Abstract
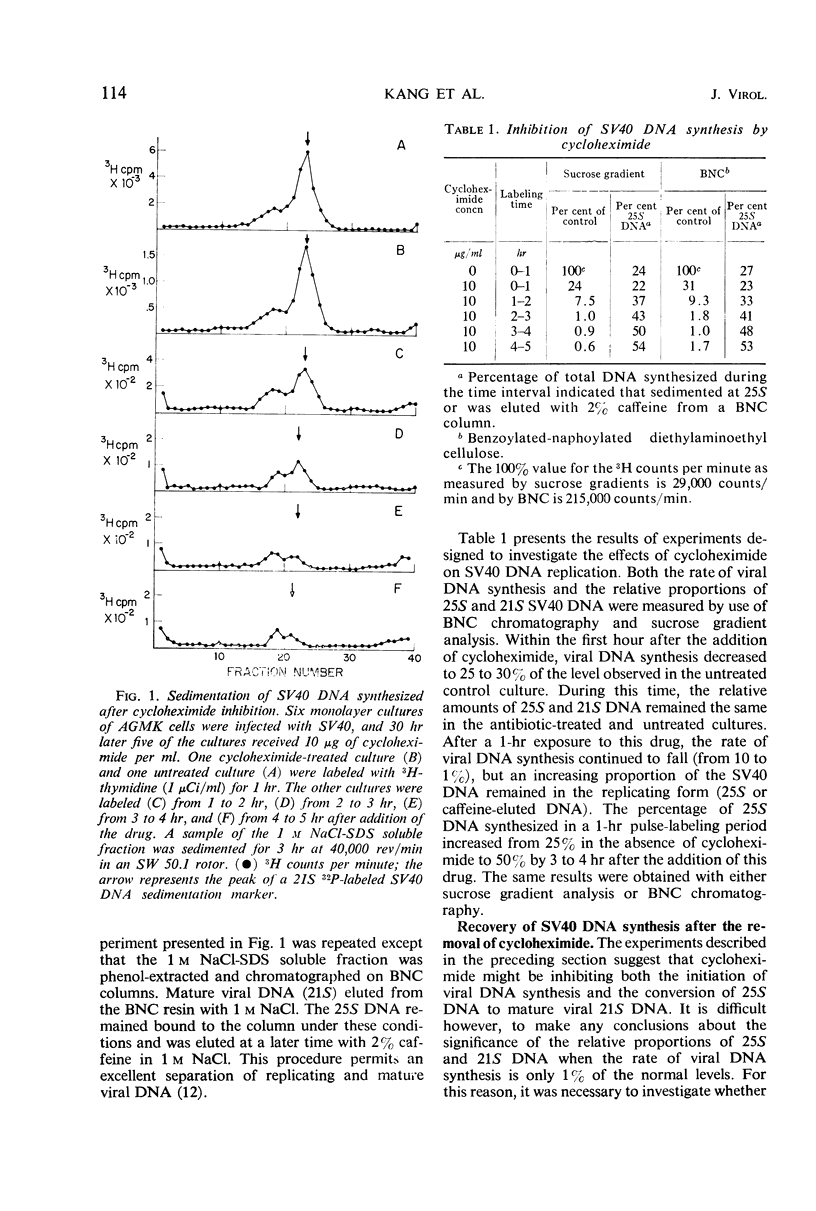
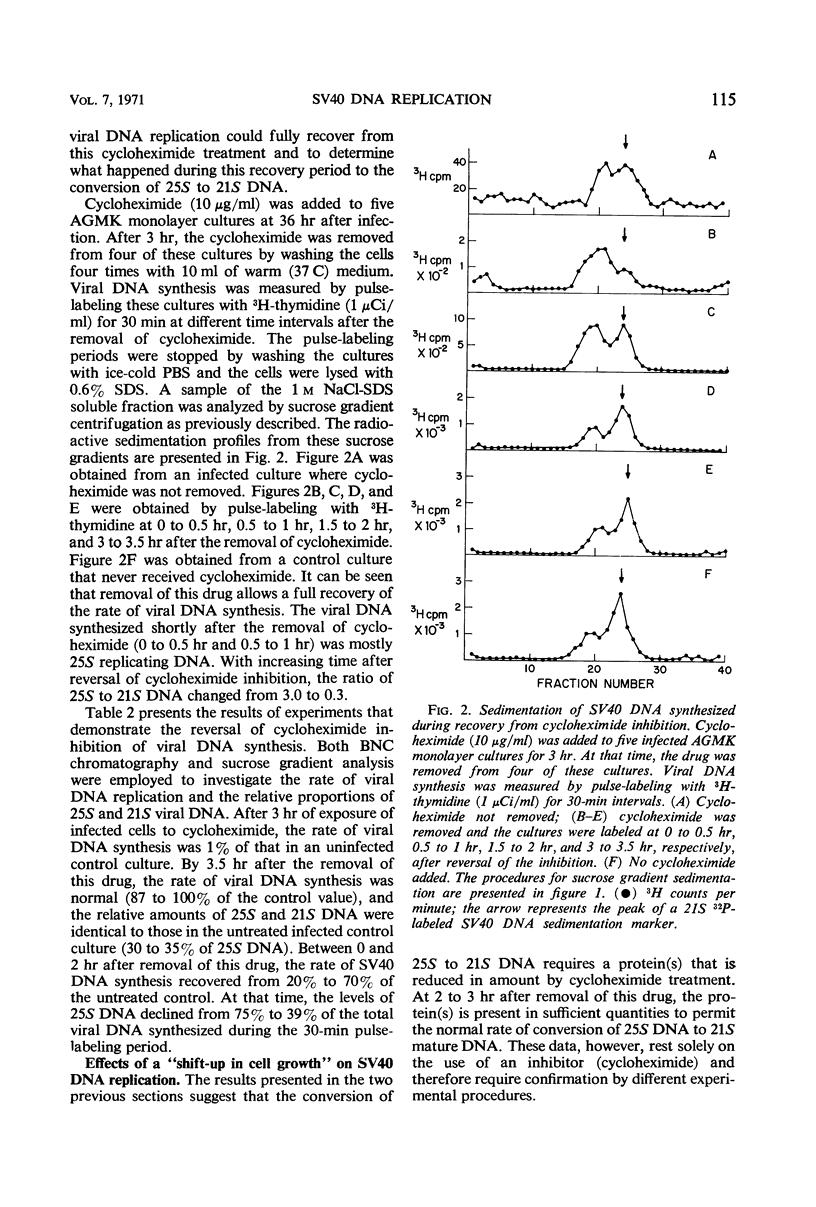
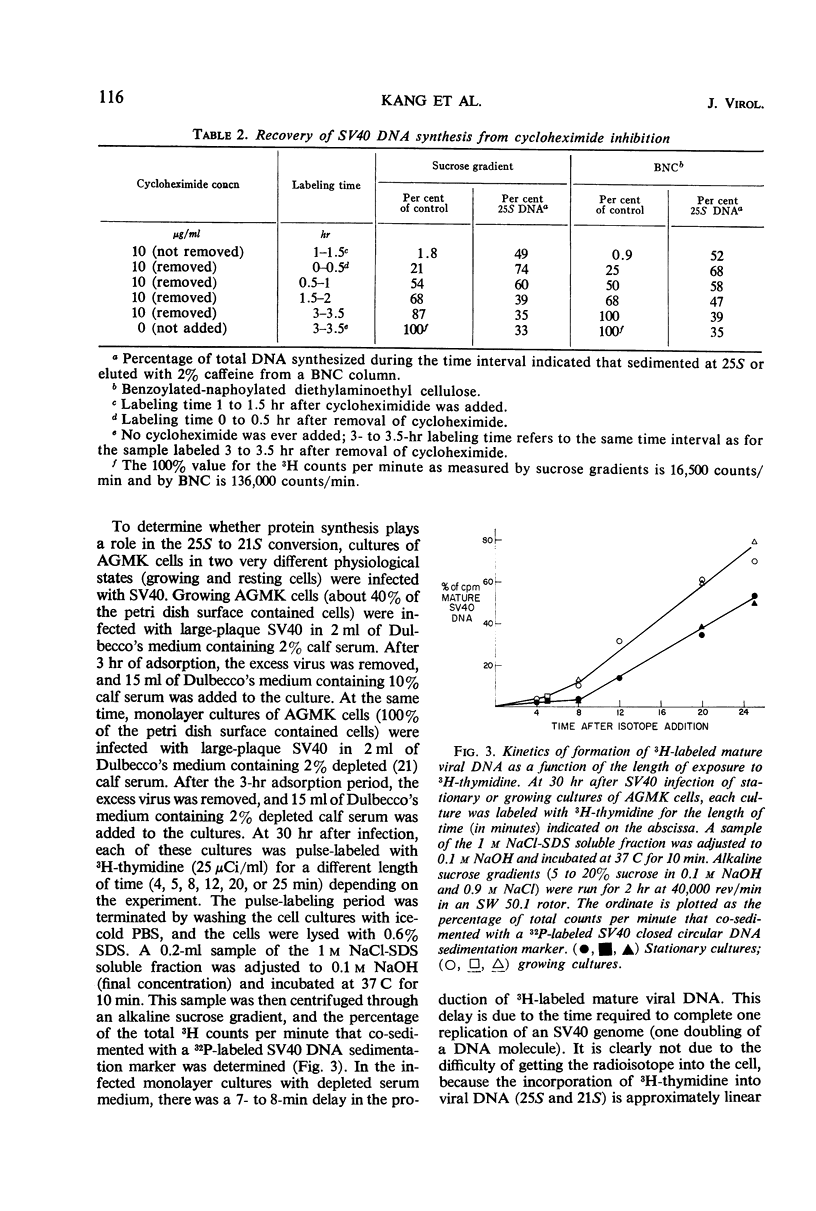
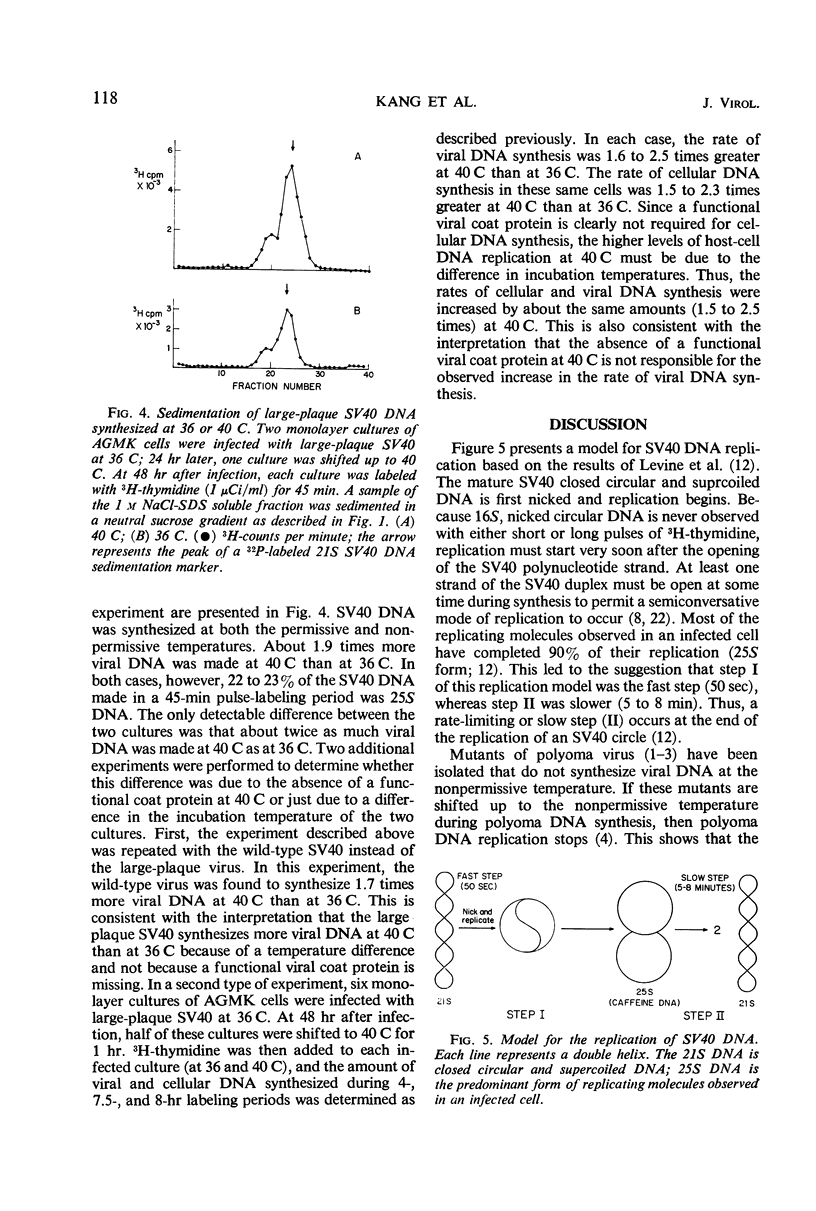
The replication of simian virus 40 (SV40) deoxyribonucleic acid (DNA) was inhibited by 99% 2 hr after the addition of cycloheximide to SV40-infected primary African green monkey kidney cells. The levels of 25S (replicating) and 21S (mature) SV40 DNA synthesized after cycloheximide treatment were always lower than those observed in an infected untreated control culture. This is consistent with a requirement for a protein(s) or for protein synthesis at the initiation step in SV40 DNA replication. The relative proportion of 25S DNA as compared with 21S viral DNA increased with increasing time after cycloheximide treatment. Removal of cycloheximide from inhibited cultures allowed the recovery of viral DNA synthesis to normal levels within 3 hr. During the recovery period, the ratio of 25S DNA to 21S DNA was 10 times higher than that observed after a 30-min pulse with 3H-thymidine with an infected untreated control culture. The accumulation of 25S replicating SV40 DNA during cycloheximide inhibition or shortly after its removal is interpreted to mean that a protein(s) or protein synthesis is required to convert the 25S replicating DNA to 21S mature viral DNA. Further evidence of a requirement for protein synthesis in the 25S to 21S conversion was obtained by comparing the rate of this conversion in growing and resting cells. The conversion of 25S DNA to 21S DNA took place at a faster rate in infected growing cells than in infected confluent monolayer cultures. A temperature-sensitive SV40 coat protein mutation (large-plaque SV40) had no effect on the replication of SV40 DNA at the nonpermissive temperature.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Di Mayorca G., Callender J., Marin G., Giordano R. Temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):126–133. doi: 10.1016/0042-6822(69)90134-2. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- FRIED M. CELL-TRANSFORMING ABILITY OF A TEMPERATURE-SENSITIVE MUTANT OF POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Mar;53:486–491. doi: 10.1073/pnas.53.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M. Characterization of a temperature-sensitive mutant of polyoma virus. Virology. 1970 Mar;40(3):605–617. doi: 10.1016/0042-6822(70)90205-9. [DOI] [PubMed] [Google Scholar]
- GERSHON D., SACHS L. THE TEMPORAL RELATIONSHIPS OF PROTEIN AND DNA SYNTHESIS IN POLYOMA VIRUS DEVELOPMENT. Virology. 1964 Dec;24:604–609. doi: 10.1016/0042-6822(64)90214-4. [DOI] [PubMed] [Google Scholar]
- Gilead Z., Ginsberg H. S. Characterization of a Tumorlike Antigen in Type 12 and Type 18 Adenovirus-Infected Cells. J Bacteriol. 1965 Jul;90(1):120–125. doi: 10.1128/jb.90.1.120-125.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Hirt B. Evidence for semiconservative replication of circular polyoma DNA. Proc Natl Acad Sci U S A. 1966 Apr;55(4):997–1004. doi: 10.1073/pnas.55.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kit S., Kurimura T., De Torres R. A., Dubbs D. R. Simian virus 40 deoxyribonucleic acid replication. I. Effect of cycloheximide on the replication of SV40 deoxyribonucleic acid in monkey kidney cells and in heterokaryons of SV40-transformed and susceptible cells. J Virol. 1969 Jan;3(1):25–32. doi: 10.1128/jvi.3.1.25-32.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Teresky A. K. Deoxyribonucleic acid replication in simian virus 40-infected cells. II. Detection and characterization of simian virus 40 pseudovirions. J Virol. 1970 Apr;5(4):451–457. doi: 10.1128/jvi.5.4.451-457.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XV. Bacteriophage DNA synthesis in abortive infections with a set of conditional lethal mutants. J Mol Biol. 1967 Nov 28;30(1):69–80. doi: 10.1016/0022-2836(67)90244-6. [DOI] [PubMed] [Google Scholar]
- Mackinlay A. G., Kaiser A. D. DNA replication in head mutants of bacteriophage lambda. J Mol Biol. 1969 Feb 14;39(3):679–683. doi: 10.1016/0022-2836(69)90155-7. [DOI] [PubMed] [Google Scholar]
- Ozer H. L., Takemoto K. K., Kirschstein R. L., Axelrod D. Immunochemical characterization of plaque mutants of simian virus 40. J Virol. 1969 Jan;3(1):17–24. doi: 10.1128/jvi.3.1.17-24.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Takemoto K. K. Site of host restriction of simian virus 40 mutants in an established African green monkey kidney cell line. J Virol. 1969 Oct;4(4):408–415. doi: 10.1128/jvi.4.4.408-415.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedat J., Lyon A., Sinsheimer R. L. Purification of Escherichia coli pulse-labeled RNA by benzoylated DEAE-cellulose chromatography. J Mol Biol. 1969 Sep 28;44(3):415–434. doi: 10.1016/0022-2836(69)90370-2. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Kirschstein R. L., Habel K. Mutants of simian virus 40 differing in plaque size, oncogenicity, and heat sensitivity. J Bacteriol. 1966 Oct;92(4):990–994. doi: 10.1128/jb.92.4.990-994.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Todaro G. J., Habel K. Recovery of SV40 virus with genetic markers of original inducing virus from SV40-transformed mouse cells. Virology. 1968 May;35(1):1–8. doi: 10.1016/0042-6822(68)90299-7. [DOI] [PubMed] [Google Scholar]
- Todaro G., Matsuya Y., Bloom S., Robbins A., Green H. Stimulation of RNA synthesis and cell division in resting cells by a factor present in serum. Wistar Inst Symp Monogr. 1967;7:87–101. [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J. Physical and topological properties of circular DNA. J Gen Physiol. 1966 Jul;49(6):103–125. doi: 10.1085/jgp.49.6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]



