Abstract
Objective
Triptolide and tripdiolide are thought to be active components of the Chinese antirheumatic herbal remedy Tripterygium wilfordii Hook F, which has been shown to be effective in treating murine lupus nephritis. This study was undertaken to examine the therapeutic effect of triptolide and tripdiolide on established lupus nephritis in (NZB X NZW)F1 mice.
Methods
(NZB X NZW)F1 mice were treated with vehicle, triptolide, or tripdiolide for 15 weeks beginning at the age of 29 weeks (after the development of lupus nephritis). Body weight, proteinuria, and anti-doublestranded DNA (anti-dsDNA) antibodies were monitored, and the kidney and spleen were assessed histologically. Culture supernatants of spleen mononuclear cells were assayed for cytokines.
Results
By 28 weeks, most (NZB X NZW)F1 mice had developed lupus nephritis. Vehicle-treated mice exhibited progressive proteinuria, hypoalbuminemia, elevated blood urea nitrogen (BUN) levels, and evidence of severe nephritis. In contrast, proteinuria and BUN levels were significantly reduced in mice treated with either triptolide or tripdiolide as compared with those treated with vehicle. There was no hypoalbuminemia or apparent evidence of lupus nephritis in mice treated with either of the 2 diterpenoids. At 44 weeks of age, the survival rate in mice treated with vehicle (35.7%) was markedly lower than that in mice treated with either triptolide (87.5%) or tripdiolide (88.2%). The mean level of anti-dsDNA antibody in mice treated with tripdiolide was lower than that in the vehicle-treated mice upon completion of the treatment course. Production of tumor necrosis factor, interleukin-6, and monocyte chemoattractant protein 1 by spleen cells was also decreased after diterpenoid therapy.
Conclusion
Therapy with triptolide or tripdiolide significantly ameliorated lupus nephritis in (NZB X NZW)F1 mice, reduced cytokine and chemokine production, and prolonged survival.
Extracts of the Chinese antirheumatic herbal remedy Tripterygium wilfordii Hook F (TWHF) have been shown to be of therapeutic benefit in patients with a variety of autoimmune and inflammatory diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and psoriasis (1). Although TWHF has toxic potential, careful extraction procedures have generated preparations with an acceptable frequency of adverse reactions, which are largely related to the gastrointestinal tract and amenorrhea (1). Although the extracts of TWHF contain a variety of components, including diterpenoids, triterpenoids, and alkaloids, evidence suggests that most, if not all, of the therapeutic activity of extracts of TWHF can be accounted for by the content of 2 diterpenoids, triptolide and tripdiolide (2). However, it has been assumed that individual components do not work as well as the extracts because of unknown effects of mixtures of components. For example, one open clinical study compared triptolide with an ethyl acetate (EA) extract of TWHF in the treatment of RA and reported that triptolide was less effective in improving clinical manifestations of the disease and was associated with more side effects than the extract of TWHF (3).
Despite the belief in a synergistic benefit from mixtures of components of TWHF extracts, investigation into the biologic activities of individual constituents of TWHF has proceeded. As one of the major active components of TWHF, triptolide has been reported to be able to suppress the production of a wide range of proinflammatory cytokines, including interleukin-2 (IL-2), interferon-γ (IFNγ), IL-6, and tumor necrosis factor (TNF), as well as inhibit the up-regulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) (4,5). Previous studies have shown that triptolide exerts its anti-inflammatory and immunosuppressive actions by directly suppressing the transcription of the genes that encode these proteins through interference with the function of transcription factors such as NF-κB, activator protein 1, nuclear factor of activated T cells, and Oct-1 (6–8). The immunosuppressive and anti-inflammatory activities of extracts of TWHF have been documented in several animal models, including collagen-induced arthritis (CIA) in mice and the air-pouch model of carageenan-induced inflammation in rats (9,10), whereas triptolide has been shown to suppress CIA when given prophylactically (11) or after the onset of disease (12). Moreover, triptolide has been claimed to be effective in the treatment of RA and psoriasis, although this has not been documented in randomized clinical trials (3,13), and the therapeutic effect in RA may be lower than that of the extract of TWHF (3).
Structurally, tripdiolide is similar to triptolide, with an α,β-unsaturated lactone ring and 3 epoxide adducts. The only difference is that the hydrogen at the C2 position of triptolide is replaced by a hydroxide group in tripdiolide. Previous studies have shown that tripdiolide is as potent as triptolide in suppressing in vitro cell proliferation and IL-2 production by mitogenstimulated T cells (2). Only 1 study tested the in vivo biologic activity of tripdiolide, and prolonged survival in leukemia-bearing mice as a result of treatment with tripdiolide was reported (14). However, the impact of tripdiolide on animal models of autoimmunity or inflammation has not been examined.
Previously, studies have shown that 2 extracts of TWHF improved the clinical manifestations of lupus in MRL/lpr mice (15,16). However, the composition of these extracts was not examined. In addition, the extracts were used prophylactically to prevent disease development but were not active as a therapy for established disease. Recently, we used the EA extract of TWHF in the treatment of established lupus nephritis in (NZB X NZW)F1 mice and found significant amelioration of disease activity (17). No previous study has examined the impact of purified components of TWHF on animal models of lupus nephritis.
The current study was therefore performed to evaluate the therapeutic effects of the 2 individual diterpenoids on established nephritis in the (NZB X NZW)F1 mouse. We found that renal disease remarkably improved, and there was greater survival of (NZB X NZW)F1 mice treated with either triptolide or tripdiolide than mice treated with vehicle. These results support the conclusions that the therapeutic value of extracts of TWHF in lupus nephritis can be accounted for by the triptolide and tripdiolide contents, that cooperation between components is not required for therapeutic benefit, and that either diterpenoid could be effective therapy for lupus nephritis.
MATERIALS AND METHODS
Reagents
Triptolide and tripdiolide were isolated from TWHF by repeated preparative high-performance liquid chromatography (HPLC), and the structures were identified by thin-layer chromatography, HPLC, mass spectroscopy, and nuclear magnetic resonance techniques, as described previously (18). Triptolide and tripdiolide were 99% and 98% pure, respectively, as assessed by HPLC. Phytohemagglutinin (PHA) and phorbol myristate acetate (PMA) were purchased from Sigma (St. Louis, MO).
Animals and treatment regimens
Eight-week-old female (NZB X NZW)F1/J mice, NZB/B1NJ, and C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). At 29 weeks of age, the animals were randomly divided into 3 treatment groups (vehicle, triptolide, and tripdiolide). Treatment was administered orally by gavaging 0.4 ml/day/animal of either vehicle (1% DMSO/1% Tween 20 in water) only or vehicle containing 6 μg of triptolide or tripdiolide. The amount was equivalent to about one-seventh and one-eighth the concentration of triptolide and tripdiolide, respectively, that caused 50% of C57BL/6J mice to die. Gavaging was carried out once a day from Monday to Friday for a total of 15 weeks. If an animal lost more than 15% of its body weight, treatment was terminated and the animal was euthanized. Otherwise, mice were killed after 15 weeks of treatment.
The study proposal (A-005-04-10) was approved and all procedures monitored by the Animal Care and Use Committee of the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Urine from individual mice was collected biweekly using metabolic cages, beginning at age 20 weeks (17). Proteinuria was quantitated by spectrophotometer using a bicinchoninic acid–based protein assay kit (Pierce, Rockford, IL) and was standardized with bovine serum albumin.
Plasma levels of blood urea nitrogen (BUN) and albumin were examined in the Diagnostic and Research Service Branch, Veterinary Resources Program, National Institutes of Health (Bethesda, MD).
Anti-double-stranded DNA (anti-dsDNA) antibodies were analyzed using an enzyme-linked immunosorbent assay kit according to the manufacturer’s directions (Diamedix, Miami, FL), as modified for the detection of murine antibodies (17). Plasma samples from NZB/B1NJ and C57BL/6J mice were used as additional normal controls.
Assessment of kidney pathology
Kidneys and spleens were harvested from the mice after their spontaneous death or euthanization. One-half of the kidney from each mouse was immersed in TBS tissue freezing medium (Triangle Biomedical Sciences, Durham, NC) and snap-frozen in ethanol–dry ice for immunochemical staining. The rest of the kidney was fixed in buffered 10% formalin (Fisher Scientific, Fair Lawn, NJ), embedded in paraffin blocks (Sugipath Medical, Richmond, IL), and stained with hematoxylin and eosin (HistoServ, Gaithersburg, MD).
Sections were graded semiquantitatively for glomerular, interstitial, and vascular lesions according to a previous reported grading method (17). Two experienced observers (VH and NSL) who were blinded to the treatment plan evaluated the samples. Kidney cryosections were stained with fluorescein isothiocyanate–conjugated goat anti-mouse IgG antibody (Cappel/MP Biomedicals, West Chester, PA) or horseradish peroxidase–conjugated goat anti-mouse C3 antibody (MP Biomedicals, Aurora, OH) followed by color development with the Vectastain ABC kit and NovaRED substrate kit (Vector, Burlingame, CA). The slides were then counterstained with hematoxylin (Vector).
To detect plasma cells, sections were stained with rat anti-mouse CD138 antibody (Dako, Fort Collins, CO). After dual endogenous block, rabbit anti-rat immunoglobulin (Dako) was added. After washing, color was developed with 3,3′-diaminobenzidine. The sections were then counterstained with hematoxylin (Dako).
Cell separation, cell culture, and cytokine assay
Spleen cells were obtained from freshly killed mice by gently pressing the organ with an inoculation loop. After deletion of red blood cells with ACK lysing buffer (Quality Biological, Gaithersburg, MD), mononuclear cells were isolated by centrifugation on a sodium diatrizoate–Ficoll gradient (Sigma). The cells (2 × 106/ml) were incubated for 16 hours in RPMI 1640 medium supplemented with 10% fetal calf serum, in the presence or absence of PHA (1 μg/ml) plus PMA (5 ng/ml). Culture supernatants were collected, lyophilized, and stored at −80°C until they were used.
Cytokine content was assayed using an immune sandwich–based cytokine array kit. Each array consists of 14 capture antibodies in quadruplicate, including antibodies to IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-12p70, IL-13, IFNγ, TNF, granulocyte–macrophage colony-stimulating factor, monocyte chemoattractant protein 1 (MCP-1), and vascular endothelial growth factor (Allied Biotech, Ijamsville, MD). The assay was performed as described in the manufacturer’s manual. Fluorescence imaging was performed with an Agitech Microarray Scanner (Agilent Technologies, Santa Clara, CA), and the fluorescence intensity was measured with an Alpha imager (Alpha Innotech, San Leandro, CA). Fluorescence intensity was normalized against 4 control spots included in each array.
Statistical analysis
An intent-to-treat analysis was performed that included all (NZB X NZW)F1 mice that completed at least 4 weeks of treatment. All statistical tests were 2-sided. Comparison of the mean values of individual variables measured at each time point with the corresponding baseline values for mice of the same group was performed using Student’s t-test. Kruskal-Wallis test was used to compare each variable between groups before and after treatment. For assessment of proteinuria, a last observation carried forward approach was used for the mice that died before the end of the study.
RESULTS
Outcome
Initially, 17 mice were included in each group. Four mice (3 in the vehicle group and 1 in the triptolide group) died within 4 weeks of starting treatment and were excluded from the study. There was a significant prolongation in the mean lifespan and significant improvement in the survival rates in the 2 diterpenoid treatment groups compared with the vehicle group (Figure 1A).
Figure 1.
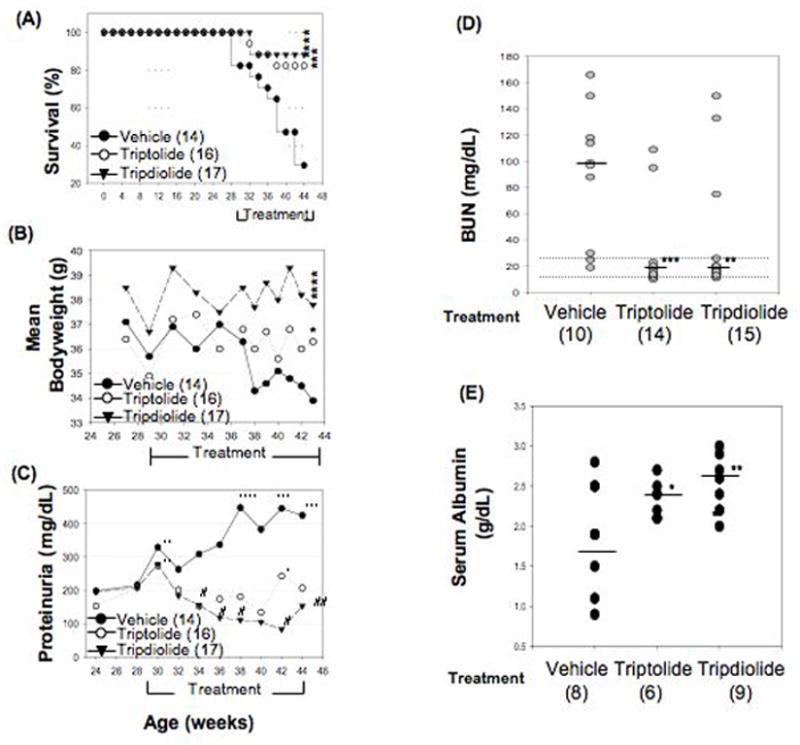
Effect of treatment with triptolide or tripdiolide on survival rate, body weight, and proteinuria, blood urea nitrogen (BUN), and serum albumin levels in (NZB X NZW)F1 mice. A, Survival rates in mice completing ≥4 weeks of treatment. **** = P < 0.0001; *** = P < 0.001 for tripdiolide versus vehicle. B, Mean body weight in mice completing ≥4 weeks of treatment. **** = P < 0.0001; * =P < 0.05 for triptolide versus vehicle. C, Proteinuria in mice completing ≥4 weeks of treatment, as determined by spectrophotometry (see Materials and Methods for details). * = P < 0.05; ** = P < 0.01; *** = P < 0.001; **** = P < 0.0001 for the significance of the increase after the indicated treatment period versus before treatment; # = P < 0.05; ## = P < 0.01 for the significance of the decrease after the indicated treatment period versus before treatment. D, Levels of BUN in blood collected immediately after death or at the end of study. Dotted lines show the normal range (17–28 mg/dl). Horizontal lines show the median. *** = P < 0.001; ** = P < 0.01 versus vehicle. E, Serum albumin levels at the end of study (normal 3.89 gm/dl in female C57BL/6J mice). Horizontal bars show the median. * = P < 0.05; ** = P < 0.01 versus vehicle. Values in A–C are the mean. Numbers in parentheses in A–E are the number of mice.
In contrast to the results of the previous study of the EA extract of TWHF, which noted that treatment caused a loss in body weight (17), neither triptolide nor tripdiolide affected body weight (Figure 1B), whereas body weight diminished significantly in the vehicle group.
Effect of therapy on proteinuria, BUN, and serum albumin levels
Significant proteinuria (≥30 mg/dl) was detected in 85% of the mice in all 3 groups before starting treatment. At this time, the mean level of urinary protein was not significantly different in the 3 groups (Figure 1C). By the end of the study, 12 of 14 animals (85.7%) in the vehicle-treated group developed severe proteinuria (>500 mg/dl). In contrast, the level of proteinuria was maintained or improved to the same low level (30–100 mg/dl) throughout the study in 9 of 16 triptolide-treated mice and in 11 of 17 tripdiolide-treated mice. Four animals in the triptolide group and 3 in the tripdiolide group exhibited severe proteinuria (>500 mg/dl) before the start of treatment that did not change during the treatment course. Worsening of proteinuria was noted within 4 weeks after the start of treatment in 3 mice in the triptolide group (18.8%) and 3 in the tripdiolide group (17.6%). Proteinuria was significantly greater in the vehicle-treated group than in the groups treated with triptolide or tripdiolide (P = 0.004) after 6 weeks of treatment and thereafter.
BUN levels were increased in the 3 treatment groups and were correlated with the severity of proteinuria. As shown in Figure 1D, 10 of 12 animals in the vehicle group had increased levels of BUN, ranging from 32 mg/dl to 159 mg/dl (normal <27 mg/dl). In contrast, in 12 of 14 mice in the triptolide group and 12 of 15 mice in the tripdiolide group, BUN levels remained within the normal range. At the end of treatment, the mean level of BUN was 99 mg/dl, 18.5 mg/dl, and 19.9 mg/dl in the vehicle, triptolide, and tripdiolide groups, respectively (P < 0.001 triptolide versus vehicle; P < 0.01 triptolide versus vehicle).
We found a negative correlation between serum albumin levels and the severity of proteinuria. Serum albumin levels were significantly decreased in mice treated with vehicle as compared with those treated with either triptolide or tripdiolide (Figure 1E). Mice treated with tripdiolide exhibited significantly higher serum levels of albumin (P < 0.05 vehicle versus tripdiolide).
Effect of therapy on kidney pathology
At the end of the study, kidneys available for histopathologic analysis were obtained from 13, 15, and 15 animals of the vehicle, triptolide, and tripdiolide groups, respectively. All kidneys from the vehicle group had glomerular, interstitial, and vascular lesions (Figure 2A). Glomeruli were the most severely affected. Glomerular lesions ranged from proliferative glomerulonephritis, with influx of mononuclear cells and rare neutrophils, to diffuse glomerular sclerosis, with crescent formation, fibrinoid necrosis, and proteinuria. Interstitial disease consisted of infiltration of mononuclear cells between tubules, with tubular atrophy, dilation, and thickened tubular basement membranes. Occasional tubules had necrotic tubular epithelium. Perivascular disease consisted of mononuclear cell infiltrates in a follicular pattern around large arteries and interlobular arteries. In the most severely affected mice, the mononuclear cells around blood vessels were confluent, with interstitial inflammation. At the end of treatment, as shown in Figure 2B, most mice treated with either triptolide or tripdiolide had less severe kidney disease, with significantly diminished glomerular and interstitial disease (P <0.01 triptolide versus vehicle; P < 0.001 tripdiolide versus vehicle).
Figure 2.
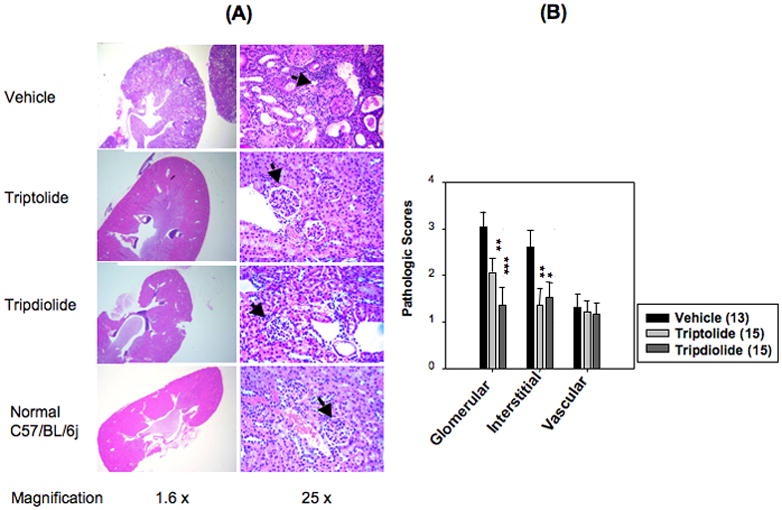
Changes in renal pathology after treatment with triptolide or tripdiolide in (NZB X NZW)F1 mice. A, Kidney sections from mice in the 3 treatment groups and a normal control group of C57BL/6J mice, shown at low and high magnification. Sections from the vehicle-treated group show glomerulonephritis, tubular dilation and atrophy, and heavy cellular infiltration in the perivascular and interstitial region. Sections from the triptolide and tripdiolide treatment groups show normal glomeruli and tubules. Representative glomeruli are indicated by arrows. Results are representative of 13, 15, and 15 mice in the vehicle, triptolide, and tripdiolide groups, respectively. B, Glomerular, interstitial, and perivascular disease in the 3 treatment groups, scored on a scale of 0–4+ as described elsewhere (17). Values are the mean and SEM. Numbers in parentheses are the number of mice. * = P < 0.05; ** = P < 0.01; *** = P > 0.001 versus vehicle.
As shown in Figure 3, IgG and C3 staining was observed only in the glomeruli, and the intensity of IgG and C3 staining was correlated with the severity of proteinuria. Remarkable deposition of both IgG and C3 was observed in the renal glomeruli from all mice in the vehicle group. In contrast, significantly less or no glomerular deposition of either IgG or C3 was noted in the mice treated with triptolide or tripdiolide, which appeared to be similar to the findings in the C57BL/6J normal control mice.
Figure 3.
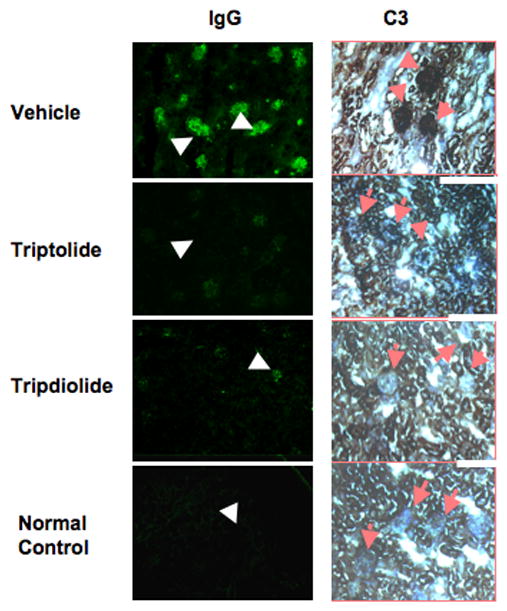
Immunohistochemical analysis of kidneys from (NZB X NZW)F1 mice treated with triptolide or tripdiolide. Kidney sections from mice in the 3 treatment groups and a normal control group of C57BL/6J mice were examined for deposition of IgG and C3. Representative glomeruli are indicated by arrows. Results are representative of 5 mice per group. (Original magnification X 16.)
Decreased spleen weight and accumulation of plasma cells in the spleens of mice treated with triptolide or tripdiolide
The spleens were harvested and weighed at the end of treatment or right after the animals died. As shown in Figure 4A, the mean spleen weight was significantly higher in the vehicle group (150 mg) than in the group treated with either triptolide (94.7 mg; P < 0.01 versus vehicle) or tripdiolide (92.8 mg; P < 0.001 versus vehicle). In our previous study of animals treated with an extract of TWHF, the decline in spleen weight was associated with a decrease in the area of the white pulp, with a marked decrease in CD11c+ myeloid cells and IgD+ B cells (17). In the current study, we focused on the effect of diterpenoid therapy on plasma cell infiltration. As shown in Figure 4B, there was a dense accumulation of CD138+ plasma cells around the central vessels in the spleens of the vehicle-treated group, but these were absent in the tripdiolide-treated group.
Figure 4.
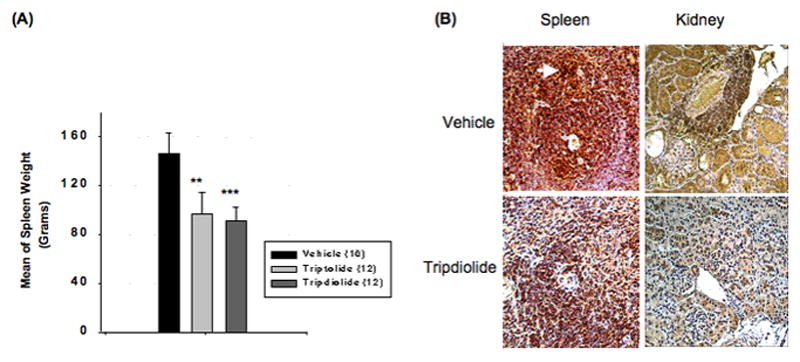
Spleen weight and CD138 staining of kidney and spleen sections from (NZB X NZW)F1 mice treated with triptolide or tripdiolide. A, Spleen weight after treatment. Values are the mean and SEM. Numbers in parentheses are the number of mice. ** = P < 0.01; *** = P < 0.001 versus vehicle. B, Sections of spleen and kidney stained for CD138+ cells. Accumulation of CD138+ plasma cells around central vessels is seen in the spleen in the vehicle-treated group (arrow), but not in the tripdiolide-treated group. Results are representative of 5 mice per group. (Original magnification X 25.)
Decrease in renal plasma cells in diterpenoid-treated (NZB X NZW)F1 mice
Previously, we observed that treatment of (NZB X NZW)F1 mice with an extract of TWHF caused a marked decrease in CD3+ T cells, CD11c+ myeloid cells, and IgD+ B cells within the kidneys (17). In the current study, we focused on CD138+ plasma cells. Staining of kidney sections showed marked infiltration of CD138+ plasma cells around small arteries in the kidneys of the vehicle-treated mice. In contrast, there was no significant accumulation of CD138+ plasma cells in the kidneys of mice treated with tripdiolide (Figure 4B).
Changes in serum levels of anti-dsDNA antibody
Compared with the normal C57BL/6J mice and the NZB/B1NJ mice, sera from the (NZB X NZW)F1 mice from the 3 treatment groups contained higher titers of IgG anti-dsDNA antibody at 28 weeks of age (Figure 5). The levels of IgG anti-dsDNA antibody were significantly increased at the end of the treatment course as compared with those at age 28 weeks in the vehicle-treated mice (52.8 IU/ml versus 63.5 IU/ml; P < 0.001). In contrast, the mean level of anti-dsDNA antibody was moderately increased 6 weeks after the beginning of treatment (60.6 IU/ml), but decreased significantly (P < 0.05) at the end of therapy (53.8 IU/ml) in tripdiolide-treated mice. In the triptolide-treated group, the mean level of IgG anti-DNA antibody was significantly increased 6 weeks after beginning treatment (44.2 IU/ml versus 69.5 IU/ml) and was slightly, but not significantly, reduced at the end of treatment (65.2 IU/ml).
Figure 5.
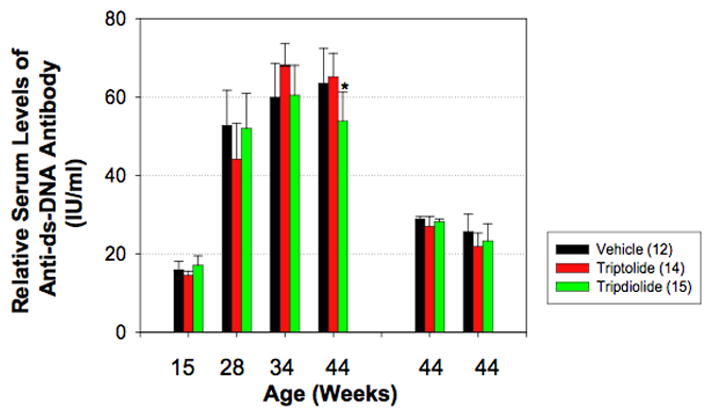
Serum anti–double-stranded DNA (anti-dsDNA) antibody titers in (NZB X NZW)F1 mice before and after treatment with triptolide or tripdiolide. Mice were treated for 15 weeks beginning at age 29 weeks (after the development of lupus nephritis). Serum was collected at the indicated times. Anti-dsDNA antibody levels were determined by enzyme-linked immunosorbent assay. The mean relative titer of anti-dsDNA antibody in sera from normal control C57BL/6J mice was 23 IU/ml. The two groups of bars at the right show serum levels of the IgA and IgM isotypes of anti-dsDNA, respectively, at the end of treatment. Values are the mean and SEM. Numbers in parentheses are the number of mice. * = P < 0.05 versus vehicle and versus triptolide at the same time point.
Serum levels of the IgA and IgM isotypes of anti-dsDNA were also determined at the end of the treatment course. The relative titers of anti-dsDNA of either the IgA or IgM isotype were about one-half the relative titers of the IgG isotype. There was no significant difference between the 3 groups in either IgA or IgM anti-dsDNA antibody levels.
Suppression of cytokine and chemokine production by spleen mononuclear cells after treatment with tripdiolide
Culture supernatants of spleen mononuclear cells from 8 (NZB X NZW)F1 mice from the vehicle and the tripdiolide treatment groups and from 3 C57BL/6J mice were assayed for production of cytokines. As shown in Figure 6, there was a significant difference in the production of IL-6 in mice treated with vehicle as compared with mice treated with tripdiolide (P < 0.01). Similarly, TNF production was significantly lower in tripdiolide-treated mice than in vehicle-treated mice (P < 0.05). In addition, spleen cells from several mice in the vehicle-treated group produced significantly higher amounts of MCP-1, whereas none of the animals in the tripdiolide-treated group produced detectable amounts of MCP-1 (P < 0.01). The levels of IL-6 and TNF produced by spleen mononuclear cells from the tripdiolide-treated (NZB X NZW)F1 mice were very similar to those produced by spleen mononuclear cells from the normal C57BL/6J mice. In addition, C57BL/6J splenocytes did not produce detectable MCP-1 (data not shown).
Figure 6.
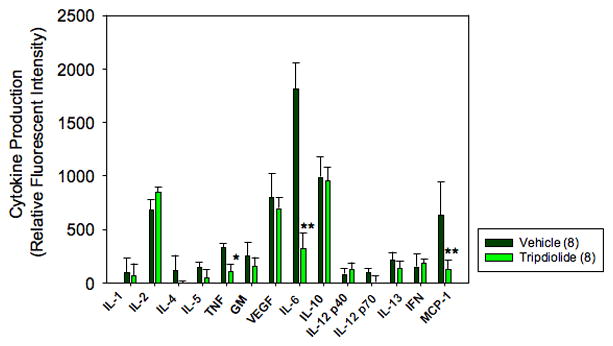
Cytokine production by spleen cells from (NZB X NZW)F1 mice after treatment with tripdiolide. Spleen mononuclear cells (2 × 106) from each mouse were incubated for 16 hours in 1 ml of complete medium with 1 μg/ml of phytohemagglutinin plus 10 ng/ml of phorbol myristate acetate. Cell-free supernatants were collected and lyophilized. After the lyophilized supernatant was reconstituted with 50 μl of sample buffer, the protein content and cytokine array analyses were performed as described in Materials and Methods. The relative fluorescence intensity was determined from the mean fluorescence intensity of 4 spots per cytokine per mouse and was normalized against the protein content in the cell supernatant from the same animal. Values are the mean and SEM of 8 mice per group. * = P < 0.05; ** = P < 0.01 versus vehicle. IL-1 = interleukin-1β; TNF = tumor necrosis factor; GM = granulocyte–macrophage colony-stimulating factor; VEGF = vascular endothelial growth factor; IFN = interferon-γ; MCP-1 = monocyte chemoattractant protein 1.
DISCUSSION
In this study, we explored the therapeutic effect of the putative active components of TWHF, triptolide and tripdiolide, on a murine model of lupus nephritis. Results of the study show that 85.7% of mice treated with vehicle had severe glomerulonephritis, which was documented by the presence of progressive proteinuria, elevated BUN levels, and histologic abnormalities at the end of study. In contrast, progressive proteinuria and pathologic changes in the kidneys were halted or improved in most mice treated with either triptolide or tripdiolide. In addition, mice treated with either of the diterpenoids had a prolonged survival rate compared with mice treated with vehicle. These results indicate that these 2 diterpenoid components exerted a therapeutic effect on established murine lupus nephritis that was similar to the effect of the EA extract of TWHF (17). Notably, the finding that either triptolide or tripdiolide is effective therapy for established (NZB X NZW)F1 murine lupus nephritis and is just as active as the whole extract suggests that there is no necessary interaction between components of the extract and indicates that these diterpenoids may be effective individually for treatment of SLE in humans.
Considerable effort has been focused on the isolation and identification of active components of TWHF. There is general agreement that the active components responsible for the therapeutic effect of extracts of TWHF are a group of diterpenoids (2,19). Although many diterpenoid compounds have been isolated from TWHF, triptolide and tripdiolide have been documented to be responsible for most of the biologic activity of the EA extract (1,2). However, there is a strong bias that individual components may not be as effective as the whole extract because of unknown interactions between the active components. As a result, only a few clinical trials have used individual diterpenoids such as triptolide as a single component to treat patients with autoimmune inflammatory diseases. Unfortunately, those studies could not generate conclusive results, since the trial in RA was uncontrolled and the number of patients enrolled was too small to be analyzed statistically (3). No clinical trials have examined tripdiolide.
Triptolide has been widely used as a representative of the TWHF diterpenoids for in vitro and animal model studies (1,2,4–16). However, no studies of the effects of individual diterpenoids in murine models of lupus have been reported. The aim of the current study was to explore whether a single diterpenoid could convey therapeutic benefit to mice with established lupus nephritis without intolerable side effects. The dose of the EA extract used to treat the (NZB X NZW)F1 mice in our previous study contained a total of 110 μg/kg of triptolide and tripdiolide in the high-dose group (17,18). In the current study, treatment of (NZB X NZW)F1 mice with 170 μg/kg of either triptolide or tripdiolide resulted in significant improvement in lupus nephritis. These results further documented that triptolide and tripdiolide are likely to be the active components that account for the therapeutic impact of the EA extract of TWHF. More interestingly, mice in the vehicle group in the present study and in the EA extract group in our previous study (17) had similarly significant loss of body weight. In contrast, mice treated with either triptolide or tripdiolide in the present study maintained stable body weight throughout the treatment course. These results suggest improved tolerability of purified triptolide or tripdiolide in (NZB X NZW)F1 mice with lupus nephritis.
Tripdiolide is a structural analog of triptolide. The effect of tripdiolide has not been extensively studied in vitro or in vivo. Only 1 study showed the therapeutic effect of tripdiolide in a murine model of leukemia (14). In vitro, tripdiolide was shown to exert inhibitory effects on the production of multiple cytokines that were as potent as those of triptolide (2). As expected from these considerations, data from the present study showed that treatment with tripdiolide significantly reduced the severity of lupus nephritis and prolonged the survival rate in the mice with lupus and was at least as effective as triptolide. Moreover, reductions in serum levels of anti-dsDNA antibody were noted in the mice treated with tripdiolide, but not those treated with triptolide. These results suggest that tripdiolide may have a therapeutic advantage over triptolide.
The important role of anti-dsDNA antibodies in the pathogenesis of autoimmune glomerulonephritis in lupus mice has been documented (20,21). However, a treatment benefit was not necessarily paralleled by a decrease in serum levels of anti-dsDNA antibodies (17,22,23). The current data show a statistically significant reduction in anti-dsDNA antibody levels in mice treated with tripdiolide, and a trend toward a reduction in this antibody was noted in the triptolide-treated mice at the end of treatment, although these effects were modest. The observations that treatment largely abolished immune complex deposition in the kidney but exerted only a modest effect on serum levels of anti-dsDNA suggest that the autoantibody measured in the serum may not account for immune complex deposition in the organs or that the diterpenoid may alter the environment of the kidney structure, such that immune complex deposition is impeded. Consistent with this possibility, a direct effect of triptolide on kidney function mediated by an interaction with the calcium-channel polycystin 2 has recently been reported (24).
Plasma cells are known to infiltrate the kidneys and spleens of (NZB X NZW)F1 mice and could thereby produce autoantibodies locally, resulting in an enhancement of the local concentration of autoantibodies and immune complexes (25). Approximately 0.1% of IgG-secreting kidney plasma cells produce anti-DNA antibodies (Muntaz I, Hiepe F, Manz RA: personal communication), as compared with ~4% of IgG-secreting spleen cells in (NZB X NZW)F1 mice (26). Autoantibody-producing plasma cells are also known to infiltrate the kidneys of other strains of mice that spontaneously develop lupus, such as MRL/lpr (27). The current study also identified increased numbers of CD138+ plasma cells in the kidney and spleen of vehicle-treated (NZB X NZW)F1 mice and markedly fewer in animals treated with either triptolide and tripdiolide. The loss of plasma cells in the kidney paralleled the decrease in IgG and C3 deposition. These results suggest that the infiltrating plasma cells in the (NZB X NZW)F1 mice could play a major role in immune complex deposition in the kidney. A similar suggestion has been made with regard to MRL/lpr mice (27).
Treatment with diterpenoids may therefore reduce immune complex deposition in the kidney by eliminating infiltrating plasma cells. Notably, kidney plasma cells are thought to be generated elsewhere and to migrate into the inflamed kidney (25). Since one site of their generation is the spleen (26), the effect of therapeutic diterpenoids on the generation of splenic plasma cells could also play a role in the treatment effect. Previous studies have shown that active diterpenoids of TWHF can inhibit the generation of antibody-secreting plasma cells in vitro (1).
The mechanism by which triptolide and tripdiolide exerted their therapeutic benefit in (NZB X NZW)F1 mice with lupus nephritis could be related to their immunosuppressive and direct antiinflammatory actions. It has been documented that triptolide inhibits the up-regulation of multiple proinflammatory genes, including IL-2, IFNγ, TNF, IL-6, COX-2, and iNOS (2,4,5). Several studies have also shown that triptolide inhibited the NF-κB–mediated transcription pathway (28–30). Data from cytokine array assays performed in the present study documented that spleen mononuclear cells from treated (NZB X NZW)F1 mice produced significantly less IL-6 and TNF than did those from vehicle-treated mice. These findings were consistent with our previous report that treatment of rats with extracts of TWHF significantly suppressed the production of IL-6, TNF, prostaglandin E2, and nitric oxide by cultured spleen mononuclear cells (9,10,31). It has previously been reported that levels of IL-1β, IL-6, and TNF were elevated in the serum and kidneys of mice with lupus (32–34). These cytokines, therefore, have been considered to be important in the pathogenesis of lupus nephritis. Inhibition of their production by diterpenoids of TWHF may reduce the levels of IL-6 and TNF in the circulation and in the kidney and consequently reduce kidney inflammation.
In conclusion, triptolide and tripdiolide treatment controlled and reduced lupus nephritis in (NZB X NZW)F1 mice as effectively as did the EA extract of TWHF and with better tolerability, suggesting that putative interactions of components in the extract are not required for its therapeutic benefit and that these individual bioactive diterpenoids may represent a new approach to the treatment of patients with lupus nephritis
Acknowledgments
We would like to thank Dr. Kristina Zale for technical support with the immunohistochemical staining and the acquisition and analysis of the imaging data.
Abbreviations
- TwHF
Tripterygium wilfordii Hook F
- EA
ethyl acetate
- LD50
lethal dose 50 %
- BUN
Blood urea nitrogen
- SLE
systemic lupus erythematosus
- RA
rheumatoid arthritis
- IgG
immunoglobulin G
- FITC
fluorescein isothiocyanate
- C3
complement 3
- HRP
horseradish peroxidase
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Lipsky had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Tao, Lipsky.
Acquisition of data. Tao, Fan, Hoffmann, Gao, Longo, Zerfas.
Analysis and interpretation of data. Tao, Fan, Hoffmann, Gao, Longo, Lipsky.
Manuscript preparation. Tao, Fan, Hoffmann, Longo, Lipsky.
Statistical analysis. Tao.
References
- 1.Tao XL, Lipsky PE. The Chinese anti-inflammatory and immunosuppressive herbal remedy, Tripterygium wilfordii Hook F. Rheum Dis Clin North Am. 2000;20:29–50. doi: 10.1016/s0889-857x(05)70118-6. [DOI] [PubMed] [Google Scholar]
- 2.Tao XL, Cai JJ, Lipsky PE. The identity of immunosuppressive components of the ethyl acetate extract and chloroform methanol extract (T2) of Tripterygium wilfordii Hook F. J Pharmacol Exp Ther. 1995;272:1305–12. [PubMed] [Google Scholar]
- 3.Su DF, Li RL, Sun YJ. Comparative study of triptolide and the ethyl acetate extract of Tripterygium wilfordii Hook F in the treatment of rheumatoid arthritis. Zhong Cao Yao. 1990;10:144–6. [PubMed] [Google Scholar]
- 4.Tao XL, Davis LS, Hashimoto S, Lipsky PE. The Chinese herbal remedy, T2, inhibits mitogen-induced cytokine gene transcription by T cells, but not initial signal transduction. J Pharmacol Exp Ther. 1996;276:316–25. [PubMed] [Google Scholar]
- 5.Lu Y, Liu Y, Fukuda K, Nakamura Y, Kumagai N, Nishida T. Inhibition by triptolide of chemokine, proinflammatory cytokine molecule expression induced by lipopolysaccharide in corneal fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:3796–800. doi: 10.1167/iovs.06-0319. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Liu ZH, Dai CS, Liu D, Li CH. Triptolide down-regulates tumor necrosis factor-α and interferon-γ-induced overexpression of monocyte chemoattractant protein-1 in human proximal tubular epithelial cells. Hong Kong J Nephrol. 2002;1:29–32. [Google Scholar]
- 7.Wang B, Ma L, Tao XL, Lipsky PE. Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheum. 2004;50:2995–3003. doi: 10.1002/art.20459. [DOI] [PubMed] [Google Scholar]
- 8.Tao XL, Schulze-Koops H, Ma L, Cai J, Mao YP, Lipsky PE. Effects of Tripterygium wilfordii Hook F extracts on induction of cyclooxygenase 2 activity and prostaglandin E2 production. Arthritis Rheum. 1998;41:130–8. doi: 10.1002/1529-0131(199801)41:1<130::AID-ART16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Gu WZ, Brandwein SR, Banerjee S. Inhibition of type II collagen-induced arthritis in mice by an immunosuppressive extract of Tripterygium wilfordii Hook F. J Rheumatol. 1992;19:682–8. [PubMed] [Google Scholar]
- 10.Tao XL, Ma L, Mao YP, Lipsky PE. Suppression of carageenan-induced inflammation in vivo by an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F. Inflamm Res. 1999;48:139–48. doi: 10.1007/s000110050437. [DOI] [PubMed] [Google Scholar]
- 11.Gu WZ, Brandwein SR. Inhibition of type II collagen-induced arthritis in rats by triptolide. Int J Immunopharmacol. 1998;20:389–400. doi: 10.1016/s0192-0561(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 12.Lin N, Liu C, Xiao C, Jia H, Imada K, Wu H, et al. Triptolide, a diterpenoid tripoxide, suppresses inflammation and destruction in collagen-induced arthritis mice. Biochem Pharmacol. 2007;73:136–46. doi: 10.1016/j.bcp.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Shanghai Cooperative Group. Treatment of psoriasis with triptolide ointment. Chin J Dermatol. 1988;21:381–2. [Google Scholar]
- 14.Kupchan SM, Court WA, Daicy RG, Jr, Gilmore CJ, Bryan RF. Triptolide and tripdiolide, novel antileukemic diterpenoid tripoxides from Tripterygium wilfordii. J Am Chem Soc. 1972;94:7194–5. doi: 10.1021/ja00775a078. [DOI] [PubMed] [Google Scholar]
- 15.Gu WZ, Banerjee S, Brandwein SR. Suppression of renal disease and arthritis, and prolongation of survival in MRL-lpr mice treated with an extract of Tripterygium wilfordii Hook F. Arthritis Rheum. 1992;35:1381–6. doi: 10.1002/art.1780351122. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XY, Tsuchiya N, Dohi M, Yamamoto K, Ishihara K, Okudaira H, et al. Prolonged survival of MRL-lpr/lpr mice treated with Tripterygium wilfordii Hook-F. Clin Immunol Immunopathol. 1992;62:66–71. doi: 10.1016/0090-1229(92)90023-h. [DOI] [PubMed] [Google Scholar]
- 17.Tao X, Fan F, Hoffmann V, Longo NS, Lipsky PE. Therapeutic impact of the ethyl acetate extract of Tripterygium wilfordii Hook F on nephritis in NZB/W F1 mice. Arthritis Res Ther. 2006;8:R24. doi: 10.1186/ar1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai JJ, Tao XL, Lipsky PE. High performance liquid chromatographic determination of triptolide and tripdiolide in an ethyl acetate extract. J Liquid Chromatogr. 1994;17:4479–87. [Google Scholar]
- 19.Zheng JR, Feng K, Gu K. Screening of anti-inflammatory, immunosuppressive and antifertility components of Tripterygium wilfordii Hook F. V. Effects of seven diterpene lactone epoxide compounds on the proliferation of T and B lymphocytes in vitro. Acta Acad Med Sinicae. 1994;16:24–8. [PubMed] [Google Scholar]
- 20.Dixon FJ, Lidstone MB, Tonietti G. Pathogenesis of immune complex glomerulonephritis of New Zealand mice. J Exp Med. 1971;134:65–75. [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, et al. Spontaneous murine lupus-like syndromes: clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borel Y, Lewis RM, Andre-Schwatz J, Stollar BD, Diener E. Treatment of lupus nephritis in adult (NZB X NZW)F1 mice by cortisone-facilitated tolerance to nucleic acid antigens. J Clin Invest. 1978;61:276–86. doi: 10.1172/JCI108937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiffer L, Sinha J, Wang X, Huang W, Gonsdroff GV, Schiffer M, et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171:489–97. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 24.Leuneroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu ZH, Somlo S, et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci U S A. 2007;104:4389–94. doi: 10.1073/pnas.0700499104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassese G, Lindenau S, Boer B, Arce S, Hauser A, Riemekasten G, et al. Inflamed kidneys of NZB/W mice are a major site for the homeostasis of plasma cells. Eur J Immunol. 2001;31:2726–32. doi: 10.1002/1521-4141(200109)31:9<2726::aid-immu2726>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voight C, Eilat D, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–84. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekine H, Watanabe H, Gilkeson GS. Enrichment of anti-glomerular antigen antibody producing cells in the kidneys of MRL/MpJ-Faslpr mice. J Immunol. 2004;172:3913–21. doi: 10.4049/jimmunol.172.6.3913. [DOI] [PubMed] [Google Scholar]
- 28.Kim YH, Lee SH, Lee JY, Choi SW, Park JW, Kwon TK. Triptolide inhibits murine-inducible nitric oxide synthase expression by down-regulating lipopolysaccharide-induced activity of nuclear factor-κB and c-Jun NH2-terminal kinase. Eur J Pharmacol. 2004;494:1–9. doi: 10.1016/j.ejphar.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 29.Zhuang WJ, Fong CC, Cao J, Ao L, Leung CH, Xiao PG, et al. Involvement of NF-κB and c-myc signaling pathways in the apoptosis of HL-60 cells induced by alkaloids of Tripterygium hypoglaucum (levl) Hutch Phytomedicine. 2004;11:295–302. doi: 10.1078/0944711041495128. [DOI] [PubMed] [Google Scholar]
- 30.Jiang XH, Wong BC, Lin MC, Zhu GH, Kung HF, Jiang SH, et al. Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor-κB activation in gastric cancer cells. Oncogene. 2001;20:8009–18. doi: 10.1038/sj.onc.1204981. [DOI] [PubMed] [Google Scholar]
- 31.Tao XL, Ma L, Cai JJ, Lipsky PE. Treatment with an ethyl acetate extract of Tripterygium wilfordii Hook F improves joint inflammation in HLA B27 transgenic rats [abstract] Arthritis Rheum. 1996;39(Suppl 9):S298. [Google Scholar]
- 32.Aringer M, Smolen JS. Tumor necrosis factor and other pro-inflammatory cytokines in systemic lupus erythematosus: a rationale for therapeutic intervention. Lupus. 2004;13:344–7. doi: 10.1191/0961203303lu1024oa. [DOI] [PubMed] [Google Scholar]
- 33.Alleva DG, Kaser SB, Beller DI. Aberrant cytokine expression and autocrine regulation characterize macrophages from young MRL+/+ and NZB/W F1 lupus-prone mice. J Immunol. 1997;159:5610–9. [PubMed] [Google Scholar]
- 34.Moore KJ, Yeh K, Naito T, Kelley VR. TNF-α enhances colony-stimulating factor-1-induced macrophage accumulation in autoimmune renal disease. J Immunol. 1996;157:427–32. [PubMed] [Google Scholar]


