Summary
Background
Radiation often causes depletion of immunocytes in tissues and blood, which results in immunosuppression. Molecular hydrogen (H2) has been shown in recent studies to have potential as a safe and effective radioprotective agent through scavenging free radicals. This study was designed to test the hypothesis that H2 could protect immunocytes from ionizing radiation (IR).
Material/Methods
H2 was dissolved in physiological saline or medium using an apparatus produced by our department. A 2-[6-(4′-hydroxy) phenoxy-3H-xanthen-3-on-9-yl] benzoate (HPF) probe was used to detect intracellular hydroxyl radicals (•OH). Cell apoptosis was evaluated by annexin V-FITC and Propidium iodide (PI) staining as well as the caspase 3 activity. Finally, we examined the hematological changes using an automatic Sysmex XE 2100 hematology analyzer.
Results
We demonstrated H2-rich medium pretreatment reduced •OH level in AHH-1 cells. We also showed H2 reduced radiation-induced apoptosis in thymocytes and splenocytes in living mice. Radiation-induced caspase 3 activation was also attenuated by H2 treatment. Finally, we found that H2 rescued the radiation-caused depletion of white blood cells (WBC) and platelets (PLT).
Conclusions
This study suggests that H2 protected the immune system and alleviated the hematological injury induced by IR.
Keywords: radioprotection, apoptosis, hydrogen, immunosuppression
Background
Exposure to ionizing radiation (IR) often causes immunosuppression, which enhances the probability of infection and affects the recovery from radiation sickness [1,2]. Immunosuppression also limits the further application of radiotherapy for cancer. Although it is known that IR affects functions of radioresistant immunocytes like macrophages [3], dendritic cells (DC) [4], and regulatory T cells [5], apoptosis of radiosensitive immune cells is also an important pathway for radiation-induced immunosuppression [6,7]. In different developmental stages of the immune system, cell apoptosis regulates the body’s homeostasis in physiological and pathological conditions [8]. Apoptosis induced by IR in thymocytes, splenocytes and peripheral blood lymphocytes affect the body’s immune status as well as human health.
IR causes tissue damage mainly by free radicals [9]. For decades, free radical scavengers have been studied for radioprotection of the immune system. But from thiol compounds to plant extractions, they all face significant shortcomings, including relatively high toxicity and unfavorable routes of administration, which affect their applications and efficacies. Therefore the search for safer and more effective radioprotectors continues.
Recently, Ohsawa et al. reported that molecular hydrogen (H2) could reduce reactive oxygen species such as •OH and ONOO− etc [10]. Our department demonstrated H2 treatment protected cultured cells and mice from radiation damage, and exerted protective effects on the gastrointestinal tract, cardiovascular system and spermatogenic epithelium from γ-irradiation [11–14]. These encouraging results prompted us to study whether H2 treatment could protect the immune system against IR.
Material and methods
H2-rich saline/medium production
H2 was dissolved into physiological saline or RPMI1640 medium (Invitrogen, California, USA) for 6 h under high pressure (0.4MPa) to a supersaturated level using a H2-rich water producing apparatus produced by our department. The saturated H2-rich saline/medium was stored under atmospheric pressure at 4°C in an aluminum bag with no dead volume. H2-rich saline/medium was freshly prepared 1 day before irradiation, which ensured that a concentration of more than 0.6 mmol/L was maintained. Gas chromatography (Biogas Analyzer Systems-1000, Mitleben, Japan) was used to confirm the content of H2 in saline/medium by the method described by Ohsawa et al. [10].
Irradiation
60Co-gamma rays at the Irradiation Center (Faculty of Naval Medicine, Second Military Medical University, China) were used for the irradiation. Mice and cells (with or without H2 pre-treatment) were exposed to different radiation doses depending upon the requirement of the present study.
Mice and treatment
All the experiments were approved by the Second Military Medical University, China in accordance with the Guide for Care and Use of Laboratory Animals published by the US NIH (publication No. 96-01). Male wild-type BALB/c mice, 4–6 weeks old, were purchased from the China Academy of Science (Shanghai, China). All mice were housed in individual cages in a temperature-controlled room with a 12 h light/dark cycle. Food and water were provided ad libitum. Twenty minutes before irradiation, mice were treated intraperitoneally (IP) with 0.2 ml physiological saline or saturated H2-rich saline every 5 minutes for 4 injections. Mice were irradiated in a holder designed to immobilize unanaesthetized mice such that the abdomens were exposed to the beam. At different time points after irradiation, mice were killed by cervical dislocation and used in subsequent experiments.
Cell culture and detection of •OH
Human lymphocyte AHH-1 cells (American Type Culture Collection, Manassas, VA, USA) were maintained in RPMI1640 (Invitrogen, California, USA) with 10% fetal bovine serum and 1% penicillin-streptomycin-glutamine at 37°C in a 5% CO2 humidified chamber. To detect cellular •OH, we treated AHH-1 cells with H2-rich or normal medium and added 5 μM 2-[6-(4′-hydroxy) phenoxy-3H-xanthen-3-on-9-yl] benzoate (HPF) (a maker of oxidation of •OH and ONOO−) (Daiichi Pure Chemicals Co., Tokyo, Japan). Immediately after being exposed to 8Gy radiation, the cells were centrifuged and washed twice with phosphate buffer saline (PBS, pH 7.4). Cell suspensions were smeared onto slides. Cellular images were obtained using an Olympus BX60 fluorescent microscope equipped with a Retiga 2000R digital camera. We quantified fluorescent signals for 100 cells for each group using ImageJ software (version 1.44p, Wayne Rasband, NIH, US).
Apoptosis assay
The mice were killed at 24 h after irradiation, after which spleen and thymus were immediately removed. Cells were dispersed by passage through a fine wire mesh into a 35×10mm petri dish containing 1ml PBS. Isolated splenocytes and thymocytes were washed 3 times with PBS, and then stained with Annexin V-FITC and Propidium Iodide (PI) by Apoptosis Detection Kit (Bipec Biopharma, Massachusetts, USA), according to the manufacturer’s instructions. Subsequently, cells were analyzed by flow cytometry (Beckman Coulter, California, USA).
Detection of caspase 3 activity
Isolated spleens were homogenized in Radio Immunoprecipitation Assay (RIPA) lysis buffer (Beyotime Biotechnology, Shanghai, China) with 1mM of serine protease inhibitor phenyl methanesulfonyl fluoride (PMSF) (Beyotime Biotechnology, Shanghai, China) at 8 h after irradiation, and they were centrifuged at 12000 g for 10 min at 4°C. Level of caspase 3 in supernatants was determined by a Caspase 3 Assay Kit (Sigma, St. Louis, MO, USA), according to the manufacturer’s instruction. The experiments were repeated 3 times.
Hemograms analysis
Blood samples were taken from the retro-orbital sinus/plexus using EDTA-coated blood collection tubes at 24 h after irradiation. Whole blood samples from all groups were analyzed using an automated Sysmex XE 2100 hematology analyzer (Sysmex, Kobe, Japan).
Statistical analysis
Data are expressed as means±SEM for each experiment. The number of samples is indicated in the description of each experiment. We used an analysis of variance (ANOVA) followed by a Student-Newman-Keuls post-hoc test for statistical analysis. We performed experiments for quantification in a blinded fashion.
Results
H2 reduced radiation-induced •OH level in cultured cells
Radiation increased the intracellular HPF fluorescence intensity compared to the control group. The HPF fluorescence intensity in the H2 treated group was much lower than in the single-radiation group (Figure 1A). H2 significantly reduced the •OH level produced by irradiation (Figure 1A, B).
Figure 1.
H2 reduced radiation-induced •OH in cultured cells. (A) representative images of fluorescence of the ROS (•OH and ONOO−) marker HPF were taken immediately after 8Gy γ-irradiation. The fluorescence intensity represented the •OH and ONOO− level in cells of each group. (B) HPF fluorescence in cells treated with 8 Gy γ-radiation in H2(−) or H2(+) medium were qualified for 100 cells. Value are given as mean ±SEM (n=5), * P<0.01.
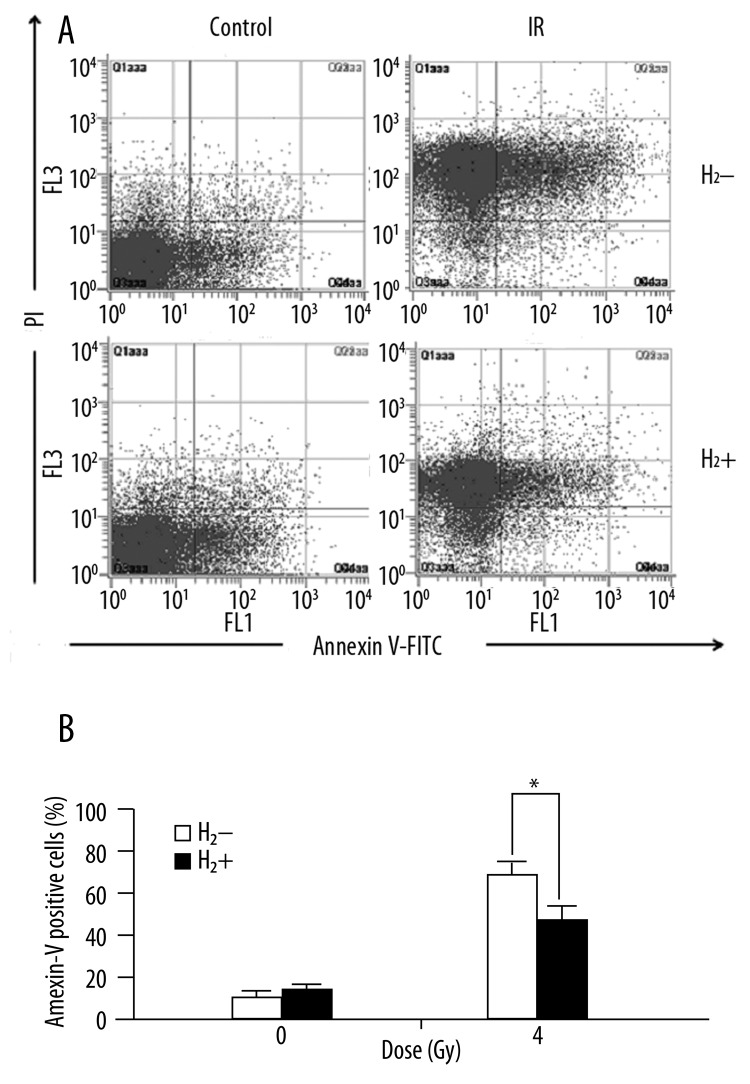
H2-rich saline protected immunocytes from radiation-induced apoptosis
After whole body irradiation (WBI), apoptosis of thymocytes and splenocytes were significantly enhanced. The splenocyte apoptosis decreased significantly in mice pretreated with H2-rich saline after irradiation (Figure 2A, B). The apoptosis of thymocytes were also reduced significantly after H2 treatment (Figure 3A, B).
Figure 2.
H2-rich saline reduced radiation-induced apoptosis in splenocytes after WBI. (A) representative diagrams of distribution of stained cells. (B) a bar graph of apoptotic cells (Annexin V positive) expressed by percentage of total cells. Values are given as mean±SEM (n=5), * P<0.01.
Figure 3.
H2-rich saline reduced radiation-induced apoptosis in thymocytes after WBI. (A) distribution of stained thymocytes. (B) a bar graph of apoptotic cells expressed by percentage of total cells. Values are given as mean ±SEM (n=5), * P<0.01.
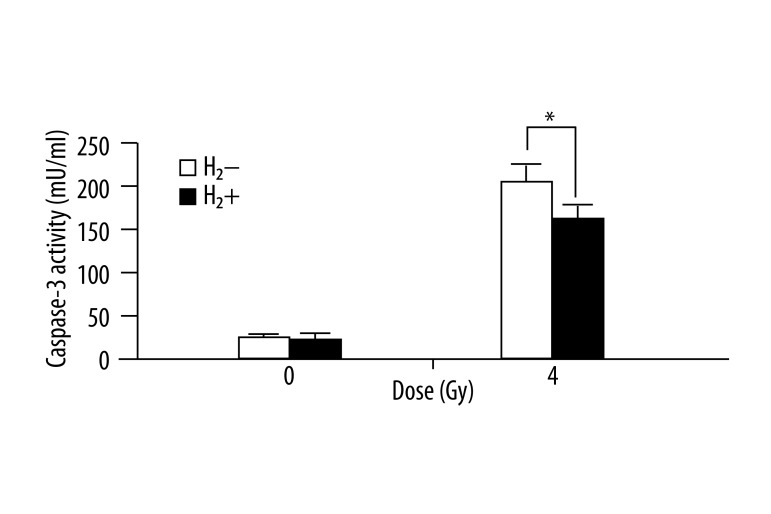
H2 attenuated radiation-induced activation of caspase 3
The caspase 3 activity in spleen tissue of irradiated mice was up-regulated compared to the controls. H2 treatment significantly attenuated the radiation-induced activation of caspase 3 in spleen tissues (Figure 4), while single H2 treatment had no obvious effect on the baseline activity of caspase 3.
Figure 4.
H2-rich saline attenuated radiation induced activation of caspase 3 in mice spleen tissues. Caspase 3 activity of each group was determined and expressed in a bar graph. Values are given as mean ±SEM (n=5), * P<0.01.
H2-rich saline mitigated radiation-induced hematological injury
At 24 h after irradiation, the numbers of white blood cells (WBC) and platelet (PLT) were reduced in the irradiated mice. H2 treatment significantly mitigated radiation-caused reduction of WBC and PLT, but had no influence on other indexes (Table 1).
Table 1.
Effects of H2 on peripheral blood in mice.
| Groups | WBC (×109/L) | PLT (×109/L) | RBC (×1012/L) | HGB (g/L) | MCV (fL) | MCHC (g/L) |
|---|---|---|---|---|---|---|
| Control | 3.89±0.14 | 900.50±40.31 | 8.90±0.06 | 136.66±4.04 | 46.44±0.14 | 336.50±0.71 |
| H2 | 4.18±0.54 | 872.50±31.82 | 9.28±0.12 | 138.33±7.23 | 46.12±1.13 | 333.00±5.66 |
| IR | 0.94±0.21** | 169.05±35.36** | 9.46±0.24 | 140.33±10.97 | 45.55±1.34 | 340.50±10.60 |
|
| ||||||
| IR+H2 | 1.81±0.16* | 335.50±23.12* | 8.31±0.53 | 131.67±8.51 | 46.05±0.78 | 336.00±1.41 |
WBC – white blood cells; PLT – platelets; RBC – red blood cells; HGB – hemoglobin; MCV – mean corpuscular volume; MCHC – mean corpuscular hemoglobin concentration; IR – ionizing radiation. The data are expressed as mean ±SEM (n=5).
P<0.01 vs. the control group and
P<0.05 vs. the irradiated control group.
Discussion
To our knowledge, this is the first study demonstrating that H2 has protective effects on the immune system of irradiated mice. Radiation-induced apoptosis in radiosensitive immunocytes causes depletion of cells in immune organs and blood, which leads to immunosuppression [15], but until now no ideal radioprotector for immune system has met the requirements of both efficacy and safety. We previously demonstrated that H2 exerted a protective effect against γ-irradiation in cultured cells and mice [12]. In this study, we found H2 reduced the hydroxyl radical level in AHH-1 cells after radiation. We also found that H2-rich saline effectively reduced radiation-induced apoptosis in thymocytes and splenocytes in living mice after WBI. In the execution phase of cell apoptosis, caspase 3 was often activated and radiation-induced caspase 3 activation was also partially inhibited by H2 treatment. Finally, we studied the hematological change after H2 treatment and found that H2 rescued the radiation-caused depletion of WBC and PLT, but did not find any influence on other indexes.
Recently, Ohsawa et al. reported that H2 could effectively reduce the most cytotoxic of ROS, •OH [10]. Inhalation of H2 was also reported to protect cerebral, myocardial and hepatic IR injury, and ameliorated oxidative stresses in lung and intestinal transplantation [16–18]. To our knowledge, 60–70% ionizing radiation-induced damage was caused by •OH [19]. •OH could easily react with DNA, proteins, lipids, etc and exert strong cytotoxic effects. In this study, our data showed that incubation of AHH-1 cells with H2-rich medium reduced the intracellular •OH produced by IR. The reaction between H2 and •OH was also previously demonstrated in a cell-free Fenton system [10]. Thus, in AHH-1 cells, H2 may react with •OH and protect cells from radiation damage, which may account for some mechanisms active in mitigating radiation damage.
IR could induce cell apoptosis in radiosensitive cells, like CD4+ T helper (Th) cells, CD8+ cytotoxic T lymphocyte (CTL), or the antibody-producing B cells [20], which act as effectors in cell-mediated and humoral immune responses [24]; thus the apoptosis or depletion of these cells directly caused immunosuppression. We found that the H2 could effectively inhibit radiation-induced apoptosis in thymocytes and splenocytes of living mice. The reduction of apoptosis in thymocytes and splenocytes suggests that H2 could also alleviate radiation-induced thymus and spleen damage. We also found that H2 attenuated radiation-induced caspase 3 activation, which plays a central role in the execution phase of cell apoptosis, but the change in caspase 3 may be due to the reduction in •OH by H2 through the ROS signaling pathway.
We then examined whether H2 had a protective effect on hematological injury induced by irradiation. We found that H2 attenuated the decrease in WBCs caused by radiation, which suggested a marked enhancement of immune function. The protective effects of H2 on WBC may act similarly to the inhibition of apoptosis on lymphocytes in thymus and spleen, as H2 is so easy to diffuse into cells in blood and tissues. H2 also has protective effects on PLT against radiation damage, but the underlying mechanism is unclear. However, no significant change was observed in the red blood cells (RBC), hemoglobin (HBG), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC). This is not surprising, because the nucleus, which is absent in RBCs, is an important target of radiation.
Besides efficacy, safety is another important requirement for an ideal radioprotector. H2 is produced by bacteria in the body and then released into the circulation [21], and the reaction between H2 and •OH produces water [22], indicating that low concentration H2 could be safe for human health. Therefore dissolving it in PBS, physiological saline or medium would provide a convenient method of application. Immunosuppression is the most frequent consequence of routine irradiation. H2 exhibits great potential as novel, safe and effective radioprotector for use by radiation workers, radiologists and doctors, as well as clinical uses.
Conclusions
We found that H2-rich saline or water could inhibit radiation-induced apoptosis in immune cells and ameliorate the homological injuries. The protective effect was attributed to its free radicals scavenging capacity. We suggest H2 is a safe and effective radioprotector for the immune system. But the direct target of H2 and the exact mechanism requires further study.
Acknowledgements
Special thanks to Dr. Qingqiang Xu from the Department of Microbiology, and Dr. Bing Yu from Department of Cell Biology of our university for providing additional help.
Footnotes
Declaration of interest
The author has no conflict of interest to disclose.
Source of support: This work was supported by a grant from the National Natural Science Foundation of China (No. 81072241) and by a grant from Natural Science Foundation of Shanghai, China (No. 11ZR1446400)
References
- 1.Goel HC, Prakash H, Ali A, et al. Podophyllum hexandrum modulates gamma radiation-induced immunosuppression in Balb/c mice: implications in radioprotection. Mol Cell Biochem. 2007;295:93–103. doi: 10.1007/s11010-006-9277-5. [DOI] [PubMed] [Google Scholar]
- 2.Shankar B, Premachandran S, Bharambe SD, et al. Modification of immune response by low dose ionizing radiation: role of apoptosis. Immunol Lett. 1999;68:237–45. doi: 10.1016/s0165-2478(99)00074-7. [DOI] [PubMed] [Google Scholar]
- 3.Ibuki Y, Goto R. Ionizing radiation-induced macrophage activation: augmentation of nitric oxide production and its significance. Cell Mol Biol (Noisy-le-grand) 2004:50. Online Pub: L617–26. [PubMed] [Google Scholar]
- 4.Liao YP, Wang CC, Butterfield LH, et al. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immunol. 2004;173:2462–69. doi: 10.4049/jimmunol.173.4.2462. [DOI] [PubMed] [Google Scholar]
- 5.Cao M, Cabrera R, Xu Y, et al. Gamma irradiation alters the phenotype and function of CD4+CD25+ regulatory T cells. Cell Biol Int. 2009;33:565–71. doi: 10.1016/j.cellbi.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao YP, Wang CC, Butterfield LH, et al. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immunol. 2004;173:2462–69. doi: 10.4049/jimmunol.173.4.2462. [DOI] [PubMed] [Google Scholar]
- 7.Lombard C, Nagarkatti M, Nagarkatti P. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: potential role for CB2-selective ligands as immunosuppressive agents. Clin Immunol. 2007;122:259–70. doi: 10.1016/j.clim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu SZ, Zhang YC, Mu Y, et al. Thymocyte apoptosis in response to low-dose radiation. Mutat Res. 1996;358:185–91. doi: 10.1016/s0027-5107(96)00119-4. [DOI] [PubMed] [Google Scholar]
- 9.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 10.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–94. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 11.Qian L, Cao F, Cui J, et al. The potential cardioprotective effects of hydrogen in irradiated mice. J Radiat Res. 2010;51:741–47. doi: 10.1269/jrr.10093. [DOI] [PubMed] [Google Scholar]
- 12.Qian L, Cao F, Cui J, et al. Radioprotective effect of hydrogen in cultured cells and mice. Free Radic Res. 2010;44:275–82. doi: 10.3109/10715760903468758. [DOI] [PubMed] [Google Scholar]
- 13.Chuai Y, Zhao L, Ni J, et al. A possible prevention strategy of radiation pneumonitis: combine radiotherapy with aerosol inhalation of hydrogen-rich solution. Med Sci Monit. 2011;17(4):Y1–4. doi: 10.12659/MSM.881698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuai Y, Shen J, Qian L, et al. Hydrogen-rich saline protects spermatogenesis and hematopoiesis in irradiated BALB/c mice. Med Sci Monit. 2012 doi: 10.12659/MSM.882513. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharjee M, Bose I, Sarkar P, et al. A sequential scanning of the immune efficiency in astrocytoma (Grade I to Grade Iii), meningioma and secondary glioma patients with and without therapeutic scheduling. Cancer Invest. 2006;24:502–13. doi: 10.1080/07357900600814839. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura T, Huang CS, Tochigi N, et al. Inhaled hydrogen gas therapy for prevention of lung transplant-induced ischemia/reperfusion injury in rats. Transplantation. 2010;90:1344–51. doi: 10.1097/TP.0b013e3181fe1357. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Yu G, Liu SY, et al. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J Surg Res. 2011;167:e339–44. doi: 10.1016/j.jss.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Sun Q, He B, et al. Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int J Cardiol. 2011;148:91–95. doi: 10.1016/j.ijcard.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 19.Vijayalaxmi, Reiter RJ, Tan DX, et al. Melatonin as a radioprotective agent: a review. Int J Radiat Oncol Biol Phys. 2004;59:639–53. doi: 10.1016/j.ijrobp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Harrington NP, Chambers KA, Ross WM, et al. Radiation damage and immune suppression in splenic mononuclear cell populations. Clin Exp Immunol. 1997;107:417–24. doi: 10.1111/j.1365-2249.1997.272-ce1158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3:1129–34. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 22.Chuai Y, Gao F, Li B, et al. Hydrogen-rich saline attenuates radiation-induced male germ cell loss in mice through reducing hydroxyl radicals. Biochem J. 2012;442(1):49–56. doi: 10.1042/BJ20111786. [DOI] [PubMed] [Google Scholar]