Summary
Background
Neoplasms are the second leading cause of death in Poland after vessel diseases, despite the huge progress in medical sciences in the last 20 years. Recently, gastric cancer morbidity has decreased, but mortality is still at a high level.
Material/Methods
Tissues from 24 patients with a histopathologically diagnosed mucosal and adenomucosal gastric cancer were tested. Patients were divided into 2 equal groups: patients without metastases (G1) and patients with metastases in the liver (G2). In all tested tissues of G1 and G2, the expression of VEGF (vascular endothelial growth factor) and metalloproteinase 2, respectively, were estimated.
Results
Results revealed a statistically significant increase in the VEGF expression for G1 and G2 in relation to the margin (p1<0.001; p2<0.001). The increase of gene expression for VEGF did not significantly differ statistically in G1 and G2. The obtained results revealed a statistically significant difference in the increase of gene expression for MMP-2 in G1 in relation to the margin (p<0.05) and a very high one in G2 in relation to the average margin value (p<0.001). A highly statistically significant correlation was obtained for VEGF and MMP-2 in the tissue of patients with metastases (p<0.001; r=0.714). The highly elevated expression of MMP-2 in the tissue of gastric cancer in patients with metastases confirms its participation in the invasiveness of the neoplasmatic process.
Conclusions
The highly significant correlation between VEGF and MMP-2 suggests a connection between both mechanisms in the progression of gastric cancer.
Keywords: gastric cancer, VEGF, MMP 2, gene expression
Background
Gastric cancer is one of the most common malignant neoplasms, and it is the only one in which morbidity and mortality are not decreasing [1].
Advances in oncologic immunodiagnostics have not influenced outcomes in patient with gastric cancer. A review of the literature indicates researchers’ interest in the characteristics of the vascular supply of this cancer. There have been attempts to explain gastric cancer’s nonspecific vascularization using modern techniques of molecular biology, vascular imaging, and estimation of the activity of chosen cytokines responsible for angiogenesis and the degradation of extracellular matrix. This is an important problem because any malignant tumor above 1mm, in order to survive, requires the proliferation of new blood vessels [1–3]. Angiogenesis in solid tumors is a complex process and plays an important role in the progression of the disease and in the metastatic process. Tumor neovascularization can be initiated by neoplasm cell hypoxia, which induces the activity of the gene encoding VEGF, which is well-known as a main proangiogenic factor. That process plays a significant role in the evolution of cancer. Its influence on neovascularization has also recently been attributed to some matrix metalloproteinas. Like mainly MMP-2 and MMP-9. Metalloproteinases are capable of degrading collagen and other extracellular matrix proteins, thereby facilitating the migration of neoplasm cells, invasiveness and distant metastases.
Material and Methods
Material for the study were fragments of tissues collected from 24 patients (both male and female) during surgical procedures with a histopathologically confirmed adenocarcinoma of the stomach, of a medium size and well-differentiated. Patients were assigned to 1 of 2 groups – group I (G1) consisted of 12 patients without metastases, and group II (G2) consisted of 12 patients with liver metastases. Both groups were approximately equal in regard to age and sex. Patients’ mean age was 64.16 years in G1 and 63.25 in G2. The urease test for the presence of Helicobacter pylori was performed preoperatively on subjects in both groups, with 4 positive results in group I and 5 positive results in group II.
Neoplasm staging was defined according to the international TNM classification and depended on clinical data, abdomen and pelvis ultrasonography, CT and chest X-ray. Patients’ specific characteristics are presented in Table 1.
Table 1.
Demographic characteristics and distribution according to TNM classification.
| Group I (G1) n=12 | Group II (G2) n=12 | |
|---|---|---|
| Median age | 64.16 | 63.25 |
| Age range | 43–81 | 45–81 |
| Sex F/M | 3/9 | 3/9 |
| Staging according to TNM classification | ||
| T1N1M0 | 8 | 0 |
| T2N1M0 | 4 | 0 |
| T2N1M1 | 0 | 9 |
| T3N1M1 | 0 | 3 |
| Kind of surgical procedure: | ||
| Total gastrectomy | 12 | 4 |
During the surgical procedure, disease-altered tissue fragments and a minimum of 5 cm negative margins were collected. Samples were stored in a container with liquid nitrogen and frozen at −70°C. Distinguishing between malignant tissue and stroma was defined through an assessment of the number of necrotizing cells.
Diagnostic techniques of molecular biology included an evaluation of the expression of genes for VEGF and MMP-2. RNA was isolated from homogenates after the preliminary peeling of cells in liquid nitrogen. The nucleic acid concentration in the extract was measured using the spectrophotometric technique with the application of RNA/DNA calculator „ Gene Quant” (LKB Pharmacia Biotech). RNA extraction was performed using a modified chloroform-phenol method described by Chomczynski and Sacchi (Total RNA Prep. Plus, A&A Biotechnology) [4–6].
Inclusion criteria:
clinically diagnosed and histopathologically confirmed adenocarcinoma and mucinous adenocarcinoma of the stomach,
written informed consent.
Exclusion criteria:
tissue diagnosis of neoplasms other than gastric adenocarcinoma,
liver failure,
autoimmune diseases,
inflammatory process and ulcerations,
presence of another malignant disease,
lack of informed consent.
The data was managed and analyzed using Microsoft Excel. Verification of the statistical hypothesis was performed using the non-parametric Mann-Whitney test (comparison of healthy tissue with group G1 and G1 with G2) and the Wilcoxon test (comparison of healthy tissue with group G1). P<0.05 was considered significant.
Results
VEGF
A more intense expression of the gene for VEGF was indicated in 10 of 12 individuals (83.3%) from group G1 (without metastases). Moreover, in group G2 (with metastases) a more intense expression of that gene, in comparison to the average expression in the negative margins of specimens from G1, was obtained for 11 of 12 individuals (91.7%).
Average values were:
tissue of margin G1–0.98,
group I (G1, without metastases) – 4.32,
group II (G2, with metastases) – 5.91.
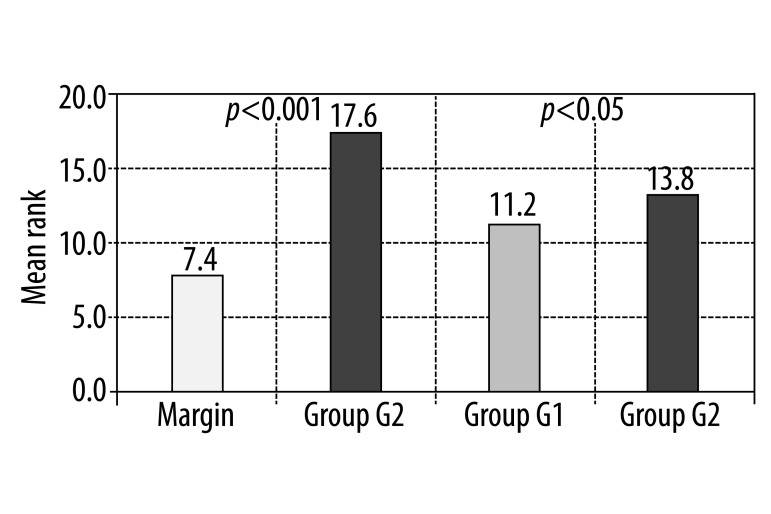
Values of VEGF gene expression in both groups studied, G1 and G2, are statistically significantly higher in comparison to the malignant-free infiltration margin (p1<0.01; p2<0.001). However, there was no statistically significant difference between the groups studied, with (G1) and or without metastases (G2). Results are presented in Figure 1 and Table 1.
Figure 1.
Results of the Mann-Whitney test for evaluation of the difference in VEGF expression between healthy margin and group G2 and between group G1 and G2.
MMP-2
In group I (G1), samples from 9 of the 12 patients without metastases (75%) revealed a more intense expression of the gene for MMP-2 in comparison to the tissue of the margins. In group II (G2) a higher level of expression was demonstrated in every specimen (100%).
Average values of MMP-2 gene expression present:
tissue of margin G1 – 4.58,
group I (G1, without metastases) – 10.55,
group II (G2, with metastases) – 16.71.
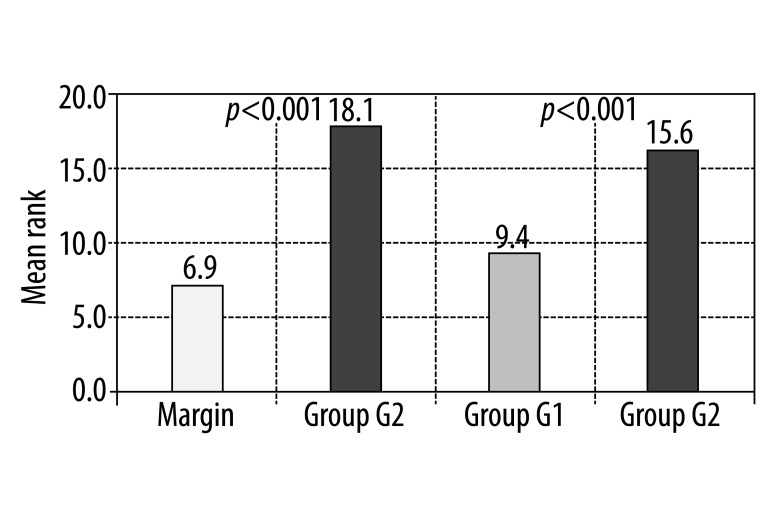
There is a statistical significance of group I (G1) in comparison to the healthy margin (p1<0.05) and a very high statistical significance of group II (G2) in comparison to the healthy margin and between both studied groups, p2<0.001 and p<0.001, respectively. Results are presented in Figure 2 and Table 3.
Figure 2.
Result of the Mann-Whitney test for evaluation of the difference in MMP-2 expression between healthy margin and group G2 and between group G1 and G2.
Table 3.
Result of the Wilcoxon test for evaluation of the difference in MMP-2 expression between healthy margin and group G1.
| Gene | Wilcoxon test | Difference |
|---|---|---|
| MMP-2 | p<0.05 | G1>Margin |
Moreover, there was an attempt to find a correlation between the expressions of both genes studied in healthy margin samples and in groups I and II. There was no proven correlation between MMP-2 and VEGF for group G1 and the healthy margin. A correlation only occurred for group G2.
Discussion
A review of the literature shows oncologists’ serious interest in tumor angiogenesis, as an aspect of the evaluation of disease progression and therapy initiation. According to Szala and Radzikowski, and many others, the process of angiogenesis begins as a result of neoplasm cell hypoxia and mutations of suppressor genes and oncogenes, subsequently leading to the activation of endothelial cells and the degradation of the basement membrane and extracellular matrix [7–10]. The ultimate consequence of that process is the activation of the gene encoding VEGF.
The presented study began with an evaluation of VEGF gene expression. For samples of both groups studied (G1 without metastases and G2 with liver metastases), a greater VEGF expression was statistically significant, in comparison to the healthy margin (p<0.01; p<0.001). There was no statistically significant difference between G1 and G2 (p>005).
Our results confirm the ability of neoplasm cells to undergo intense angiogenesis, especially in patients with a metastatic process. According to Meada et al., the process of neovascularization in solid tumors is a relevant, prognostic factor for patients with gastric cancer, and because of this they suggest an evaluation of mRNA for VEGF in tissue specimens obtained through surgical procedures. If there is no properly equipped diagnostic lab, they recommend counting the blood vessels in the tissue specimens [11].
Because few reports have addressed the causes of gastric cancer, a comparison of this study to others is quite difficult. However, the usefulness of sampling the VEGF gene expression for many neoplasms has been accepted, especially for breast cancer, colorectal cancer, prostate cancer, non-small cell lung cancer, and malignant melanoma [12–15].
If a tumor grows larger than 1 mm3, hypoxia and hypoglycemia will occur as a result of insufficient cell nutrition through the process of diffusion. Cell hypoxia induces the synthesis of the HIF-1 protein, which, jointly with products of a mutated p-53 gene, stimulates the production of the main proangiogenic cytokine, VEGF [7,16–18]. VEGF, together with others cytokines such as bFGF and PIGF, not only stimulates the endothelium but also activates the Bcl-2 gene which, through its protein, inhibits apoptosis [19,20].
In this study, the expression of VEGF was evaluated. It is necessary not only to stress the statistically higher expression in tissue samples from both groups studied, but also the fact that the significance was very high in individuals with metastases (p<0.001). In that group (G2), angiogenesis facilitates the metastatic process through neoplasm cells entering the circulation, settling and proliferating in the site of metastasis [21].
According to D’Amore et al, progression into invasive cancer, which is associated with metastasizing, is related to gaining a new, angiogenic phenotype [22]. In this study, this was observed in patients from group II. An evaluation of VEGF expression confirmed the ability of cancer tissue to undergo neovascularization, although the number of blood vessels is quite small.
The study review concerning metalloproteinases reveals the involvement of metalloproteinase 2 (MMP-2), whose elevated expression increases a cancer’s ability to metastasize. Many studies have focussed on metalloproteinase 9 (MMP-9) and tissue metalloproteinase inhibitors [23–26]. In connection with the above, in this study, the expression of the MMP-2 gene was additionally measured. An evaluation of the data showed a significant difference in MMP-2 elevated expression between individuals without metastases and margin tissue (p<0.05) and a very high, statistically significant expression of MMP-2 in the group of individuals with metastases (G2) in comparison to an average expression in the margin (p<0.001). The highly significant difference between the studied groups (p<0.01) was remarkable.
Lee et al. obtained similar results [26]. According to their research, the elevated expression of MMP-2 and MMP-9 in gastrointestinal cancers increases their ability to metastasize. Moreover, Bae et al. found that MMP-2 expression, stimulated by Bcl-2, increases the invasiveness of neoplasm cells in gastrointestinal cancers [27,28].
Results of these studies confirm the above suggestions and the relation between MMP-2 and a cancer’s invasiveness and metastatic process. Highly significant differences were found in samples from patients with metastases. Feng et al., Cai et al., and Whatling et al. stress the value of MMP-2 sampling in tissue specimens obtained during surgical procedures as a predictor of the course of a disease [25,29,30].
The results of this study confirmed the presence of a correlation between VEGF and MMP-2 in the cancer tissue of patients with metastases (G2). This was of high statistical significance (p<001) and a very high correlation coefficient (r=0.714). But this correlation occurred only in samples from group II (G2 – with metastases). Thus, our results only partly confirm other studies, and although a correlation between VEGF and MMP-2 was not visible, the indirect but close cooperation of both factors was demonstrated. In 1992, Deschemaeker et al noticed the influence of VEGF on inducing metalloproteinases expression, including MMP-2 and MMP-9, both connected with a cancer’s invasiveness, which stimulate angiogenesis through the degradation of the extracellular matrix, and the alternation of a phenotype to an angiogenic one [7,16,19,21,23,31]. These observations have great clinical and therapeutic significance. Both parameters are due to the invasiveness of a neoplasm and the metastatic process, beginning with the extracellular matrix and vessel wall degradation and continuing to the formation of tumor vascular supply.
Conclusions
In spite of an elevated expression of VEGF, the number of blood vessels in gastric cancer tissue remains low, which might be an antiangiogenic effect of metalloproteinases and their inhibitors.
A highly elevated expression of MMP-2 in the tissue of gastric cancer in patients with metastases confirms its participation in the invasiveness of the neoplasmatic process.
The highly significant correlation between VEGF and MMP-2 suggests a connection between both mechanisms in the progression of gastric cancer.
Table 2.
Results of the Wilcoxon test for evaluation of the difference in VEGF expression between healthy margin and group G1.
| Gene | Wilcoxona test | Difference |
|---|---|---|
| VEGF | p<0.01 | G1>Margin |
Footnotes
Source of support: The study was carried out as a research project supported by a grant from the Medical University of Silesia (NN-1-159/06)
References
- 1.Szczeklik A. Choroby wewnętrzne. In: Zatoński WA, Didkowska J, Olszewski W, editors. Epidemiologia nowotworów i badania przesiewowe Kraków. II. 2005. p. 1983. [in Polish] [Google Scholar]
- 2.Gonzalez CA, Sala N, Capella G. Genetic susceptibility and gastrin cancer risk. Int J Cancer. 2002;100:249–60. doi: 10.1002/ijc.10466. [DOI] [PubMed] [Google Scholar]
- 3.Hemminki K, Jiang Y. Familial and second gastric carcinomas: a nationwide epidemiologic study from Sweden. Cancer. 2002;94:1157–65. [PubMed] [Google Scholar]
- 4.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiology. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- 5.Chomczyński P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–59. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Chomczyński P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cells and tissue samples. BioTechniques. 1993;15:532–37. [PubMed] [Google Scholar]
- 7.Szala S, Radzikowski C. Podłoże molekularne angiogenezy nowotworów. Nowotwory. 1997;47:1–19. [in Polish] [Google Scholar]
- 8.Zhang Y, Li M, Yao Q, Chen C. Recent advances in tumor hypoxia: Tumor progression, molecular mechanisms, and therapeutic implications. Med Sci Monit. 2007;13(10):RA175–80. [PubMed] [Google Scholar]
- 9.Nam SY, Ko YS, Jung J, et al. A hypoxia-dependent upregulation of hypoxia-inducible factor-1 by nuclear factor-κB promotes gastric tumour growth and angiogenesis. Br J Cancer. 2011;104(1):166–74. doi: 10.1038/sj.bjc.6606020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zielonka T. Angiogeneza – Część I. Mechanizmy powstawania naczyń krwionośnych. Aler Ast Imm. 2003;8(4):169–74. [in Polish] [Google Scholar]
- 11.Maeda K, Chung YS, Takatsuka S, et al. Tumor angiogenesis as a predictor of recurrence in gastric carcinoma. J Clin Oncol. 1995;13:477–81. doi: 10.1200/JCO.1995.13.2.477. [DOI] [PubMed] [Google Scholar]
- 12.Fuhrmann-Benzakein E, Ma MN, Rubia-Brandt L, et al. Elavated levels of angiogenic cytokines in the plasma of cancer patients. Int J Cancer. 2000;85:40–45. doi: 10.1002/(sici)1097-0215(20000101)85:1<40::aid-ijc7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Benoy I, Salgado R, Colpaert C, et al. Serum interleukin 6, plasma VEGF, serum VEGF and VEGF platelet load in breast cancer patients. Clin Breast Cancer. 2002;2:311–15. doi: 10.3816/cbc.2002.n.008. [DOI] [PubMed] [Google Scholar]
- 14.Cascinu S, Staccioli M, Gasparini G. Expression of Vascular Endothelial Growth Factor can predict even-free survival in stage II colon cancer. Clinical Cancer Research. 2000;6:2803–7. [PubMed] [Google Scholar]
- 15.Jezierska A, Motyl T. Matrix Metalloproteinase-2 involvement in breast cancer progression: A mini-review. Med Sci Monit. 2009;15(2):RA32–40. [PubMed] [Google Scholar]
- 16.Ferrara N, Gerber HP, Lecuter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–75. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 17.Metges JP, Funderila C, Doncet L, et al. Angiogenesis and p53 status in gastric cancer, prospective serum and immunohistochemical study. Ann Oncol. 2000;11(4):64–65. [Google Scholar]
- 18.Jośko J, GwoŸdŸ B, Jędrzejowska-Szypułka H, et al. Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med Sci Monit. 2000;6(5):1047–52. [PubMed] [Google Scholar]
- 19.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:10011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 20.Ma ZL, Deng H. Research development of VEGF and its effect on angiogenesis in tumour. Jiangsu Yixue Zazhi. 2004;30:50–51. [Google Scholar]
- 21.Ponta H, Hoffman M, Herlisch P. Recent advances in genetics of metastasis. Eur J Cancer. 1994;30:1195–2001. doi: 10.1016/0959-8049(94)00393-j. [DOI] [PubMed] [Google Scholar]
- 22.D’Amore PA, Shima DT. Tumor angiogenesis a physiological process or genetically determined? Cancer Metastasis Rev. 1996;15:205–12. doi: 10.1007/BF00437473. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Li G, Wu J, et al. Clinicopathological significance of E-cadherin, VEGF, and MMPs in gastric cancer. Tumour Biol. 2010;31(6):549–58. doi: 10.1007/s13277-010-0068-y. [DOI] [PubMed] [Google Scholar]
- 24.Mrena J, Wihstey JP, Nordling S. MMP-2 but not MMP-9 associated with COX-2 and survival in gastric cancer. J Clin Pathol. 2006;59(6):618–23. doi: 10.1136/jcp.2005.033761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whatling C, McPheat W, Camejo EH. Matrix Management Assigning different roles for MMP-2 and MMP-9 in Vascular Remodeling. Arter Thromb Vasc Biol. 2004;24:10–11. doi: 10.1161/01.ATV.0000100562.63144.C1. [DOI] [PubMed] [Google Scholar]
- 26.Lee LY, Wu CM, Wang CC, et al. Expression of matrix metalloproteinases MMP-2 and MMP-9 in gastric cancer and their relation to clandin-4 expression. Histol Histopatol. 2008;23(5):515–21. doi: 10.14670/HH-23.515. [DOI] [PubMed] [Google Scholar]
- 27.Bae JH, Park MJ, Yoon SH, et al. Bcl-w promotes gastric cancer cell invasion by inducing matrix metalloproteinase-2 expression via phosphoinositide 3-kinase, akt and Sp1. Cancer Res. 2006;66(10):4991–95. doi: 10.1158/0008-5472.CAN-05-4254. [DOI] [PubMed] [Google Scholar]
- 28.Wang LB, Jiang ZN, Fan MY, et al. Changes of histology and expression of MMP-2 and nm23-H1 in primary and metastatic gastric cancer. World J Gastroenterol. 2008;14(10):1612–16. doi: 10.3748/wjg.14.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Ji, Chen YL, Jin WL, et al. Relationship between matrix metalloproteinase-2 mRNA expression and clinicopathological and urokinase-type plasminogen activator system parameters and prognosis in human gastric cancer. World J Gastroenterol. 2005;11(21):3222–26. doi: 10.3748/wjg.v11.i21.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai H, Kong ZR, Chen HM. Matrix metalloproteinase-2 and angiogenesis in gastric cancer. Aizheng. 2002;21:25–28. [PubMed] [Google Scholar]
- 31.Descheemacker KA, Wyns S, Nelles L, et al. Interaction of AP-1, AP-2 and Sp-1-like proteins with two distinct sites in the upstream regulatory region of the plasminogen activator inhibitor-1 gene mediates the phorbol 12-myristate 13-acetate response. J Biol Chem. 1992;267:15086–91. [PubMed] [Google Scholar]