Summary
Background
Chronic mitral regurgitation (MR) results in a state of chronic left ventricular (LV) volume overload, resulting in compensatory dilatation. Mitral valve (MV) surgery for regurgitation reduces LV preload but increases LV afterload. Few data are available documenting subsequent changes in LV size and function over time following MV surgery for severe regurgitation in unselected populations.
Material/Methods
Pre- and postoperative echocardiograms (n=454) acquired from 108 consecutive patients with chronic MR who underwent MV surgery were analyzed.
Results
LV diastolic diameter was 4 mm smaller on postoperative compared to preoperative exams, whereas LV fractional shortening (FS) was unchanged. Linear regression analysis showed no change in LV diastolic diameter over time postoperatively, whereas LV FS increased over time following surgery. Improvement in LV FS occurred at an average rate of 1.6% per year (95% CI, 0.2–2.9). Subgroups were small, but the same secular trends were generally noted in groups with or without coronary artery bypass graft surgery (CABGS) and in those with or without mitral leaflet disease.
Conclusions
Following MV surgery for MR, LV diastolic diameter reduces by 2 mm at the time of surgery, but then remains stable over time. Improvement in LV function over time postoperatively was only seen in those without concomitant CABGS, possibly related to less baseline myocardial scarring in this group.
Keywords: mitral regurgitation, echocardiography, mitral valve surgery, left ventricular diastolic diameter, left ventricular fractional shortening, coronary artery bypass surgery
Background
Chronic mitral regurgitation (MR) results in a state of chronic volume overload for the left ventricle (LV), resulting in compensatory dilatation. This process, known as remodeling, is similar to changes occurring after myocardial infarction or with dilated cardiomyopathy, ultimately resulting in LV failure [1–5]. Mitral valve surgery for regurgitation acutely removes the condition of LV volume overload, but also increases LV afterload. Relatively few data are available documenting the changes in LV size and function over time following mitral surgery for severe regurgitation in unselected populations. We therefore examined for echocardiographic changes in LV size and function following mitral surgery in consecutive patients referred for mitral surgery. We also investigated the effect of clinical and surgical factors on changes in LV size and function over time following surgery.
Material and Methods
The cardiology database at our institution (Apollo Advance; LumeDx, Oakland, CA) was queried for subjects having mitral valve surgery performed between 1/1/01 and 12/31/06. Operative reports were reviewed to determine if concomitant coronary artery bypass graft surgery (CABGS) was performed. Subjects were included if the preoperative echocardiogram demonstrated severe MR and if they had at least one pre- and one post-operative echocardiogram. Subjects were excluded if a preoperative echocardiogram showed aortic valve stenosis (more than mild), aortic insufficiency (more than mild), mitral stenosis (any), or any prior valve prosthesis or repair. Subjects were also excluded if repair or replacement was performed on any valve other than the mitral valve. Postoperative echocardiograms demonstrating MR (more than mild) were not included since we were interested in the effect of removing MR on changes in LV size and function.
All echocardiograms were performed on Sonos 5500 or 7500 (Philips). Diameter measurements were made on the parasternal long axis view per standard echocardiographic practice. MR severity was graded per standard criteria.
From the 108 identified subjects, 454 echocardiograms were available (277 postoperative and 177 preoperative). During the first postoperative month, 108 echoes were performed. Between one month and one year, 78 echoes were performed. After the first year, 91 more echoes were performed (range, 372 to 1,962 days).
Analysis was conducted with STATA 9.0 (College Station, TX). Normal data are presented as mean ±1 SD and compared with t-test or paired t-test as appropriate. Non-normal data are presented as median (inter-quartile range) and are compared using the Mann-Whitney test. Comparison of categorical data was performed using chi-square analysis. Linear regression was used to assess the change in cardiac chamber sizes and LV fractional shortening over time, both before and after cardiac surgery. Multiple linear regression was performed to test for confounding and interaction. Variables of interest were age, sex, race, CABGS at the time of mitral surgery, presence of mitral leaflet disease (opposed to functional MR), mitral valve replacement (opposed to repair), and low ejection fraction (<50%).
Results
The clinical characteristics and demographics of the study sample at the time of surgery are shown in Table 1. Compared to those without CABGS, subjects with CABGS more often had functional MR. Subjects with and without CABGS were similar with respect to age, sex, prevalence of mitral replacement (as opposed to repair), LV ejection fraction (eyeball method), LV fractional shortening (measured), LV systolic and diastolic diameters, left atrial (LA) diameter, body mass index (BMI), and race.
Table 1.
Patient demographics, clinical characteristics, echo findings at the time of surgery. Normal data are presented ± SD. Non-normal data are presented [inter-quartile range]. Data are also present stratified by whether or not CABG was performed at the time of mitral valve surgery.
| Overall (n=108) | CABG (n=43) | No CABG (n=65) | p-value | |
|---|---|---|---|---|
| Age (years) | 64±13 | 67±11 | 62±13 | 0.05 |
| Male (%) | 38 | 42 | 35 | 0.48 |
| Mitral replacement (%) | 61 | 51 | 68 | 0.08 |
| Mitral leaflet disease (%) | 40 | 16 | 35 | 0.03 |
| Median LVEF (%) | 46 [36,60] | 42 [35,60] | 50 [36,60] | 0.37 |
| Mean LVEF (%) | 47±14 | 45±13 | 48±15 | 0.38 |
| LA diameter (mm) | 50±8 | 48±8 | 51±8 | 0.09 |
| LV diastolic diameter | 58±9 | 56±6 | 59±10 | 0.11 |
| LV systolic diameter | 45±12 | 45±10 | 45±13 | 0.96 |
| Renal Insufficiency (%) | 27 | 23 | 29 | 0.49 |
| Hematocrit (%) | 36 [32,41] | 36 [32,39] | 36 [32,40] | 0.90 |
| Body Mass Index | 26±5 | 26±4 | 27±5 | 0.74 |
| Race | ||||
| White (%) | 26 | 35 | 20 | 0.08 |
| Black (%) | 30 | 19 | 37 | 0.04 |
| Hispanic (%) | 10 | 7 | 12 | 0.37 |
| Other/unkown (%) | 34 | 40 | 31 | 0.35 |
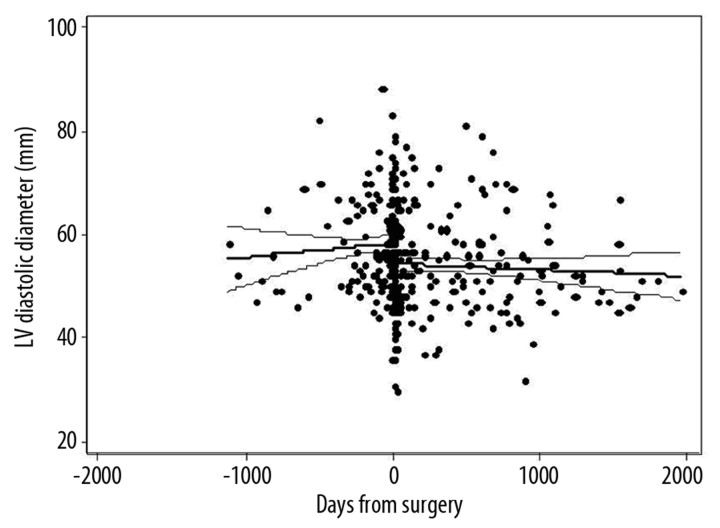
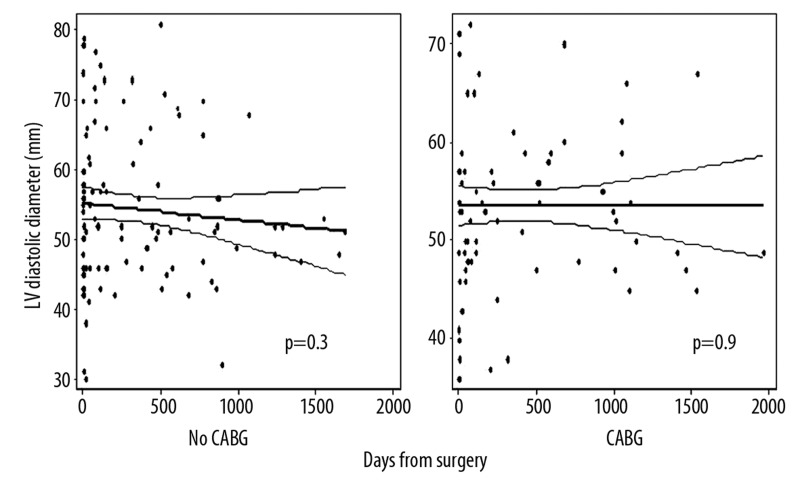
Figure 1 displays the LV diastolic diameter (LVDD) of all subjects relative to the number of days before and days after mitral valve surgery. LVDD was smaller on postoperative echoes compared to preoperative exams (53±9 mm vs. 57±9 mm, p<0.001), largely reflecting a change in the LVDD occurring at the time of surgery. Although there was a slight downward trend, the regression line reflecting the change in LVDD over time following mitral valve surgery (beta=−0.5 mm/year [95% CI: −1.5, 0.5], p=0.4) did not show a significant secular trend. When patients were stratified by CABGS performance, the regression line reflecting the change in LVDD over time did not demonstrate a significant secular trend in either group [CABG (β= −0.02 mm/year [95% CI: −1.2, 1.2], p=0.98); No CABG (β= −0.9 mm/year [95% CI: −2.5, 0.8], p=0.3)]. Using multiple linear regression, the interaction term for CABG and change in LV diameter was not significant (p=0.42), indicating that the change in LV diameter over time was not significantly different between those with and without CABG. Despite the lack of statistical significance, the downward trend over time in the group without CABG is noteworthy, especially since no such trend is seen in the group without CABG (Figure 2). Because the sample sizes are fairly small in the subgroups, it is plausible that there is a true difference between the groups that was not detected. No significant secular trends in postoperative LVDD were seen when stratified by MR etiology (functional vs. degenerative), by surgery type (repair vs. replacement), by the presence or absence of mitral leaflet disease, or by ejection fraction. Using multivariate linear regression to adjust for potential confounders, the coefficient for LVDD still did not show a significant secular change (Table 2).
Figure 1.
Left ventricular (LV) diastolic diameter before and after mitral valve surgery.
Figure 2.
Left ventricular (LV) diastolic diameter following mitral valve surgery. Graphs stratify patients into group with concomitant coronary artery bypass grafting (CABG) vs. those without CABG.
Table 2.
Multivariate linear regression models. Model #1 examines the association between LV diastolic diameter and years after surgery, adjusting for multiple potential confounders. Model #2 examines the association between LV fractional shortening and years after surgery, adjusting for multiple potential confounders.
| Dependent variable | Independent variables | Coefficient (β) | 95% Conf Interval | p-value | |
|---|---|---|---|---|---|
| Model #1 | LV diast diam (mm) | ||||
| Years after surgery | −0.12 mm/year | [−0.97, 0.73] | 0.79 | ||
| Age (years) | −0.07 | [−0.16, 0.02] | 0.13 | ||
| Male | 3.57 | [1.42, 5.71] | 0.001 | ||
| Black (c/w white) | 0.78 | [−2.34, 3.91] | 0.62 | ||
| Hispanic (c/w white) | 5.59 | [1.97, 9.23] | 0.003 | ||
| Unkn Race (c/w white) | 1.62 | [−1.40, 4.64] | 0.29 | ||
| CABG | −2.70 | [−5.03, −0.38] | 0.02 | ||
| EF < 50% | 10.03 | [7.76, 12.32] | <0.001 | ||
| Mitral leaflet disease | −2.19 | [−4.74, 0.36] | 0.09 | ||
| Mitral repair | −0.34 | [−2.56, 1.87] | 0.76 | ||
| Model #2 | Fractional shortening (%) years after surgery | ||||
| 3.8 %/year | [0.67, 6.88] | 0.02 | |||
| Age (years) | 0.001 | [−0.002, 0.004] | 0.54 | ||
| Male | −0.06 | [−0.13, 0.02] | 0.17 | ||
| Black (c/w white) | 0.02 | [−0.09, 0.14] | 0.67 | ||
| Hispanic (c/w white) | 0.03 | [−0.10, 0.16] | 0.66 | ||
| Unkn Race (c/w white) | −0.03 | [−0.14, 0.08] | 0.60 | ||
| CABG | −0.02 | [−0.11, 0.06] | 0.58 | ||
| EF < 50% | −0.007 | [−0.09, 0.08] | 0.86 | ||
| Mitral leaflet disease | 0.09 | [−0.004, 0.18] | 0.06 | ||
| Mitral repair | 0.01 | [−0.07, 0.09] | 0.10 |
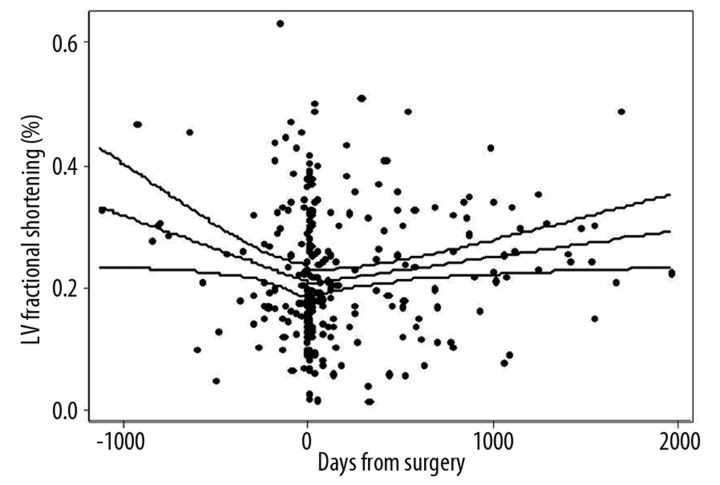
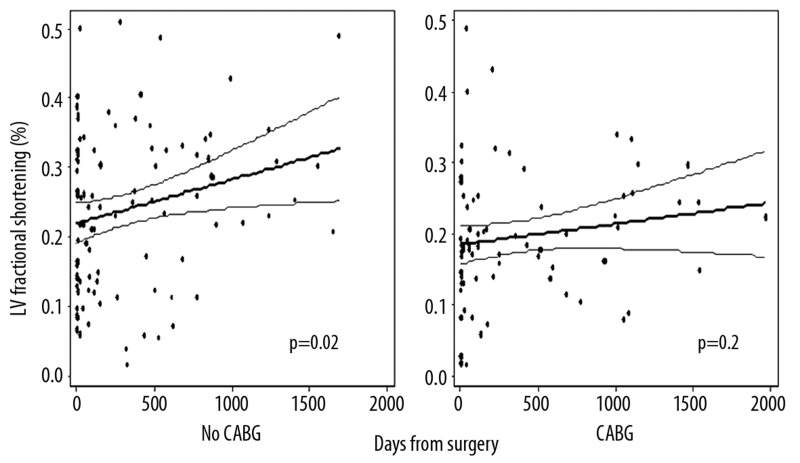
LV fractional shortening (FS) was unchanged on postoperative echoes compared to preoperative exams (22±12% vs. 22±11%, p=0.71). Overall, LV fractional shortening increased by 1.6% per year (95% CI: 0.3, 2.9, p=0.02) after surgery (Figure 3). Postoperative secular trends in LV FS, stratified by CABGS performance are displayed in Figure 4. The regression line reflecting the change in LV FS over time following surgery was significant for an increase in LV FS in those without CABGS (beta=2.3% per year [95% CI: 0.4, 4.2], p=0.02). LV FS did not demonstrate a significant change over time in those with CABG (β=1.1% per year, 95% CI: −0.5, 2.7, p=0.20). Using multiple linear regression, the interaction term for CABGS and change in LV FS over time was not significant (p=0.42), indicating that the change in LV FS over time was not significantly different between those with and without CABGS. This is intuitive from the regression lines, which appear to have a similar slope in those with and without CABGS (Figure 4). Increase in LV FS over time was confined almost entirely to those who had decreased LVEF at baseline [Low LVEF (β=7.7% per year [95% CI: 2.1, 13.3], p=0.07); Normal LVEF (β=0.4% per year [95% CI: −2.7, 3.6], p=0.78)]. No significant secular trends in postoperative LV FS were seen when stratified by MR etiology (functional vs. degenerative), by surgery type (repair vs. replacement), or by the presence or absence of mitral leaflet disease. Using multivariate linear regression to adjust for potential confounders, the coefficient for LV FS remained significant for an increase over time after mitral valve surgery (Table 2).
Figure 3.
Left ventricular (LV) fractional shortening before and after mitral valve surgery.
Figure 4.
Left ventricular (LV) fractional shortening following mitral valve surgery. Graphs stratify patients into group with concomitant coronary artery bypass grafting (CABG) vs. those without CABG.
The same analysis performed above for LVDD was repeated to examine for changes in LA diameter over time, both before and after MV surgery. Regression of LA diameter with time after surgery (beta=0.3 mm/year [95% CI: −0.7, 1.3], p=0.4) did not show significant secular trends. Mean LA diameter was unchanged on postoperative echoes (49±9 mm) compared to preoperative exams (51±7 mm) (p<0.07).
Discussion
We found that LV diastolic size decreased at the time of mitral valve surgery, but no subsequent change was observed during follow-up postoperatively. Postoperative LV function remained unchanged from the preoperative exams; however, function did steadily improve over time following surgery. Increase in LF function over time after surgery was mainly seen in those with reduced LV function before surgery.
Our findings are generally consistent with prior literature. Gelsomino et al. [6,7] also found that LVDD on echocardiography decreased at the time of surgery for patients with functional regurgitation undergoing CABGS with MV repair. LVDD remained stable on follow-up exams at 1, 3 and 5 years after surgery. Similar to patients in our study, their cohort did not experience change in LV systolic function immediately following surgery. However, they did report that ejection fraction remained unchanged at 1, 3 and 5 years postoperatively. Although their study sample was large, with 251 patients, they did not include subjects with degenerative disease nor did they include subjects who did not require CABGS. They did, however, include subjects with moderate regurgitation.
Westenberg et al. [8] performed cardiac magnetic resonance imaging in 20 subjects with dilated cardiomyopathy before and after mitral valve repair without CABGS. They similarly found that LV diastolic size decreased significantly early after surgery but did not have subsequent change on repeat imaging at one year. They also found that LV ejection fraction had increased at 2 months postoperatively, with sustained improvement at one year.
Geidel et al. [9] studied 121 patients who underwent mitral repair for severe functional MR with or without CABGS. Using echocardiography, they also found that subjects had reduction in LV diastolic size immediately following surgery and that LV size did not change postoperatively by 2.5 years. LV FS was similar to preoperative values immediately after surgery, with subsequent improvement noted at 2.5 years. In contrast to our results, however, they found that the improvement in LV function that occurred during the follow-up period was limited to those that received concomitant CABGS at the time of MV surgery.
The literature is therefore consistent that LV diastolic size generally reduces at the time of mitral surgery for MR and then tends to remain stable subsequently. Similarly, all of the aforementioned studies agree that LV function generally remains similar to preoperative function in the early postoperative period. There remains some discrepancy, however, regarding subsequent improvement in LV function in the years following mitral valve surgery. Factors such as baseline LV function, type of surgery (repair vs. replacement), need for CABG and primary MR (compared to functional MR) likely account for differences between study populations. Gelsomino et al noted that the effect of mitral valve surgery on LV size and function can be variable. Accordingly, they described a subset of patients that experienced progressive reduction in LV size, with improved LV function over time following the early postoperative period. This group was thought to undergo a process called “reverse remodeling” and were found to have increased survival in their study.
Our investigation has several important limitations. Due to the retrospective nature of the study, echocardiograms were not acquired at pre-specified intervals. Imaging was generally acquired at the discretion of the treating clinicians. Therefore, recurrent imaging may have been preferentially acquired in those with more symptoms or in those who survived long enough to have follow-up echoes months and years after surgery.
There are several important strengths to our study that are noteworthy. Data regarding change in cardiac chamber size beyond the peri-operative period are scarce. Understanding the degree to which MV surgery interrupts the natural history of MR is extremely important. Changes in chamber size are increasingly being used as surrogate markers of prognosis in many studies of congestive heart failure [10–15]. Also, improved understanding of how the LV adapts to changing loading conditions is important when considering adoption of new therapies. For this reason, we excluded patients who had documented MR on postoperative exams, since loading conditions were incompletely modified in this group.
Conclusions
Following MV surgery for MR, LV size becomes reduced at the time of surgery, but then remains stable over time thereafter. Improvement in LV function over time postoperatively was only seen in those without concomitant CABGS, possibly related to less baseline myocardial scarring in this group.
Footnotes
Conflicts of interest
None of the authors have any conflicts of interest pertaining to this article.
Source of support: Departmental sources
References
- 1.Enriquez-Sarano M, Schaff H, Orszulak T, et al. Congestive heart failure after surgical correction of mitral regurgitation: a long-term study. Circulation. 1995;92:2496–503. doi: 10.1161/01.cir.92.9.2496. [DOI] [PubMed] [Google Scholar]
- 2.Reed D, Abbott RD, Smucker ML, Kaul S. Prediction of outcome after mitral valve replacement in patients with symptomatic chronic mitral regurgitation: the importance of left atrial size. Circulation. 1991;84:23–34. doi: 10.1161/01.cir.84.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Grigioni F, Avierinos JF, Ling LH, et al. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. J Am Coll Cardiol. 2002;40:84–92. doi: 10.1016/s0735-1097(02)01922-8. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 5.Konstam MA, Rousseau MF, Kronenberg MW, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure; SOLVD Investigators. Circulation. 1992;86(2):431–38. doi: 10.1161/01.cir.86.2.431. [DOI] [PubMed] [Google Scholar]
- 6.Gelsomino S, Lorusso R, DeCicco G, et al. Five-year echocardiographic results of combined undersized mitral ring annuloplsty and coronary artery bypass grafting for chronic ischaemic mitral regurgitation. European Heart J. 2008;29:231–40. doi: 10.1093/eurheartj/ehm468. [DOI] [PubMed] [Google Scholar]
- 7.Gelsomino S, Lorusso R, DeCicco G, et al. Left ventricular reverse remodeling after undersized mitral ring annuloplasty in patients with ischemic regurgitation. Ann Thorac Surg. 2008;85:1319–31. doi: 10.1016/j.athoracsur.2007.12.074. [DOI] [PubMed] [Google Scholar]
- 8.Westenberg JJM, vand der Geest RJ, Lamb HJ, et al. MRI to evaluate left atrial and ventricular reverse remodeling after restrictive annuloplasty in dilated cardiomyopathy. Circulation. 2005;112:1437–42. doi: 10.1161/CIRCULATIONAHA.104.525659. [DOI] [PubMed] [Google Scholar]
- 9.Geidel S, Lass M, Drauser K, et al. Early and late results of restrictive mitral valve annuloplasty in 121 patients with cardiomyopathy and chronic mitral regurgitation. The Thoracic and Cardiovasc Surgeon. 2008;56:262–68. doi: 10.1055/s-2008-1038420. [DOI] [PubMed] [Google Scholar]
- 10.St John Sutton MG, Pfeffer MA, Plappert T, et al. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction: the protective effects of captopril. Circulation. 1994;89:68–75. doi: 10.1161/01.cir.89.1.68. [DOI] [PubMed] [Google Scholar]
- 11.Yu CM, Bleeker GB, Fung JW, et al. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005;112:1580–86. doi: 10.1161/CIRCULATIONAHA.105.538272. [DOI] [PubMed] [Google Scholar]
- 12.Ypenburg C, van Bommel RJ, Borleffs CJ, et al. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol. 2009;53:483–90. doi: 10.1016/j.jacc.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 13.Burkhoff D, Klotz S, Mancini DM. LVAD-induced reverse remodeling: basic and clinical implications for myocardial recovery. J Cardiac Fail. 2006;12:227–39. doi: 10.1016/j.cardfail.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 14.St John Sutton M, Ghio S, Plappert T, et al. Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association class I/II heart failure. REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) Study Group. Circulation. 2009;120:1858–65. doi: 10.1161/CIRCULATIONAHA.108.818724. [DOI] [PubMed] [Google Scholar]
- 15.Anand IS, Florea VG, Fisher L. Surrogate end points in heart failure. J Am Col Cardiol. 2002;39:1414–21. doi: 10.1016/s0735-1097(02)01773-4. [DOI] [PubMed] [Google Scholar]