Abstract
Anti-apoptotic members of the Bcl-2 family, including Bcl-2, Bcl-xL, Mcl-1, Bcl-w and Bfl-1, inhibit the mitochondrial pathway of apoptosis. Bcl-xL and Mcl-1 are constitutively expressed in the liver. Although previous research established Bcl-xL as a critical apoptosis antagonist in differentiated hepatocytes, the significance of Mcl-1 in the liver, especially in conjunction with Bcl-xL, has not been clear. To examine this question, we generated hepatocyte-specific Mcl-1– deficient mice by crossing mcl-1flox/flox mice and AlbCre mice and further crossed them with bcl-xflox/flox mice, giving Mcl-1/Bcl-xL– deficient mice. The mcl-1flox/flox AlbCre mice showed spontaneous apoptosis of hepatocytes after birth, as evidenced by elevated levels of serum alanine aminotransferase (ALT) and caspase-3/7 activity and an increased number of terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling (TUNEL)-positive cells in the liver; these phenotypes were very close to those previously found in hepatocyte-specific Bcl-xL– deficient mice. Although mcl-1flox/+ AlbCre mice did not display apoptosis, their susceptibility to Fas-mediated liver injury significantly increased. Further crossing of Mcl-1 mice with Bcl-xL mice showed that bcl-xflox/+ mcl-1flox/+ AlbCre mice also showed spontaneous hepatocyte apoptosis similar to Bcl-xL– deficient or Mcl-1– deficient mice. In contrast, bcl-xflox/flox mcl-1flox/+ AlbCre, bcl-xflox/+ mcl-1flox/flox AlbCre, and bcl-xflox/flox mcl-1flox/flox AlbCre mice displayed a decreased number of hepatocytes and a reduced volume of the liver on day 18.5 of embryogenesis and rapidly died within 1 day after birth, developing hepatic failure evidenced by increased levels of blood ammonia and bilirubin. Conclusion: Mcl-1 is critical for blocking apoptosis in adult liver and, in the absence of Bcl-xL, is essential for normal liver development. Mcl-1 and Bcl-xL are two major anti-apoptotic Bcl-2 family proteins expressed in the liver and cooperatively control hepatic integrity during liver development and in adult liver homeostasis in a gene dose-dependent manner.
The mitochondrial pathway of apoptosis is regulated by the Bcl-2 family proteins.1,2 They are functionally divided into two basic groups: pro-apoptotic and anti-apoptotic members. Pro-apoptotic members are further divided into multi-domain members, such as Bax and Bak, and BH3-only proteins. Bax/Bak triggers release from mitochondria of cytochrome c, presumably by forming pores at the mitochondrial outer membrane. Cytochrome c released into the cytosol activates multiple caspases, which cut a variety of cellular substrates and dismantle the cell.3 The release of Bax/Bak–mediated cytochrome c is considered to be a point of no return and a commitment to cell death.4 Killing by BH3-only proteins, such as Bid, Bim, or Puma, requires Bax or Bak, placing them upstream of Bak/Bax activation. BH3-only proteins are transcriptionally or posttranslationally activated by a variety of cellular stresses. They are considered to be sensors that transmit apoptotic stimuli to mitochondria. Anti-apoptotic members, including Bcl-2, Bcl-xL, Mcl-1, Bcl-w, and Bfl-1, inhibit the mitochondrial pathway of apoptosis either by directly blocking Bak/Bax activation or by sequestering BH3-only proteins from Bak or Bax.
Mcl-1 has increasingly attracted attention because of its role in liver disease. Several reports have shown that Mcl-1 is overexpressed in a subset of human hepatocellular carcinomas and provides apoptosis resistance.5–7 The multi-kinase inhibitor sorafenib, which was recently approved by the Food and Drug Administration as a chemotherapeutic agent for hepatocellular carcinoma,8 is capable of down-regulating Mcl-1 expression and producing apoptosis in hepatoma cells.9 Cycloxygenase 2 or hepatocyte growth factor up-regulates Mcl-1 expression in hepatocytes and improves Fas-mediated liver injury.10,11 Recently, enforced expression of Mcl-1 was reported to reduce liver injury induced by anti-Fas injection in mice.12 However, little is known about the physiologic significance of Mcl-1 in hepatocytes.
We previously reported that hepatocyte-specific Bcl-xL knockout mice were born and grew up but developed spontaneous hepatocyte apoptosis, identifying Bcl-xL as a critical apoptosis antagonist in hepatocytes.13 This raises a question of whether other anti-apoptotic Bcl-2 family members, such as Mcl-1, have a significant role in regulating hepatocyte apoptosis and what the relationship is among those molecules. To this end, in the current study, we generated hepatocyte-specific Mcl-1 knockout as well as Bcl-xL/Mcl-1 double knockout mice and found that, like Bcl-xL, Mcl-1 is critical for maintaining hepatocyte integrity in adult liver, but not essential for liver development. However, both deficiencies cause a severe defect in liver development and lethality during the early neonatal period because of severe hepatic failure. The current study identifies Bcl-xL and Mcl-1 as two major anti-apoptotic Bcl-2 family proteins in the liver and demonstrates their gene dose– dependent effects for controlling hepatic integrity.
Materials and Methods
Mice
Mice carrying the mcl-1 gene encoding amino acids 1 through 179 flanked by 2 loxP (mcl-1flox/flox) were provided by Dr. You-Wen He of Duke University.14 Mice carrying a bcl-x gene with two loxP sequencers at the promoter region and a second intron (bcl-xflox/flox) were described previously.15 Heterozygous AlbCre transgenic mice expressing Cre recombinase gene under the promoter of the albumin gene were described previously.13 We generated hepatocyte-specific Mcl-1 knockout mice (mcl-1flox/floox AlbCre) by mating mcl-1flox/flox and AlbCre mice. We then used these knockout mice to generate hepatocyte-specific Bcl-xL/Mcl-1 knockout mice (bcl-xflox/flox mcl-1fl/oxflox AlbCre) by mating them with bcl-xflox/flox mice. Traditional Bid knockout mice were described previously.16 They were maintained in a specific pathogen–free facility and treated with humane care under approval from the Animal Care and Use Committee of Osaka University Medical School.
Genotyping
Genomic DNA was extracted from the tail and subjected for polymerase chain reaction (PCR) for genotyping mice. The primers used were as follows: 5′-GCCACCTCATCAGTCGGG-3′ and 5′-TCAGAAGCCGCAATATCCCC-3′ for the bcl-x allele; 5′-GGTTCCCTGTCTCCTTACTTACTGTAG-3′ and 5′-CTCCTAACCACTGTTCCTGACATCC-3′ for the mcl-1 allele; 5′-GCGGTCTGGCAGTAAAAACTATC-3′, 5′-GTGAAACAGCATTGCTGTCACTT-3′, 5′CTAGGCCACAGAATTGAAAGATCT-3′ 5′-GTAGGTGGAAATTCTAGCATCATCC-3′ for the AlbCre allele; 5′-CCGAAA TGTCCCATAAGAG-3′, 5′-GAGATGGACCACAACATC-3′, and 5′ TGCTACTTCCATTTGTCACGTCCT-3′ for the bid allele. PCR products were electrophoretically separated using 2% agarose gels. The expected sizes of the PCR products were as follows: 165 bp for the wild-type bcl-x allele, 195 bp for the floxed bcl-x allele, 200 bp for the wild-type mcl-1 allele, 300 bp for the floxed mcl-1 allele, 130 bp for the wild-type bid allele, and 350 bp for the bid knockout allele. AlbCre-negative mice showed a 350-bp band, and heterozygous AlbCre mice showed 100-bp and 350-bp double bands.
Apoptosis Assay
To measure serum ALT level and caspase-3/7 activity, blood was collected from the inferior vena cava of mice and centrifuged. Serum was stored at −20°C until use. Serum ALT levels were measured by a standard method at Oriental Kobo Life Science Laboratory (Nagahama, Japan), and serum caspase-3/7 activity was measured by a luminescent substrate assay for caspase-3 and caspase-7 (Caspase-Glo assay, Promega, Tokyo, Japan). For histological analysis, livers were formalin-fixed, embedded in paraffin, and thin sliced. The liver sections were stained with hematoxylineosin. To detect cells with oligonucleosomal DNA breaks, the sections were also subjected to terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling (TUNEL) staining, according to a previously reported procedure.17 For Fas-stimulating study, anti-Fas antibody (Jo2 clone) (PharMingen, San Diego, CA) was intraperitoneally injected into mice 3 hours before sacrifice.
Western Blot Analysis
Approximately 25 mg liver tissues was lysed with a lysis buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate and 1× protein inhibitor cocktail (Nacalai tesque, Kyoto, Japan), phosphate-buffered saline; pH 7.4). After incubation on ice for 15 minutes, the lysate was centrifuged at 10,000g for 15 minutes at 4°C. The protein content of the supernatants was determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Equal amounts of protein were electrophoretically separated by sodium dodecyl sulfate polyacrylamide gels (8% or 12%) and transferred onto polyvinylidene fluoride membrane. For immunodetection, the following antibodies were used: anti–Bcl-xL antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti–Mcl-1 antibody (Rockland, Gilbertsville, PA), anti-Bax antibody (Cell Signaling Technology, Beverly, MA), anti-Bid antibody (Cell Signaling Technology), anti-albumin antibody (Affinity Bioreagents, Golden, CO), and anti– beta actin antibody (Sigma-Aldrich, Saint Louis, MO). Detection of immunolabeled proteins was performed using a chemiluminescent substrate (Pierce).
Neonate Analysis
Neonatal mice delivered by cesarean section were suckled by a surrogate mother and sacrificed at 10 hours after birth. Blood from the neonatal mice was centrifuged, and the plasma was stored at −20°C until use. The levels of total bilirubin and ammonia were measured by Van den Bergh reaction and a standard enzymatic procedure, respectively, at Oriental Kobo Life Science Laboratory.
Real-Time Reverse-Transcription PCR
Total RNA was prepared from liver tissue using RNeasy kit (QIA-GEN, Tokyo, Japan). For complementary DNA synthesis, 1 μg total RNA was reverse-transcribed using the High Capacity RNA-to-DNA Master Mix (Applied Biosystems, Foster City, CA). Complementary DNA, equivalent to 40 ng RNA, was used as a template for real-time reverse-transcription PCR (RT-PCR) using an Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems). The messenger RNA expressions of tumor necrosis factor alpha (TNF-α), collagen-alpha1(I), and transthyretin were measured using TaqMan Gene Expression Assays (Assay ID: Mm00443260_g1, Mm00801666_g1, and Mm00443267_m1, respectively), and were corrected with the quantified expression level of beta-actin messenger RNA measured using TaqMan Gene Expression Assays (Assay ID: Mm02619580_g1).
Statistical Analysis
Data are presented as mean ± standard deviation. Comparisons between two groups were performed by unpaired t test. Multiple comparisons were performed by analysis of variance followed by Scheffe post hoc correction. P < 0.05 was considered statistically significant.
Results
Hepatocyte-Specific Mcl-1 Deficiency Leads to Spontaneous Hepatocyte Apoptosis in the Adult Liver
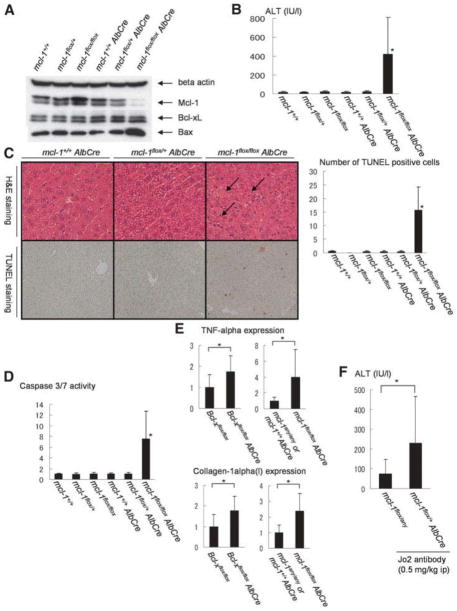
To generate hepatocyte-specific Mcl-1– deficient mice, floxed mcl-1 mice were crossed with heterozygous AlbCre mice. After mcl-1flox/+ AlbCre mice were mated with mcl-1flox/+ mice, and offspring were screened for genotyping and Mcl-1 expression. mcl-1flox/flox AlbCre mice were born and grew up. Their expression in the liver of Mcl-1 was greatly reduced compared with that of wild-type mice (Fig. 1A). The levels of Bcl-xL expression did not change in mcl-1flox/flox AlbCre liver. Bcl-xL and Mcl-1 proteins migrated as typical doublet bands of which the biochemical nature had been previously determined.18 The trace amount of Mcl-1 expression found in the knockout liver may have been attributable to expression in nonparenchymal cells, as previously observed in hepatocyte-specific Bcl-xL– deficient mice.13
Fig. 1.
Hepatocyte-specific Mcl-1 knockout mice. Offspring from mating of mcl-1flox/+ AlbCre mice and mcl-1flox/+ mice were sacrificed at the age of 6 weeks. (A) Western blot of whole liver lysate for the expression of Bcl-xL, Mcl-1, and Bax. (B) Serum ALT levels. N = 15 mice for each group. *P < 0.05 versus the other five groups. (C) Left panel shows hematoxylin-eosin and TUNEL staining of the liver section. Arrow indicates typical apoptotic cells. Right panel shows statistics of TUNEL-positive cells. The number of TUNEL-positive cells was determined in a defined area. N = 5 mice for each group. *P < 0.05 versus the other five groups. (D) Serum levels of caspase-3/7 activity. The levels were normalized to mcl-1+/+ AlbCre (−) mice. N = 15 mice for each group. *P < 0.05 versus the other five groups. (E) Real-time RT-PCR analysis for TNF-α and collagen-1alpha(1) expression. *P < 0.05. N = 12 or 9. The levels were normalized to the wild-type mice. (F) Serum ALT levels of Fas-stimulated mice. The mcl-1flox/+ AlbCre mice and mcl-1flox/+ or flox mice were sacrificed 3 hours after intraperitoneal injection of 0.5 mg/kg Jo2 antibody. *P < 0.05. N = 13 or 7.
To investigate the significance of Mcl-1 in the liver, mice were sacrificed 6 weeks after birth and subjected to analysis of serum ALT levels and caspase-3/7 activity as well as liver histology and TUNEL staining. mcl-1flox/flox AlbCre mice displayed significantly higher levels of serum ALT than control mice (AlbCre-negative or mcl-1+/+ Al-bCre mice) (Fig. 1B). Hepatocytes with typical apoptosis morphology such as cellular shrinkage and nuclear condensation were frequently observed in the liver sections of mcl-1flox/flox AlbCre mice (Fig. 1C). Consistently, the number of cells with TUNEL positivity, a hallmark of apoptotic cell death, in the liver was significantly higher in mcl-1flox/flox AlbCre mice than in control mice (Fig. 1C). Activity of caspase-3/7, executioners of apoptosis, was significantly higher in circulation of mcl-1flox/flox AlbCre mice than in control mice, which might reflect activation of those proteases in the knockout liver (Fig. 1D). Bax expression was clearly increased in mcl-1flox/flox AlbCre mice, suggesting Bax activation being involved in the apoptosis in mcl-1flox/flox AlbCre mice (Fig. 1A). Furthermore, the expression of TNF-α and collagen-alpha1(I) was significantly increased in the mcl-1flox/flox AlbCre liver compared with the wild-type liver, as found in the Bcl-xL knockout liver (Fig. 1E). Taken together, hepatocyte-specific Mcl-1 knockout mice developed spontaneous apoptosis leading to sterile inflammation and fibrotic response in the liver, like hepatocyte-specific Bcl-xL knockout mice.13
Heterozygous Deletion of the mcl-1 Gene Does Not Produce Apoptosis But Increases the Susceptibility to Fas Stimulation
Although the levels of Mcl-1 expression were significantly decreased in mcl-1flox/+ AlbCre liver (Fig. 1A, Supporting Fig. 1), mcl-1flox/+ AlbCre mice did not have apoptosis phenotypes in the liver (Fig. 1B–D). Therefore, we examined the susceptibility to Fas stimulation in these mice. We injected anti-Fas antibody into mcl-1flox/+ AlbCre mice and mcl-1flox/+ or flox mice and measured the levels of their serum ALT. mcl-1flox/+ AlbCre mice displayed significantly higher levels of serum ALT than control mice (Fig. 1F). These findings suggest that haplo-deficiency of Mcl-1 does not produce apoptosis in a physiological setting but clearly reduces apoptosis resistance under pathological conditions.
Involvement of Bid in Apoptosis Caused by Mcl-1 Deficiency
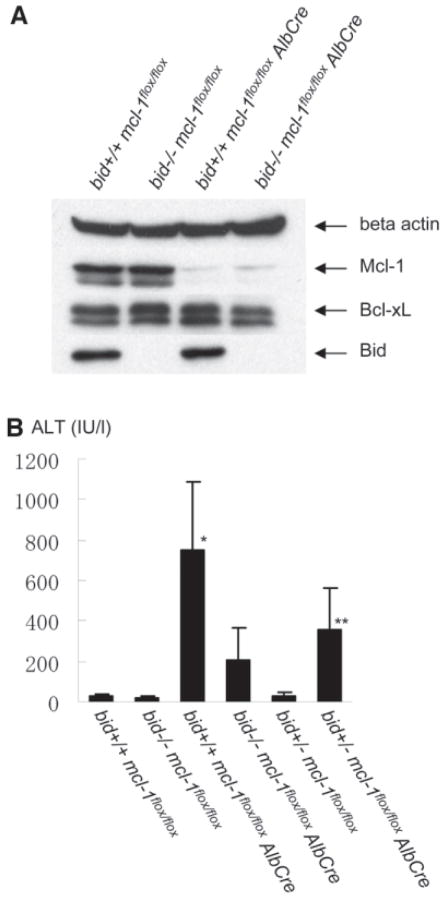
BH3-only proteins regulate life and death balance by interacting with core Bcl-2 family members. The hepatocyte is a so-called type 2 cell, which requires Bid as a sensor for Fas-mediated apoptotic stresses.19 In addition, it has been reported that the caspase-8/Bid pathway is involved in a variety of liver pathological conditions.16,20 To examine the possibility of Bid being involved in hepatocyte apoptosis caused by Mcl-1 deficiency, we crossed hepatocyte-specific Mcl-1 knockout mice with Bid knockout mice. Offspring form mating of bid+/− mcl-1flox/flox AlbCre mice with bid+/− mcl-1flox/flox mice were sacrificed at 6 weeks after birth and subjected to analysis of apoptosis phenotypes. Mice with each genotype grew up, and, as expected, the levels of Bid and/or Mcl-1 expression in the liver were correspondingly reduced with their genotypes (Fig. 2A). The levels of serum ALT were significantly lower in bid−/− mcl-1flox/flox AlbCre mice than in bid+/+ mcl-1flox/flox AlbCre mice (Fig. 2B). The results indicate that Bid was involved in hepatocyte apoptosis found in Mcl-1 knockout mice.
Fig. 2.
Mcl-1/Bid double-knockout mice. Offspring from mating of bid+/− mcl-1flox/flox AlbCre mice with bid+/− mcl-1flox/flox mice were sacrificed at 6 weeks after birth. (A) Western blot of whole liver lysate for the expression of Mcl-1, Bcl-xL, and Bid. (B) Serum ALT levels. N = 12 mice for each group. *P < 0.05 versus the other five groups; **P < 0.05 versus the AlbCre-negative groups and the bid+/+ mcl-1flox/flox AlbCre group.
Combined Deficiency of Mcl-1 and Bcl-xL in Hepatocytes Causes Lethality
Phenotypes observed in hepatocyte-specific Mcl-1 knockout mice were very similar to those in hepatocyte-specific Bcl-xL knockout mice.13 These results indicated that Bcl-xL and Mcl-1 share similar anti-apoptotic functions but do not compensate for the loss of each other. To examine whether their expression and function are completely nonredundant or just partially so, we generated hepatocyte-specific Bcl-xL/Mcl-1 double-knockout mice.
The bcl-xflox/+ mcl-1flox/+ AlbCre mice were mated with bcl-xflox/flox mcl-1flox/flox mice, and genotypes of the offspring were screened at 3 weeks after birth. AlbCre-negative and bcl-xflox/+ mcl-1flox/+ AlbCre mice were born and grew up, but not bcl-xflox/flox mcl-1flox/+ AlbCre, bcl-xflox/+ mcl-1flox/flox AlbCre, and bcl-xflox/flox mcl-1flox/flox AlbCre mice (Table 1). The lack of Bcl-xL and Mcl-1 caused a more severe phenotype than either knockout, suggesting that they partially compensate for the loss of each other at least from the viewpoint of maintaining normal development.
Table 1.
Genotyping of Offspring Obtained by Crossing bcl-xflox/+ mcl-1flox/+ AlbCre Mice and bcl-xflox/flox mcl-1flox/flox Mice
| AlbCre | bcl-x | mcl-1 | ED18.5 | 3 Weeks |
|---|---|---|---|---|
| − | flox/+ | flox/+ | 4 | 14 |
| − | flox/flox | flox/+ | 6 | 17 |
| − | flox/+ | flox/flox | 12 | 17 |
| − | flox/flox | flox/flox | 7 | 17 |
| + | flox/+ | flox/+ | 11 | 22 |
| + | flox/flox | flox/+ | 8 | 0 |
| + | flox/+ | flox/flox | 9 | 0 |
| + | flox/flox | flox/flox | 10 | 0 |
| Total | 67 | 87 |
ED, embryonic day.
Note that each genotype is expected to account for one-eighth of the offspring from this mating.
Mice Lacking Single Alleles for Both Bcl-xL and Mcl-1 Develop Spontaneous Apoptosis in the Adult Liver Similar to Bcl-xL or Mcl-1 Knockout Mice
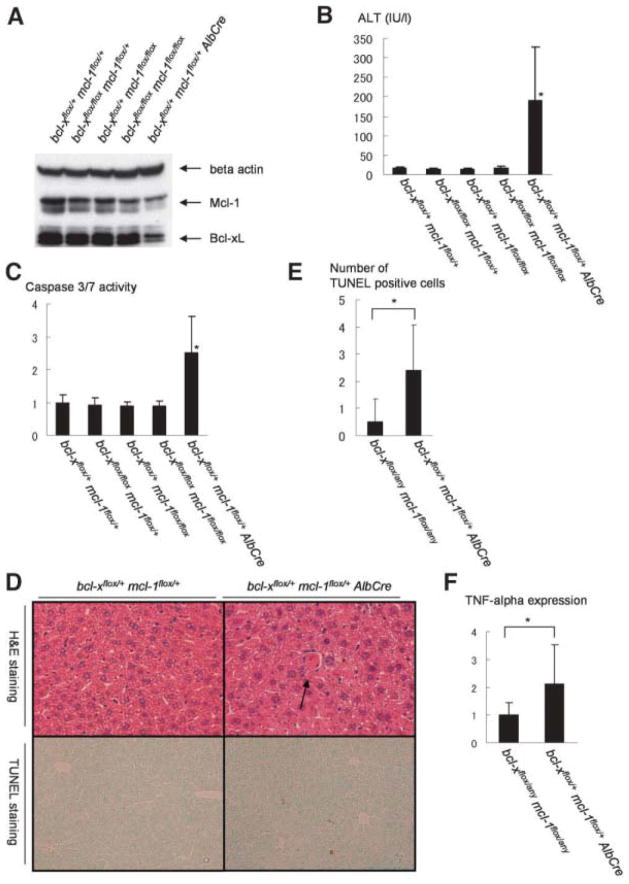
Offspring from mating of bcl-xflox/+ mcl-1flox/+ AlbCre and bcl-xflox/flox mcl-1flox/flox were sacrificed at 6 weeks after birth and subjected to analysis of Bcl-xL/Mcl-1 expression and apoptosis phenotypes. As expected, bcl-xflox/+ mcl-1flox/+ AlbCre liver expressed reduced levels of expression for both Bcl-xL and Mcl-1 (Fig. 3A). Interestingly, bcl-xflox/+ mcl-1flox/+ AlbCre mice developed spontaneous hepatocyte apoptosis as evidenced by an increase in serum ALT levels and caspase-3/7 activity (Fig. 3B,C). In agreement with this, hepatocytes with typical apoptotic morphology and positive for TUNEL staining were found scattered in the liver lobules in these mice (Fig. 3D,E). Furthermore, bcl-xflox/+ mcl-1flox/+ AlbCre mice showed higher expression of TNF-α than wild-type mice (Fig. 3F). The phenotypes were very similar to hepatocyte-specific Bcl-xL or Mcl-1knockout mice.
Fig. 3.
Hepatocyte-specific Bcl-xL/Mcl-1– deficient mice. Offspring from mating bcl-xflox/+ mcl-1flox/+ AlbCre mice and bcl-xflox/flox mcl-1flox/flox mice were sacrificed at the age of 6 weeks. (A) Western blot of whole liver lysate for the expression of Bcl-xL and Mcl-1. (B) Serum ALT levels. N = 9 mice for each group. *P < 0.05 versus the other five groups. (C) Serum levels of caspase-3/7 activity. The levels were normalized to bcl-xflox/+ mcl-1flox/+ mice. N = 9 mice for each group. *P < 0.05 versus the other five groups. (D) Hematoxylin-eosin and TUNEL staining of the liver sections for bcl-xflox/+ mcl-1flox/+ AlbCre mice. Findings for bcl-xflox/+ mcl-1flox/+ mice are shown as a control. (E) Statistics of TUNEL-positive cells. The number of TUNEL-positive cells was determined in a defined area. N = 5 or 6. *P < 0.05. (F) RT-PCR analysis for TNF-α expression. The levels were normalized to the group of bcl-xflox/+ or flox mcl-1flox/+ or flox. *P < 0.05. N = 9.
Hepatocyte-Specific Mcl-1/Bcl-xL-Deficient Mice Show Impaired Development of the Liver and Liver Failure During the Neonatal Period
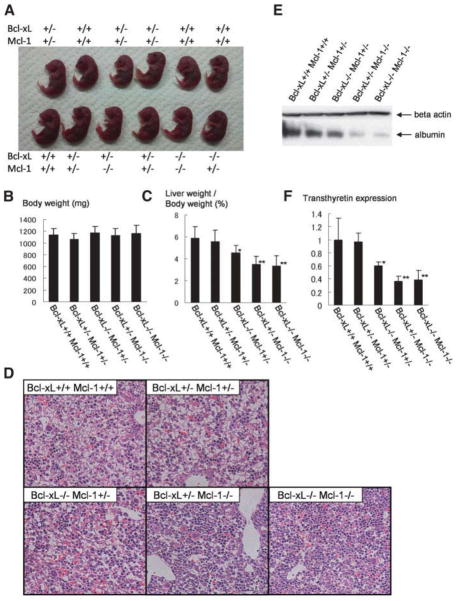
To examine the impact of Bcl-xL/Mcl-1deficiency at an earlier time point, offspring obtained from crossing bcl-xflox/+ mcl-1flox/+ AlbCre mice and bcl-xflox/flox mcl-1flox/flox mice were analyzed on gestational day 18.5. Live-obtained embryo followed expected Mendelian frequencies (Table 1). Overall, they looked normal, and their body weight did not differ among genotypes (Fig. 4A,B). However, the livers obtained from live pups with genotype of bcl-xflox/flox mcl-1flox/+ AlbCre, bcl-xflox/+ mcl-1flox/flox Alb-Cre, or bcl-xflox/flox mcl-1flox/flox AlbCre were clearly smaller. The ratios of liver weight to body weight were significantly lower in those pups than in AlbCre-negative or bcl-xflox/+ mcl-1flox/+ AlbCre pups (Fig. 4C). The ratios of liver weight to body weight were also examined in mcl-1flox/flox with AlbCre or without AlbCre mice, and there was no significant difference between the two (6.0 ± 0.8 versus 5.5 ± 0.9, N = 5, P = 0.34), excluding the possibility that Mcl-1 knockout itself affects the liver size at this time point. Histological analysis revealed that there were a number of hepatocytes with rectangular morphology and hematopoietic cells in the developing liver of the AlbCre-negative pups (Fig. 4D). Whereas the number of rectangular hepatocytes in bcl-xflox/+ mcl-1flox/+ AlbCre livers was similar to that in the AlbCre-negative livers, it was lower in bcl-xflox/flox mcl-1flox/+ AlbCre, bcl-xflox/+ mcl-1flox/flox AlbCre, and bcl-xflox/flox mcl-1flox/flox AlbCre livers. Rectangular cells were rarely observed in bcl-xflox/flox mcl-1flox/flox Alb-Cre livers. Furthermore, the expression of albumin and transthyretin was examined in the liver as a marker for hepatocyte differentiation.21 Consistent with histological findings, both expressions were gradually reduced from the AlbCre-negative livers to the bcl-xflox/flox mcl-1flox/flox AlbCre livers (Fig. 4E,F).
Fig. 4.
Hepatocyte-specific Bcl-xL/Mcl-1– deficient embryos. Offspring from mating bcl-xflox/+ mcl-1flox/+ AlbCre mice and bcl-xflox/flox mcl-1flox/flox mice were sacrificed on day 18.5 of gestation. Mice were classified into five groups. The bcl-xflox/+ or flox mcl-1flox/+ or flox are indicated by Bcl-xL +/+ Mcl-1 +/+; bcl-xflox/+ mcl-1flox/+ AlbCre are indicated by Bcl-xL +/− Mcl-1 +/−; bcl-xflox/flox mcl-1flox/+ AlbCre are indicated by Bcl-xL −/− Mcl-1 +/−; bcl-xflox/+ mcl-1flox/flox AlbCre are indicated by Bcl-xL +/− Mcl-1 −/−; bcl-xflox/flox mcl-1flox/flox AlbCre are indicated by Bcl-xL −/− Mcl-1 −/−. The numbers of embryos analyzed were 30 for Bcl-xL +/+ Mcl-1 +/+, 11 for Bcl-xL +/− Mcl-1 +/−, 8 for Bcl-xL −/− Mcl-1 +/−, 9 for Bcl-xL +/− Mcl-1 −/−, and 10 for Bcl-xL −/− Mcl-1 −/−. (A) Gross appearance of embryos. Representative photo for a litter is shown. (B) Body weight. (C) The ratios of liver weight to body weight. *P < 0.05 versus Bcl-xL +/+ Mcl-1 +/+; **P < 0.05 versus Bcl-xL +/+ Mcl-1 +/+ and Bcl-xL +/− Mcl-1 +/−. (D) Hematoxylin-eosin staining of the liver sections. (E) Western blot of whole-liver lysate for albumin expression. (F) Real-time RT-PCR analysis for transthyretin expression. The levels were normalized to the group of Bcl-xL +/+ Mcl-1 +/+. *P < 0.05 versus Bcl-xL +/+ Mcl-1 +/+; **P < 0.05 versus Bcl-xL +/+ Mcl-1 +/+ and Bcl-xL +/− Mcl-1 +/−.
We noticed that offspring obtained from crossing bcl-xflox/+ mcl-1flox/+ AlbCre mice and bcl-xflox/flox mcl-1flox/flox mice frequently died within 1 day after birth. To examine the cause of the early neonatal death, offspring were sacrificed at 10 hours after birth. They were divided into two groups according to the data shown in Table 1: expected survivors including AlbCre-negative and bcl-xflox/+ mcl-1flox/+ AlbCre pups, and expected nonsurvivors including bcl-xflox/flox mcl-1flox/+ AlbCre, bcl-xflox/+ mcl-1flox/flox AlbCre, and bcl-xflox/flox mcl-1flox/flox AlbCre pups. The levels of total bilirubin and ammonia in circulation were determined and compared between the groups. Both blood bilirubin levels and ammonia levels were significantly higher in the expected nonsurvivors than in the expected survivors (Fig. 5A,B). These results suggested that bcl-xflox/flox mcl-1flox/+ AlbCre, bcl-xflox/+ mcl-1flox/flox AlbCre, and bcl-xflox/flox mcl-1flox/flox AlbCre mice died quickly after birth because of hepatic failure, in agreement with the findings of impaired liver development.
Fig. 5.
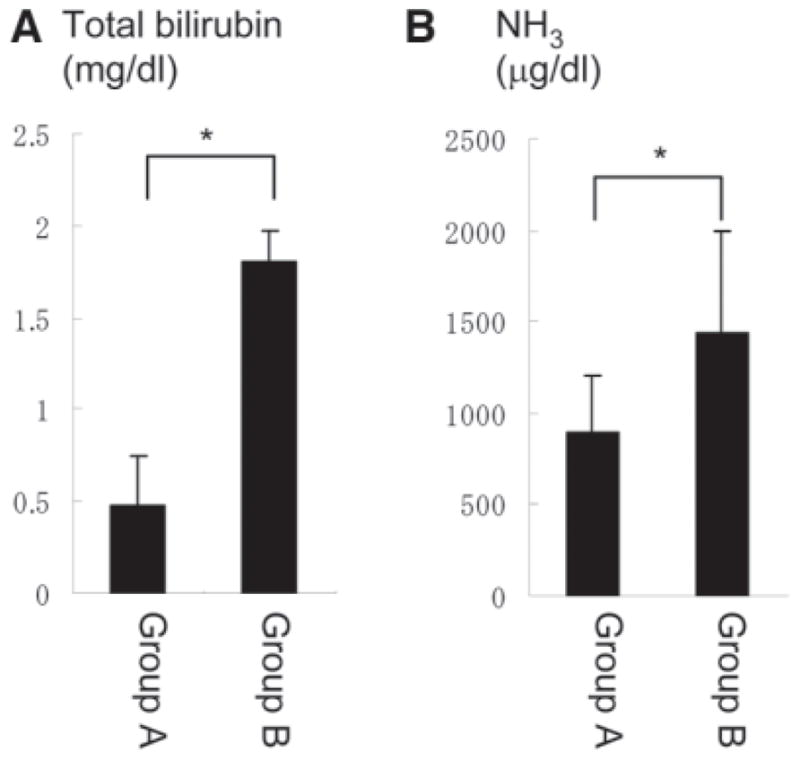
Plasma biochemistry of hepatocyte-specific Bcl-xL/Mcl-1– deficient neonates 10 hours after birth. Group A (N = 13) was defined as expected survivors including AlbCre-negative mice and bcl-xflox/+ mcl-1flox/+ AlbCre mice. Group B (N = 6) was defined as expected nonsurvivors including bcl-xflox/flox mcl-1flox/+ AlbCre, bcl-xflox/+ mcl-1flox/flox AlbCre, bcl-xflox/flox mcl-1flox/flox AlbCre. (A) Plasma total bilirubin levels. *P < 0.05. (B) Plasma ammonia levels in both groups. *P < 0.05.
Discussion
Five members of the anti-apoptotic Bcl-2 family have been found: Bcl-2, Bcl-xL, Bcl-w, Bfl-1, and Mcl-1. Traditional knockout of Bcl-2, a prototype of this family, displays growth retardation, hair color abnormality, lymphocyte decrease, and polycystic kidney.22,23 In agreement with the finding that Bcl-2 is not expressed in hepatocytes,13 these mice did not show any liver phenotypes. Similarly, Bcl-w24,25 or Bfl-1 knockout mice26 were generated but no liver phenotypes have been reported. Traditional knockout of Bcl-xL or Mcl-1 caused more severe phenotypes. Deletion of the bcl-x gene resulted in embryonic lethality because of abnormal neuronal development and hematopoiesis.27 The mcl-1 knockout embryo fails to be implanted in utero.28 Thus, study of traditional knock-out mice could not reveal the significance of Bcl-xL or Mcl-1 in the liver.
We previously reported that hepatocyte-specific knockout of Bcl-xL caused spontaneous apoptosis in hepatocytes after birth and established that Bcl-xL is critically important for the integrity of hepatocytes.13 The current study demonstrated that Mcl-1 plays an anti-apoptotic role in differentiated hepatocytes similar to that of Bcl-xL. During the preparation of this manuscript, a report by Vick et al.29 appeared on the Web, demonstrating a similar apoptosis phenotype in mice with specific knockout of the mcl-1 gene in hepatocytes. Our findings are in agreement with theirs and further provide evidence that deletion of a single allele for the mcl-1 gene fails to produce apoptosis phenotypes under physiological conditions, as observed in knockout of the bcl-x gene.13 Mcl-1 heterozygous disrupted mice did not produce apoptosis at least until 16 weeks of age (our unpublished data). It was demonstrated that hepatocyte-specific Mcl-1 knockout mice showed higher levels of liver injury than control mice on anti-Fas antibody injection.29 However, because mice lacking both mcl-1 alleles possess preexisting liver injury, it would be difficult to exactly compare liver injury after anti-Fas antibody injection and to conclude whether decreased Mcl-1 expression actually increases the susceptibility to Fas. In the current study, we took advantage of Mcl-1 heterozygous disrupted mice to address this point. They showed significantly higher levels of liver injury after Fas stimulation than wild-type mice, formally proving the significance of Mcl-1 expression under pathological conditions. Furthermore, our data on Mcl-1/Bid– deficient mice implies that the Bid pathway is involved in generating apoptosis found in Mcl-1 knockout mice. Because Bid mediates a variety of cellular stresses in hepatocytes upstream of Mcl-1,30,31 it will be interesting in future study to determine what stresses generate hepatocyte apoptosis in Mcl-1 knockout mice.
Bcl-xL and Mcl-1 share similar structures and functions.1 The observations that either deficiency similarly leads to spontaneous hepatocyte apoptosis imply that they play a non-redundant role in maintaining the integrity of hepatocytes in the adult liver. To further understand the relationship of both molecules, we generated hepatocyte-specific Bcl-xL/Mcl-1 knockout mice. Interestingly, mice lacking single alleles for both genes (bcl-x+/− mcl-1+/−) induced spontaneous hepatocyte apoptosis that could not be distinguished from that found in Bcl-xL or Mcl-1 knockout mice. This indicates that, whereas knockout of a single allele of the bcl-x or mcl-1 gene did not produce apoptosis, knockout of two alleles of any combination among both genes was sufficient to produce hepatocyte apoptosis. This finding suggests that both molecules are not independently but rather interdependently required for ensuring integrity of differentiated hepatocytes.
Bcl-xL/Mcl-1-deficient mice as well as mice only having a single allele of either bcl-x or mcl-1 gene displayed a decreased number of hepatocytes and reduced liver size on day 18.5 of gestation and appeared to develop lethal liver failure within 1 day after birth. Because the liver contains a large number of hematopoietic cells during development (Fig. 4D), it is very difficult to determine the expression levels of Bcl-xL or Mcl-1 specifically in hepatocytes in each knockout mouse. Liver development begins on day 8.5 of gestation in the mouse when the liver primordium is delineated from the endoderm.32 The albumin promoter, which is active in both hepatoblasts and hepatocytes, shows a 20-fold increase in transcriptional activity from day 9.5 to day 12.5 of gestation. The level of albumin then continues to increase as the liver develops simultaneously with the biliary tree and the hepatic bile duct being formed.33 Thus, the target genes could probably be successfully deleted during embryogenesis in the AlbCre recombination system. The observation that Bcl-xL/Mcl-1– deficient mice developed severer phenotypes than Bcl-xL– deficient or Mcl-1– deficient mice supports the idea that Cre-mediated deletion of the target genes actually took place during embryogenesis in our model. In contrast to the knockout of two alleles, knockout of three alleles and more of the bcl-x and mcl-1 genes induced lethal neonatal hepatic failure. Thus, hepatocyte integrity appeared to be strictly controlled by Bcl-xL and Mcl-1 in a gene dose– dependent manner.
Hepatocyte-specific deficiency of both Bcl-xL and Mcl-1 led to significant reduction of liver volume because of impaired hepatocyte development. However, overall, mice with these phenotypes were capable of developing normally until birth and rapidly developed liver failure and died within 1 day after birth. This finding suggests that differentiated hepatocytes are critically required for maintaining host homeostasis after birth but not during embryogenesis. The placenta plays an important role in nutritional support and de-toxification of the embryo. Our data imply that it could probably compensate for most functions of the liver cells during embryogenesis, whereas the liver would turn to the critical organ that is essential for maintaining host homeostasis after birth. Bcl-xL/Mcl-1 knockout mice provide interesting implications for the difference in the impact of differentiated hepatocytes between embryogenesis and the early neonatal period.
In conclusion, Mcl-1 and Bcl-xL are two major Bcl-2 family proteins inhibiting hepatocyte apoptosis. Together with previous work on traditional knockout mice, our data imply that other members, if any, could not compensate for their functions. Mcl-1 and Bcl-xL cooperatively maintain hepatocyte integrity during liver development and in adult liver homeostasis, and their effects are gene-dose dependent. Recent studies also have established that Mcl-15–7 and Bcl-xL18,34 are frequently overexpressed and confer resistance to apoptosis in hepatocellular carcinoma. Therefore, Mcl-1 and Bcl-xL are important apoptosis antagonists in a variety of pathophysiological conditions of the liver.
Supplementary Material
Acknowledgments
We thank Dr. You-Wen He (Department of Immunology, Duke University Medical Center, Durham, NC) for providing the mcl-1 floxed mice.
Supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to T.Tak.).
Abbreviations
- ALT
alanine aminotransferase
- PCR
polymerase chain reaction
- RT-PCR
reverse transcription polymerase chain reaction
- TNF-α
tumor necrosis factor alpha
- TUNEL
terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 2.Tsujimoto Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol. 2003;195:158–167. doi: 10.1002/jcp.10254. [DOI] [PubMed] [Google Scholar]
- 3.Fischer U, Jänicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sieghart W, Losert D, Strommer S, Cejka D, Schmid K, Rasoul-Rockenschaub S, et al. Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J Hepatol. 2006;44:151–157. doi: 10.1016/j.jhep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Fleischer B, Schulze-Bergkamen H, Schuchmann M, Weber A, Biesterfeld S, Müller M, et al. Mcl-1 is an anti-apoptotic factor for human hepatocellular carcinoma. Int J Oncol. 2006;28:25–32. [PubMed] [Google Scholar]
- 7.Schulze-Bergkamen H, Fleischer B, Schuchmann M, Weber A, Weinmann A, Krammer PH, et al. Suppression of Mcl-1 via RNA interference sensitizes human hepatocellular carcinoma cells towards apoptosis induction. BMC Cancer. 2006;6:232. doi: 10.1186/1471-2407-6-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 10.Casado M, Mollá B, Roy R, Fernández-Martínez A, Cucarella C, Mayoral R, et al. Protection against Fas-induced liver apoptosis in transgenic mice expressing cyclooxygenase 2 in hepatocytes. Hepatology. 2007;45:631–638. doi: 10.1002/hep.21556. [DOI] [PubMed] [Google Scholar]
- 11.Schulze-Bergkamen H, Brenner D, Krueger A, Suess D, Fas SC, Frey CR, et al. Hepatocyte growth factor induces Mcl-1 in primary human hepatocytes and inhibits CD95-mediated apoptosis via Akt. Hepatology. 2004;39:645–654. doi: 10.1002/hep.20138. [DOI] [PubMed] [Google Scholar]
- 12.Baskin-Bey ES, Huang W, Ishimura N, Isomoto H, Bronk SF, Braley K, et al. Constitutive androstane receptor (CAR) ligand, TCPOBOP, attenuates Fas-induced murine liver injury by altering Bcl-2 proteins. Hepatology. 2006;44:252–262. doi: 10.1002/hep.21236. [DOI] [PubMed] [Google Scholar]
- 13.Takehara T, Tatsumi T, Suzuki T, Rucker EB, III, Hennighausen L, Jinushi M, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner KU, Claudio E, Rucker EB, 3rd, Riedlinger G, Broussard C, Schwartzberg PL, et al. Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development. 2000;127:4949–4958. doi: 10.1242/dev.127.22.4949. [DOI] [PubMed] [Google Scholar]
- 16.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 17.Takehara T, Hayashi N, Tatsumi T, Kanto T, Mita E, Sasaki Y, et al. Interleukin 1β protects mice from Fas-mediated hepatocyte apoptosis and death. Gastroenterology. 1999;117:661–668. doi: 10.1016/s0016-5085(99)70460-9. [DOI] [PubMed] [Google Scholar]
- 18.Takehara T, Takahashi H. Suppression of Bcl-xL deamidation in human hepatocellular carcinomas. Cancer Res. 2003;63:3054–3057. [PubMed] [Google Scholar]
- 19.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, et al. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137–145. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tosh D, Shen CN, Slack JM. Differentiated properties of hepatocytes induced from pancreatic cells. Hepatology. 2002;36:534–543. doi: 10.1053/jhep.2002.35060. [DOI] [PubMed] [Google Scholar]
- 22.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama K, Nakayama K, Negishi I, Kuida K, Sawa H, Loh DY. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci U S A. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, et al. Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci U S A. 1998;95:12424–12431. doi: 10.1073/pnas.95.21.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, et al. Testicular degeneration in Bclw-deficient mice. Nat Genet. 1998;18:251–256. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- 26.Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Nakayama K, et al. Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J Exp Med. 1998;188:1985–1992. doi: 10.1084/jem.188.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 28.Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- 29.Vick B, Weber A, Urbanik T, Maass T, Teufel A, Krammer PH, et al. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology. 2009;49:627–636. doi: 10.1002/hep.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin XM. Bid, a BH3-only multi-functional molecule, is at the cross road of life and death. Gene. 2006;369:7–19. doi: 10.1016/j.gene.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 31.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaestner KH. The making of the liver: developmental competence in foregut endoderm and induction of the hepatogenic program. Cell Cycle. 2005;4:146–1148. doi: 10.4161/cc.4.9.2033. [DOI] [PubMed] [Google Scholar]
- 33.Cascio S, Zaret KS. Hepatocyte differentiation initiates during endodermal-mesenchymal interactions prior to liver formation. Development. 1991;113:217–225. doi: 10.1242/dev.113.1.217. [DOI] [PubMed] [Google Scholar]
- 34.Takehara T, Liu X, Fujimoto J, Friedman SL, Takahashi H. Expression and role of Bcl-xL in human hepatocellular carcinomas. Hepatology. 2001;34:55–61. doi: 10.1053/jhep.2001.25387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.