index ≤100% (n=10/22 patients, 45.5%); (B) grouped with respect to COX-2 index ≤ median (n=10/30 patients, 33.3%); COX-2 index > median (n=12/30 patients, 40.0%)
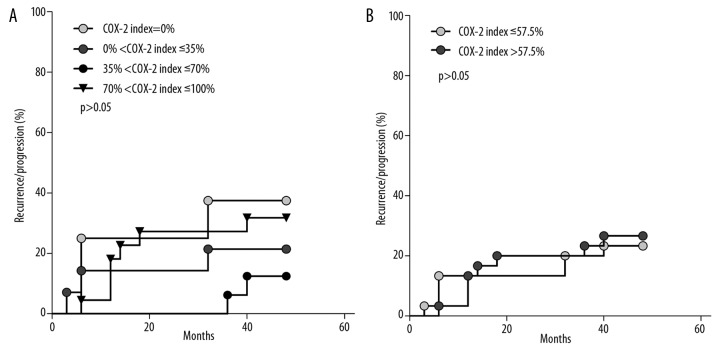
In 6/60 (10%) of our patient group, recurrence was stated by MRI within the first year of observation, the respective percentages after the second, third and fourth year of observation being 13.3%, 5%, and 8.3%. Recurrence of hyperfunction of anterior pituitary was stated in 15/60 patients (25%), in 8 of them within the first year of observation (Figure 2).
Figure 2.
Kaplan-Maier plots of hormone secretion recurrence of pituitary adenoma against COX-2 index in patients of the studied group (n=60); (A) grouped with respect to values of COX-2 index: COX-2 index =0% (n=3/8 patients, 37.5%); 0% <COX-2 index ≤35% (n=3/14 patients, 21.4%);35% <COX-2 index ≤70%(n=2/16 patients, 12.5%); 70% <COX-2 index ≤100% (n=7/22 patients, 31.8%); (B) grouped with respect to COX-2 index ≤ median (n=7/30 patients, 23.3%); COX-2 index > median (n=8/30 patients, 26.7%).
COX-2 expression: general characteristics
COX-2 expression, as determined by immunohistochemistry was stated in 52 (86.7%) of 60 cases of pituitary adenoma. COX-2 immunoreactivity was present in cell cytoplasm, restricted to several areas of the tumour. Nuclei were stained with hematoxylin (Figure 3). Values of the COX-2 index ranged between 0% and 100%, with a median value for all specimens of 57.5% [IQR=60.5]. The median value of COX-2 index for 52 COX-2 immunopositive adenomas was 67% [IQR=53.5].
Figure 3.

Expression of COX-2 in pituitary adenomas (optical microscope, magnification 200×); (A) female, 66 yrs: gonadotropinoma, COX-2 index =0%; (B) female, 75 yrs: gonadotropinoma, COX-2 index =100%.
No significant difference between COX-2 expression between female and male patients was found (56.0% [IQR=59.5] vs. 62.0% [IQR=63.0], p>0.05), nor was any correlation found between values of COX-2 indices and age of patients.
Hormone immunonegative adenomas and gonadotropinomas showed the highest median values of COX-2 index – 70.0% [IQR=37.0], and 70.0% [IQR=96.5] respectively – while in prolactinomas the values of this index were the lowest, at 32.0% [IQR=75.0]. No statistical differences in median values of this index were stated between groups of patients classified according to their final diagnosis (Figure 4).
Figure 4.
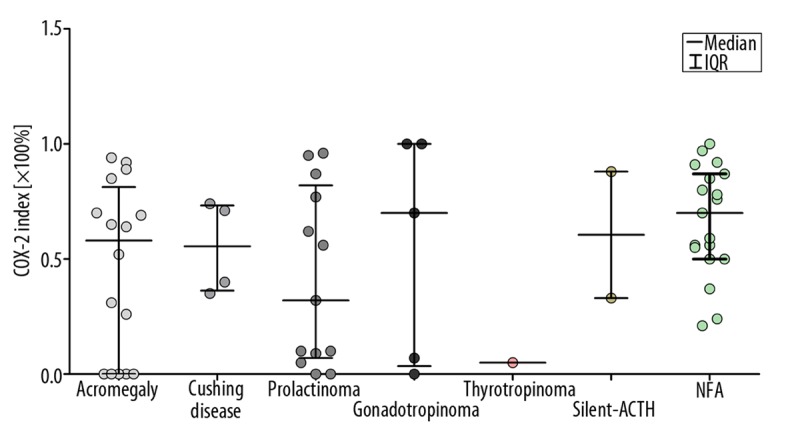
Expression of COX-2 in patients with pituitary adenoma grouped according to their final diagnosis. Median values of COX-2 index (IQR) are given: Acromegaly 58.0% (IQR=81.3),Cushing disease 55.5% (IQR=37.0), prolactinoma 32.0% (IQR=75.0), gonadotropinoma 70.0% (IQR=96.5), thyrotropinoma 5.0% (IQR=0.0), silent-ACTH 60.5.0% (IQR=55.0), NFA 70.0% (IQR=37.0).
No significant differences in COX-2 expression were observed between patients with pituitary hormone immuno-positive and -negative adenomas, defined here as tumours with no expression of ACTH, GH, PRL, TSH, LH and FSH (52.0% [IQR=75.0] vs. 70.0% [IQR=37], p>0.05), independently of whether patients were treated with somatostatin analogues or with dopamine agonists.
COX-2 expression and expression of specific pituitary hormones
Basing on our immunohistochemistry results, we studied the relation between expression of COX-2 and presence of immunopositive staining for specific anterior pituitary hormones. In terms of the median and IQR values of the COX-2 index, expression of COX-2 appeared not to be related to the expression of specific pituitary hormones. However, in the case of GH-positive and PRL-positive tumours, we noted a tendency for COX-2 expression to be either quite low or very high, suggesting that these distributions are not well described by their median and IQR values.
COX-2 expression and MR imaging
No correlation of expression of COX-2 in patients with pituitary adenoma was found with respect to tumour size, nor was any significant difference found between values of COX-2 indices between groups of patients with macro- and micro-adenomas (57.5% [IQR=61.5] vs. 62.5% [IQR=71.2], p>0.05).
We studied COX-2 expression in patients grouped separately according to various indications of tumour invasiveness (destruction of sella turcica, tumour suprasellar extension, cavernous sinus penetration, and optic chiasm compression) against groups of patients in whom no such features were visible, finding no significant changes in COX-2 expression between groups so defined (Fisher’s exact test).
Next, statistical analysis (Mann Whitney U test) was performed to compare COX-2 expression between the group of patients without any of the above signs of tumour invasiveness against patients with 1 or more such signs. In this comparison (Figure 5), the median values of COX-2 indices were: 65.0% non-invasive tumours [IQR=72.0], and 56.0% invasive tumours [IQR=57.0], p>0.05.
Figure 5.

Expression of COX-2 in patients in whom no signs of tumour invasiveness were observed in MRI and in patients in whom one or more of such signs were observed (n=60).
COX-2 expression and recurrence of disease or tumour regrowth in MRI
In general, we found no statistically significant relation between expression of COX-2 and recurrence of disease or tumour regrowth as evidenced by MRI. No difference in disease recurrence frequency was stated between the group of patients with no expression (2/8 patients) and the group with positive COX-2 expression (20/52 patients), median COX-2 indices: 0.0% [IQR=0] and 67% [IQR=53.5]. We next studied the COX-2 indices in relation to the frequency and time distribution of MRI-observed tumour recurrence/progression in our patients, presented in the form of Kaplan-Meier plots. In these plots, frequencies and times of recurrence were plotted for groups of patients selected according to values of their COX-2 indices: over 4 ranges of COX-2 index values (Figure 1A) or above and below the median value of COX-2 index over the whole group of 60 patients (Figure 1B). In our analysis, again using Kaplan-Meier plots, of the relation between frequency and time of recurrence/progression and COX-2 indices in patients with positive expression of ACTH, GH, PRL, TSH, LH, and FSH and no expression of these hormones, we found no such relation.
COX-2 expression and hormone secretion recurrence
In terms of the COX-2 index and frequency and time of recurrence of tumour hormonal secretion, no statistically significant differences were found (Figure 2A, B).
COX-2 expression and pharmacotherapy
Administration of somatostatin analogues in 8 of 16 acromegalic patients prior to surgery did not affect the expression of COX-2 in their tumour cells compared to patients in whom no such pharmacotherapy was applied (COX-2 index median values: 58.0% [IQR=84.2] vs. 67.0% [IQR=74.7], respectively, p>0.05). We found the same result in 5 of 13 patients with prolactinoma treated with dopamine agonists prior to surgery (COX-2 index median values: 77.0% [IQR=87.5] vs. 44.0% [IQR=70.7] in treated and untreated patients, respectively, p>0.05).
Discussion
There are relatively few studies of markers of proliferation and angiogenesis in pituitary tumours, especially those concerning expression of cyclooxygenase-2 (COX-2) as a potential marker of such tumours. While COX-2 is believed to play a role in the progression of cancer of the colon, breast, pancreas, prostate and lung [6,8,10,11,18,30], its role in pituitary adenomas is not clear.
We investigated COX-2 expression in pituitary tumours in patients with different types of pituitary adenoma, operated upon, whether or not having received any medical treatment with somatostatin analogues or dopamine agonists, pre- or post-surgery. In our retrospective analysis we assessed the predictive value of the COX-2 index as a marker of pituitary tumour size, invasiveness, time of recurrence of disease or tumour progression. Our analysis of COX-2 expression was supplemented by clinical and demographic patient data filed in our Department of Endocrinology and by morphology data (histopathology and immunohistochemistry for anterior pituitary hormones) filed at the Department of Pathomorphology.
In our group of 60 patients, COX-2 was expressed in pituitary adenomas in 52 (87%) cases, in agreement with other authors who quote percentages ranging between 83% [25] and 100% [27]. Our results agree with those of Bloomer et al [25] who found the average percentage of pituitary macroadenoma COX-2 positive cells to be 78%. Our median value of COX-2 index was 57.5%, [IQR=60.5], while in normal pituitary cells COX-2 expression of immunopositive cells does not exceed 0.2% [26,27]. In colorectal carcinomas COX-2 was expressed in neoplastic epithelial cells and correlated with the size of colonic polyps [31,32].
Onguru et al. [27] showed high COX-2 expression in clinically non-functioning pituitary tumours and pituitary carcinomas. They also showed statistically significant differences in COX-2 expression between functioning and non-functioning pituitary tumours and also between COX-2 expression in these tumours and pituitary carcinomas [27]. In our patient group we found no difference in median values of COX-2 indices between functioning and hormonally inactive adenomas (ie, with no expression of pituitary hormones). Vidal et al. [26] found significantly higher values of COX-2 indices in gonadotropin-producing tumours in male patients and in tumours containing no secretory granules, as evaluated by immunohistochemistry. We also found the highest values of COX-2 index in gonadotropin-expressing tumours (70%; however, only in 3 of 5 such patients) and in hormone-immunonegative adenomas (70%). On the other hand, Bloomer et al. [25] found significantly higher COX-2 index or immunohistochemical score in plurihormonal macroadenomas and TSH-expressing macroadenomas. In agreement with our results, they also found lowest COX-2 expression in prolactin-producing macroadenomas.
In our study we found no significant differences in COX-2 expression between different types of pituitary adenomas. However, our conclusion may be biased by the relatively small number of patients with different types of adenoma.
We found no differences in COX-2 expression with respect to patient gender, tumour size, or tumour invasion of bone structures adjacent to sella turcica. Moreover, we have not confirmed the correlation between COX-2 expression and patient age found by Vidal et al [26].
Over-expression of COX-2 is a prominent feature of several premalignant and malignant neoplasms as found by molecular studies [33]. Cellular and molecular changes result in response to induction of constitutive over-expression of COX-2 and prostaglandin production, leading to increased cell proliferation, survival, and inhibition of apoptosis. This results in tumour angiogenesis, invasion and metastasis. In general, higher COX-2 expression in pituitary adenomas and carcinomas as compared to normal pituitary tissue, confirmed in Western blot analysis and RT-PCR [27], suggests that high activity of this enzyme may play a role also in pituitary tumour promotion and progression. However, lack of correlation between tumour size, tumour invasiveness or recurrence and COX-2 index found in our study indicates that there are other unknown processes that determine the behavior of pituitary tumours.
Based on our results, pre-operative treatment with somatostatin analogues and dopamine agonists does not affect COX-2 expression in patients with acromegaly and prolactinoma. Vidal et al. [26] noticed that in patients treated with octreotide or with bromocriptine, higher percentages of COX-2-negative tumours occurred in GH- and PRL-producing tumours, respectively. However, both our and Vidal’s studies concern relatively small numbers of patients in whom rather broad distributions of COX-2 index values were observed, such as shown in Figure 4. In prolactinoma patients, treatment with bromocriptine does not influence tumour angiogenesis [34].
We have not found any relation between COX-2 expression and recurrence or progression of tumour size, as evaluated via MR T1-weighted imaging, although Bladowska et al suggested that T2-weighted images are superior to contrast-enhanced T1 in discrimination of tumour contours (mass) in the postoperative sella and sphenoid sinus [35].
Nor was COX-2 expression related to recurrence of hormone secretion, hence no inferences as to the necessity of re-operation or complementary radiotherapy could be drawn from our analysis of COX-2 indices. Similarly, any distinction of tumours with high growth potential on the basis of COX-2 expression appears to be unlikely. As seen in Figure 1A, the higher the value of the COX-2 index, the higher is the percentage of recurrence/progression of tumour size. However, this observation, due to the small number of patients in subgroups, cannot be supported statistically.
COX-2 immunoreactivity, being strongly correlated with microvessel density and microvessel surface density, is rather high in pituitary adenoma cells [26,27]. Invasive prolactinomas were significantly more vascular than non-invasive tumours [33]. This shows that factors related to angiogenesis may be important in determining clinical features of pituitary tumours such as invasiveness, at least in the case of macroprolactinomas. Therefore, through inhibition of this enzyme it may be possible to prevent or control progression of these tumours.
Numerous, well-documented studies concerning COX-2 expression in malignant tumours of organs other than pituitary demonstrate the beneficial effect of non-steroidal anti-inflammatory drugs (NSAIDs), which decrease the risk of development of adenocarcinoma of the digestive tract [33,36–38], and cancer of the bladder [12], prostate [33,39] and breast [9,33,40]. Therefore the possibility of applying NSAIDs or other novel COX-2 inhibitors in the therapy of certain pituitary tumours is promising, especially with respect to hormonally inactive adenomas in which expression of COX-2 appears to be the highest. The pharmacological treatment of such tumours is currently quite limited.
Conclusions
In our study of COX-2 expression in pituitary tumours we found no differences in COX-2 expression with respect to patient age, gender, tumour size, degree of tumour invasiveness or tumour function. COX-2 does not appear to be a predictive factor for recurrence or progression of tumour size. Nevertheless, due to the observed relatively high expression of COX-2 in pituitary tumours, further studies with COX-2 inhibitors are justified in the search for new approaches to the treatment of these tumours.
Acknowledgements
The authors wish to thank Ms. Edyta Radwanska, M.Sc., for her excellent technical assistance.
Footnotes
Statements
The authors declare that no conflict of interest exists.
Source of support: This work was supported by Collegium Medicum of the Jagiellonian University statutory grant No BBN/CM-4103/411/2/2005/WŁ/NKL/83/L
References
- 1.DeLellis RA, Lloyd RV, Heitz PU, Eng C. Pathology and Genetics of Tumors of Endocrine Organs. 3rd Edition. IARC Press; Lyon: 2004. World Health Organization Classification of Tumors; pp. 9–47. [Google Scholar]
- 2.Dekkers OM, Pereira AM, Roelfsema F, et al. Observation alone after transsphenoidal surgery for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 2006;91(5):1796–801. doi: 10.1210/jc.2005-2552. [DOI] [PubMed] [Google Scholar]
- 3.Dekkers OM, Pereira AM, Romijn JA. Treatment and follow-up of clinically nonfunctioning pituitary macroadenomas. J Clin Endocrinol Metab. 2008;93(10):3717–26. doi: 10.1210/jc.2008-0643. [DOI] [PubMed] [Google Scholar]
- 4.Chang EF, Sughrue ME, Zada G, et al. Long term outcome following repeat transsphenoidal surgery for recurrent endocrine-inactive pituitary adenomas. Pituitary. 2010;13(3):223–29. doi: 10.1007/s11102-010-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colao A, Loche S. Prolactinomas in Children and Adolescents. Endocr Dev. 2010;17:146–59. doi: 10.1159/000262536. [DOI] [PubMed] [Google Scholar]
- 6.Onguru O, Casey M, Kajita S, et al. Cyclooxygenase-2 and thromboxane synthase In non-endocrine and endocrine tumors: A review. Endocr Pathol. 2005;16(4):253–77. doi: 10.1385/ep:16:4:253. [DOI] [PubMed] [Google Scholar]
- 7.Cianchi F, Cortesini C, Bechi P, et al. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121(6):1339–47. doi: 10.1053/gast.2001.29691. [DOI] [PubMed] [Google Scholar]
- 8.Ristimäki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast. Cancer Res. 2002;62(3):632–35. [PubMed] [Google Scholar]
- 9.Badawi AF, Badr MZ. Chemoprevention of breast cancer by targeting cyclooxygenase-2 and peroxisosme proliferator-activated receptor gamma. Int J Oncol. 2002;20:1109–22. [PubMed] [Google Scholar]
- 10.Kokawa A, Kondo H, Gotoda T, et al. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer. 2001;91(2):333–38. doi: 10.1002/1097-0142(20010115)91:2<333::aid-cncr1006>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.Fujita H, Koshida K, Keller ET, et al. Cyclooxygenase promotes prostate cancer progression. Prostate. 2002;53(3):232–40. doi: 10.1002/pros.10152. [DOI] [PubMed] [Google Scholar]
- 12.Shirahama T, Arima J, Akiba S, Sakakura C. Relation between cyclooxygenase-2 expression and tumor invasiveness and patient survival in transitional cell carcinoma of the urinary bladder. Cancer. 2001;92:188–93. doi: 10.1002/1097-0142(20010701)92:1<188::aid-cncr1308>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Ristimaki A, Nieminen O, Saukkonen K, et al. Expression of cyclooxygenase-2 in human transitional cell carcinoma of the urinary bladder. Am J Pathol. 2001;158:849–53. doi: 10.1016/S0002-9440(10)64033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng H, Shao J, Morrow JD, et al. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58(2):362–66. [PubMed] [Google Scholar]
- 15.Costa C, Soares R, Reis-Filho JS, et al. Cyclooxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol. 2002;55(6):429–34. doi: 10.1136/jcp.55.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner HE, Nagy Z, Gatter KC, et al. Proliferation, bcl-2 expression and angiogenesis in pituitary adenomas: relationship to tumour behavior. Br J Cancer. 2000;82(8):1441–45. doi: 10.1054/bjoc.1999.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okegawa T, Pong RC, Li Y, Hsieh JT. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim Pol. 2004;51(2):445–57. [PubMed] [Google Scholar]
- 18.Dubois RN. Review article: cyclooxygenase – a target for colon cancer prevention. Aliment Pharmacol Ther. 2000;1:64–67. doi: 10.1046/j.1365-2036.2000.014s1064.x. [DOI] [PubMed] [Google Scholar]
- 19.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23(12):2840–55. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 20.van Rees BP, Saukkonen K, Ristimäki A, et al. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol. 2002;196(2):171–79. doi: 10.1002/path.1033. [DOI] [PubMed] [Google Scholar]
- 21.Buskens CJ, Van Rees BP, Sivula A, et al. Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology. 2002;122(7):1800–7. doi: 10.1053/gast.2002.33580. [DOI] [PubMed] [Google Scholar]
- 22.Xu XC. Cox-2 inhibitors in cancer treatment and prevention, a recent development. Anticancer Drugs. 2002;13(2):127–37. doi: 10.1097/00001813-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Turner HE, Harris AL, Melmed S, Wass JA. Angiogenesis in endocrine tumors. Endocrine Rev. 2003;24(5):600–32. doi: 10.1210/er.2002-0008. [DOI] [PubMed] [Google Scholar]
- 24.Turner HE, Nagy Z, Gatter KC, et al. Angiogenesis in pituitary adenomas – relationship to endocrine function, treatment and outcome. J Endocrinol. 2000;165(2):475–81. doi: 10.1677/joe.0.1650475. [DOI] [PubMed] [Google Scholar]
- 25.Bloomer CW, Kenyon L, Hammond E, et al. Cyclooxygenase – 2 (COX-2) and epidermal growth factor receptor expression in human pituitary macroadenomas. Am J Clin Oncol. 2003;26(4):75–80. doi: 10.1097/01.COC.0000074163.69381.22. [DOI] [PubMed] [Google Scholar]
- 26.Vidal S, Kovacs K, Bell D, et al. Cyclooxygenase-2 expression in Human Pituitary Tumors. Cancer. 2003;97(11):2814–21. doi: 10.1002/cncr.11387. [DOI] [PubMed] [Google Scholar]
- 27.Onguru O, Scheithauer BW, Kovacs K, et al. Analysis of Cox-2 and thromboxane synthase expression in pituitary adenomas and carcinomnas. Endocr Pathol. 2004;15(1):17–27. doi: 10.1385/ep:15:1:17. [DOI] [PubMed] [Google Scholar]
- 28.Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: A magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33(4):610–18. doi: 10.1227/00006123-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Zada G, Lin N, Laws ER. Patterns of extrasellar extension in growth hormone-secreting and nonfunctional pituitary macroadenomas. Nerosurg Focus. 2010;29(4):1–5. doi: 10.3171/2010.7.FOCUS10155. [DOI] [PubMed] [Google Scholar]
- 30.Guruswamy S, Rao CV. Multi-target approaches in colon cancer chemoprevention based on systems biology of tumor cell-signalling. Gene Regulation and Systems Biology. 2008;2:163–76. doi: 10.4137/grsb.s486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheehan KM, Sheahan K, O’Donoghue DP, et al. The relationship between cyclooxygenase expression and colorectal cancer. JAMA. 1999;282(13):1254–57. doi: 10.1001/jama.282.13.1254. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa K, Ichikawa W, Fujita T, et al. Expression cyclooxygenase-2 (COX-2) mRNA in human colorectal adenomas. Eur J Cancer. 2001;37(12):1469–74. doi: 10.1016/s0959-8049(01)00137-x. [DOI] [PubMed] [Google Scholar]
- 33.Harris RE. Cyclooxygenase-2 (cox-2) blockade in chemoprevention of cancers of the colon, breast, prostate, and lung. Immunopharmacology. 2009;17(2):55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- 34.Turner HE, Nagy Z, Gatter KC, et al. Angiogenesis in pituitary adenomas – relationship to endocrine function, treatment and outcome. J Endocrinol. 2000;165(2):475–81. doi: 10.1677/joe.0.1650475. [DOI] [PubMed] [Google Scholar]
- 35.Bladowska J, Biel A, Zimny A, et al. Are T2-weighted images more useful than T1-weighted contrast-enhanced images in assessment of postoperative sella and parasellar region? Med Sci Monit. 2011;17(10):MT83–90. doi: 10.12659/MSM.881966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1(1):11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 37.Rao M, Yang W, Seifalian AM, Winslet MC. Role of cyclooxygenase-2 in the angiogenesis of colorectal cancer. Int J Colorectal Dis. 2004;19(1):1–11. doi: 10.1007/s00384-003-0511-2. [DOI] [PubMed] [Google Scholar]
- 38.Sinicrope FA. Targeting cyclooxygenase-2 for prevention and therapy of colorectal cancer. Mol Carcinog. 2006;45(6):447–54. doi: 10.1002/mc.20232. [DOI] [PubMed] [Google Scholar]
- 39.Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191(2):125–35. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- 40.Badawi AF, Eldeen MB, Liiu Y, et al. Inhibition of rat mammary gland carcinogenesis by simultaneous targeting of cyclooxygenase-2 and peroxisosme proliferator-activated receptor gamma. Cancer Res. 2004;64(3):1181–89. doi: 10.1158/0008-5472.can-03-2556. [DOI] [PubMed] [Google Scholar]