Abstract
Bcl-2 homology domain 3 (BH3)-only protein Bid is posttranslationally cleaved by caspase-8 into its truncated form (tBid) and couples with stress signals to the mitochondrial cell death pathway. However, the physiological relevance of Bid is not clearly understood. Hepatocyte-specific knockout (KO) of Bcl-xL leads to naturally-occurring apoptosis despite co-expression of Mcl-1, which shares a similar anti-apoptotic function. We generated Bcl-xL KO, Bcl-xL/Bid double KO, Bcl-xL/Bak double KO, Bcl-xL/Bax double KO, and Bcl-xL/Bak/Bax triple KO mice and found that hepatocyte apoptosis caused by Bcl-xL deficiency was completely dependent on Bak and Bax, and surprisingly on Bid. This indicated that, in the absence of Bid, Bcl-xL is not required for the integrity of differentiated hepatocytes, suggesting a complicated interaction between core Bcl-2 family proteins and BH3-only proteins even in a physiological setting. Indeed, a small but significant level of tBid was present in wild-type liver under physiological conditions. tBid was capable of binding to Bcl-xL and displacing Bak and Bax from Bcl-xL, leading to release of cytochrome c from wild-type mitochondria. Bcl-xL–deficient mitochondria were more susceptible to tBid-induced cytochrome c release. Finally, administration of ABT-737, a pharmacological inhibitor of Bcl-2/Bcl-xL, caused Bak/Bax-dependent liver injury, but this was clearly ameliorated with a Bid KO background.
Conclusion
Bid, originally considered to be a sensor for apoptotic stimuli, is constitutively active in healthy liver cells and is involved in the Bak/Bax-dependent mitochondrial cell death pathway. Healthy liver cells are addicted to a single Bcl-2–like molecule because of BH3 stresses, and therefore special caution may be required for the use of the Bcl-2 inhibitor for cancer therapy.
Bcl-2 family proteins regulate the mitochondrial pathway of apoptosis in mammalian cells.1 They are divided into two basic groups: core Bcl-2 family proteins and Bcl-2 homology domain 3 (BH3)-only proteins. Core Bcl-2 family proteins have three or four Bcl-2 homology domains (BH1-BH4 domains), referred to as multidomain members, and structural similarity. These proteins display opposing bioactivities from inhibition to promotion of apoptosis and can be further divided into two groups: anti-apoptotic members, including Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and Bfl-1, and pro-apoptotic members, including Bax and Bak. Pro-apoptotic Bak and Bax are effector molecules of the Bcl-2 family and induce release of cytochrome c from mitochondria, presumably through their ability to form pores at the mitochondrial outer membrane. Anti-apoptotic members, which serve as regulators, inhibit Bak and Bax. The original rheostat model argues for a fine balance between Bax-like pro-apoptotic proteins and Bcl-2–like anti-apoptotic proteins in defining life and death, and this balance would be equal or favor survival in a healthy cell.2
BH3-only proteins consist of at least eight members and only share homology with each other and the core Bcl-2 family proteins through the short BH3 motif. They are transcriptionally induced or posttranslationally activated in response to a variety of apoptotic stimuli.3 When they are induced or activated, they interact with core Bcl-2 family proteins and set the rheostat balance toward apoptosis by directly activating Bax-like molecules or neutralizing Bcl-2–like molecules.4 Therefore, they serve as initial sensors of apoptotic signals that emanate from various cellular processes. Bid, a member of the BH3-only proteins, is activated via caspase-8-mediated cleavage in response to ligation of the death receptor, and its N-terminal truncated form (tBid) translocates to mitochondria and activates the mitochondrial death pathway.5 In so-called type 1 cells, such as lymphoid cells, Fas activation leads to caspase-8 activation followed by direct activation of downstream caspases such as caspase-3 and caspase-7, where Bid dose not have significant roles.6 In contrast, in type 2 cells, Fas-mediated activation of caspase-8 is not enough to activate downstream caspases. In those cells, tBid links the extrinsic or death-receptor pathway to the intrinsic or mitochondrial pathway to execute apoptosis. Hepatocytes are identified as a typical type 2 cell in which Bid plays a critical role in receptor-mediated cell death pathways.7
In our previous research, we found that genetic ablation of Bcl-xL in hepatocytes causes spontaneous apoptosis in mice.8 This indicates that Bcl-xL is a critical apoptosis antagonist in adult healthy hepatocytes, although they possess other anti-apoptotic members of the Bcl-2 family such as Mcl-1. This might be simply explained by the fact that the absence of Bcl-xL affects the rheostat balance of core Bcl-2 family proteins by increasing the ratio of Bax and Bak to anti-apoptotic Bcl-2 proteins. Indeed, neuronal cell death during development caused by Bcl-xL deficiency is ameliorated by loss of Bax.9 Platelet cell death caused by Bcl-xL deficiency is also ameliorated by loss of Bak.10 These studies indicate that the stoichiometry between Bcl-xL and Bax or Bak dictates cellular fate. However, the possibility of BH3-only proteins being involved in the apoptosis rheostat in healthy cells has not been addressed. We generated Bcl-xL/Bid double-knockout (KO) mice and demonstrated that apoptosis caused by Bcl-xL deficiency is critically dependent on Bid. A small amount of Bid appears to be activated in the liver under physiological conditions and to be significant for inducing cytochrome c release from Bcl-xL–deficient mitochondria. This study shed light on the active participation of BH3-only proteins, which are generally considered to be sensors of apoptotic stimuli, in the Bcl-2 network regulating life and death of healthy differentiated hepatocytes.
Materials and Methods
Mice
Mice carrying a bcl-x gene with 2 loxP sequencers at the promoter region and a second intron (bcl-xflox/flox) were described previously.11 Heterozygous AlbCre transgenic mice expressing Cre recombinase gene under the promoter of the albumin gene8 and traditional Bid KO mice7 also have been described previously. We purchased from the Jackson Laboratory (Bar Harbor, ME) traditional Bak KO mice, traditional Bax KO mice, and conditional Bak/Bax KO mice (bak−/− baxflox/flox).12 We generated hepatocyte-specific Bcl-xL KO mice (bcl-xflox/flox AlbCre), Bcl-xL/Bid double-KO mice (bid−/− bcl-xflox/flox AlbCre), Bcl-xL/Bak double-KO mice (bak−/− bcl-xflox/flox AlbCre), Bcl-xL/Bax double-KO mice (bax−/− bcl-xflox/flox AlbCre), and Bcl-xL/Bak/Bax triple-KO mice (bak−/− baxflox/flox bcl-xflox/flox AlbCre) by mating the strains. They were maintained in a specific pathogen-free facility and treated with humane care under approval from the Animal Care and Use Committee of Osaka University Medical School.
Apoptosis Assay
The levels of serum alanine aminotransferase (ALT) were measured by a standard method, and serum caspase-3/7 activity was measured by a luminescent substrate assay for caspase-3 and caspase-7 (Caspase-Glo assay, Promega, Tokyo, Japan). The caspase-3/7 activity was normalized by each control group. For histological analysis, the liver sections were stained with hematoxylin-eosin. To detect cells with oligonucleosomal DNA breaks, the sections were also subjected to terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining, according to a previously reported procedure.13
Western Blot Analysis
Liver tissue was lysed with a lysis buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 × protein inhibitor cocktail (Nacalai tesque, Kyoto, Japan), phosphate-buffered saline, pH 7.4). Equal amounts of protein were electrophoretically separated by sodium dodecyl sulfate polyacrylamide gels and transferred onto polyvinylidene fluoride membrane. For immunodetection, the following antibodies were used: anti-Bcl-xL antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Mcl-1 antibody (Rockland, Gilbertsville, PA), previously described anti-Bid antibody generated from glutation-S-transferase-Bid fusion protein,14 anti-full-length Bid antibody, anti-cleaved caspase-7 antibody, anti-Bax antibody, anti-Cox IV antibody (Cell Signaling Technology, Beverly, MA), anti-Bak antibody (Millipore, Billerica, MA), and anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO).
Isolation of Mitochondria-Rich and Cytosolic Fraction
After liver tissue was homogenized using isolation buffer (225 mM mannitol, 75 mM sucrose, 0.1 mM ethylene glycol tetraacetic acid, 1 mg/mL fatty acid–free bovine serum albumin, 1 × protein inhibitor cocktail, 10 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid–potassium hydroxide, pH 7.4), the lysate was centrifuged at 600g for 10 minutes, and the supernatant was centrifuged at 15,000g for 10 minutes. The pellet was regarded as a mitochondria-rich fraction and the supernatant as a cytosolic fraction.
Immunoprecipitation of Bcl-xL
Approximately 30 mg liver tissue was lysed with a TNE buffer (1% Nonidet P-40, 1 mM ethylenediaminetetra-acetic acid, 1 × protein inhibitor cocktail, 0.15 M NaCl, 10 mM Tris-HCl, pH 7.8). Equal amounts of protein samples were rotated with protein G sepharose (GE Healthcare, Tokyo, Japan) and anti-Bcl-xL antibody (Abcam, Cambridge, MA) overnight at 4°C. After centrifugation, the pellet was collected as the immunoprecipitate protein.
Incubation of tBid or Bid for Immunoprecipitation
Liver tissue (90 mg) was lysed with 800μL lysis buffer (2 mM ethylenediaminetetra-acetic acid, 10 mM ethylene glycol tetra-acetic acid, 50 mM NaF, 5 mM Na2P4O7, 10 mM β-glycerophosphate, 0.1% 2-mercaptoethanol, 1% Triton X, 1 × protein inhibitor cocktail, 50 mM Tris-HCl, pH 7.5). Equal volumes of protein samples were incubated with or without recombinant mouse tBid or full-length Bid (R&D Systems, Minneapolis, MN).
Analysis of Cytochrome C Release
The mitochondria-rich fraction was diluted in a mitochondria dilution buffer (395 mM sucrose, 0.1 mM ethylene glycol tetraacetic acid, 10 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid–potassium hydroxide, pH 7.4). The diluted mitochondria were incubated with recombinant mouse tBid or full-length Bid diluted with a reaction buffer (125 mM KCl, 0.5 mM MgCl2, 3.0 mM succinic acid, 3.0 mM glutamic acid, 10 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid–potassium hydroxide, 1 × protein inhibitor cocktail, 2.5 mM ethylenediaminetetra-acetic acid and BOC-Asp (OMe) CH2F 20 μM, pH 7.4) for 30 minutes at 37°C. The levels of cytochrome c in the buffer were determined using an enzyme-linked immunosorbent assay kit (R&D Systems). The maximum or spontaneous release of cytochrome c was defined as the level of samples incubated with 0.1% Triton X-100 or medium alone, respectively. The percentage release of cytochrome c was calculated using the following formula: % release = (experimental release − spontaneous release) × 100/(maximum release − spontaneous release).
ABT-737 Injection Study
ABT-737 was provided by Abbott Laboratories (Abbott, Park, IL). ABT-737 was dissolved with a mixture of 30% propylene glycol, 5% Tween 80, and 65% D5W (5% dextrose in water), final pH 4 to 5. Mice were given a single intraperitoneal injection of ABT-737 at 100 mg/kg and sacrificed 16 hours later. Platelets were counted using an automated cell counter (Sysmex, Kobe, Japan).
Statistical Analysis
Data are presented as mean ± standard deviation. Multiple comparisons of TUNEL-positive cells were performed by analysis of variance followed by Fisher’s post hoc correction. The other multiple comparisons were performed by analysis of variance followed by Scheffe post hoc correction. P < 0.05 was considered statistically significant.
Results
Hepatocyte Apoptosis Caused by Bcl-xL Deficiency Is Completely Lost with Bid-Deficient Background
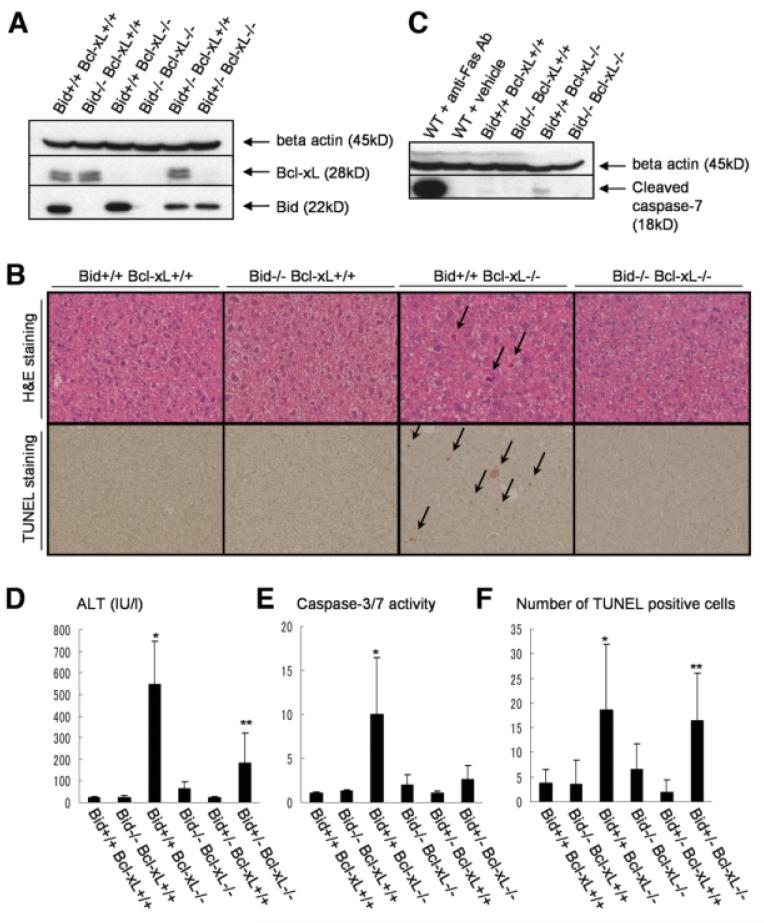
To examine the possibility of whether Bid is involved in apoptosis caused by Bcl-xL deficiency, hepatocyte-specific Bcl-xL KO mice were crossed with traditional Bid KO mice. After mating bid+/− bcl-xflox/flox AlbCre mice with bid+/− bcl-xflox/flox mice, western blot analysis confirmed lack of Bcl-xL and Bid in the liver of Bcl-xL KO mice and Bid KO mice, respectively, and intermediate expression of Bid in the Bid+/− liver (Fig. 1A). Consistent with our previous findings,8 Bcl-xL KO mice (bid+/+ bcl-xflox/flox AlbCre) produced spontaneous hepatocyte apoptosis (Fig. 1B), which was associated with caspase-7 activation in the liver (Fig. 1C). Serum ALT levels (Fig. 1D), caspase-3/7 activity (Fig. 1E), and the frequency of TUNEL-positive hepatocytes (Fig. 1F) were significantly higher in Bcl-xL KO mice than in wild-type mice (bid+/+ bcl-xflox/flox). Bid KO mice (bid−/− bcl-xflox/flox) did not produce any liver phenotypes under physiological conditions, in agreement with a previous report.7 This was further confirmed by our additional analysis on Bid KO mice and control littermates, which showed no difference in serum ALT levels (Supporting Fig. 1A), caspase-3/7 activity (Supporting Fig. 1B), and the ratios of liver weight to body weight (Supporting Fig. 1C). Of importance is the finding that serum ALT levels were reduced to the normal levels in Bcl-xL/Bid double-KO mice (bid−/− bcl-xflox/flox AlbCre). Bcl-xL KO with Bid heterozygosity (bid+/− bcl-xflox/flox AlbCre) displayed intermediate ALT levels between Bcl-xL KO mice and double-KO mice. In agreement with this observation, the number of TUNEL-positive hepatocytes in Bcl-xL/Bid double-KO mice reached background levels. In addition, the levels of caspase-3/7 activity in serum were also normalized in Bcl-xL/Bid double-KO mice. Taken together, these observations indicated that apoptosis caused by Bcl-xL deficiency is completely dependent on the BH3-only protein Bid. Bid is activated by tumor necrosis factor (TNF) receptor,15 and TNF-α, which is a ligand of TNF receptor, is produced by Myd88 signal pathway.16 To examine the possibility of involvement of Myd88 or TNF-α in this apoptosis, we generated Myd88 Bcl-xL double-KO mice by crossing myd88−/− mice with bcl-xflox/flox AlbCre mice and administered neutralizing anti–TNF-α antibody into Bcl-xL KO mice. Hepatocyte apoptosis caused by Bcl-xL deficiency was not ameliorated with Myd88 KO background or by administration of anti-TNF-α antibody (Supporting Fig. 2A, B).
Fig. 1.
Bcl-xL/Bid double-KO mice. Offspring from mating bid+/− bcl-xflox/flox AlbCre mice and bid+/− bcl-xflox/flox mice were sacrificed at 6 weeks after birth. Bcl-xL+/+ stands for bcl-xflox/flox without AlbCre, and Bcl-xL−/− stands for bcl-xflox/flox with AlbCre. (A) Western blot of whole liver lysate for the expression of Bcl-xL and Bid. (B) Representative pictures of liver histology stained with hematoxylin-eosin and TUNEL. Arrows indicate typical apoptotic cells. (C) Western blot of whole liver lysate for the expression of cleaved caspase-7. Live lysates from wild-type mice 3 hours after intraperitoneal injection of 30 μg anti-Fas antibody (clone Jo2) or vehicle were included as a positive and a negative control, respectively. (D) Serum ALT levels. N = 14 mice per group. *P < 0.05 versus the other five groups; **P < 0.05 versus the other four groups except Bid−/− Bcl-xL−/− group. (E) Serum caspase-3/7 activity. N = 13 mice per group. *P < 0.05 versus the other five groups. (F) Statistics of TUNEL-positive cells. N = 6 mice per group. * and **P < 0.05 versus all of Bcl-xL +/+ groups and Bid−/− Bcl-xL−/− group.
Hepatocyte Apoptosis Caused by Bcl-xL Deficiency Requires Both Bak and Bax
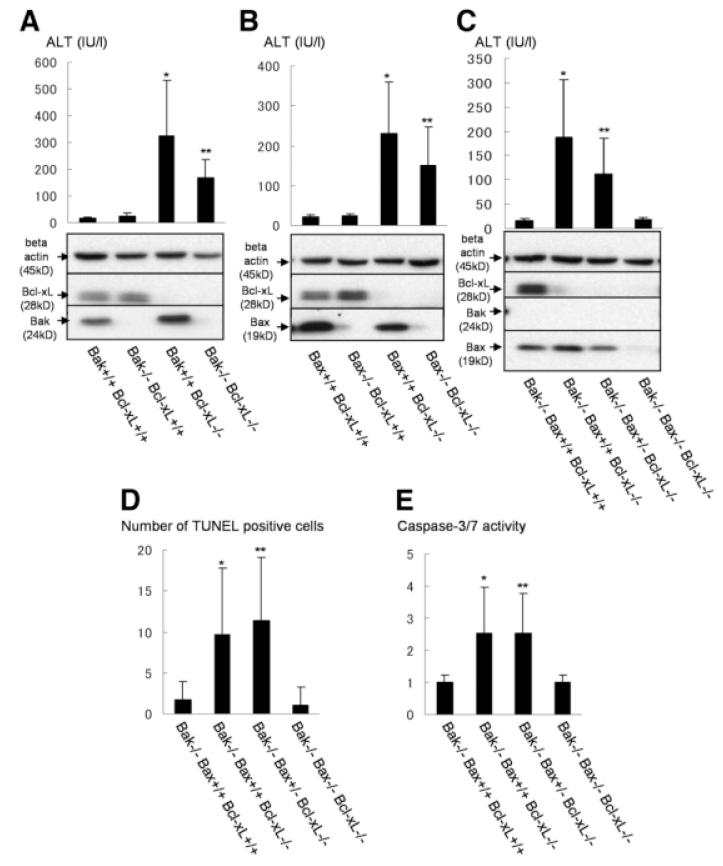
To depict the precise relationships among core Bcl-2 family proteins in regulating liver homeostasis, hepatocyte-specific Bcl-xL–deficient mice were crossed with traditional Bak or Bax KO mice. The levels of serum ALT were slightly decreased with a Bak KO background (bak−/− bcl-xflox/flox AlbCre), whereas they did not change with a Bax KO background (bax−/− bcl-xflox/flox AlbCre) (Fig. 2A, B). To examine the contribution of both Bax and Bak, Bcl-xL KO mice were crossed with conditional Bak/Bax KO mice. The levels of serum ALT were completely normalized in Bak/Bax KO background (bak−/− baxflox/flox bcl-xflox/flox AlbCre) (Fig. 2C). Hepatocyte apoptosis determined by TUNEL staining of liver sections and caspase activation determined by caspase-3/7 activity in serum also returned to background levels (Fig. 2D, E). These observations clearly indicated that apoptosis caused by Bcl-xL deficiency was generated through the Bak/Bax-dependent mitochondrial cell death pathway. To clarify the background levels of hepatocyte apoptosis, we also analyzed the liver apoptosis in bak−/− and bak−/− baxflox/flox AlbCre mice. Similarly, in bid−/− mice, there was no difference between two groups in serum ALT levels (Supporting Fig. 3A), caspase-3/7 activity (Supporting Fig. 3B), and the ratios of liver weight to body weight (Supporting Fig. 3C), which suggests that healthy hepatocytes in wild-type mice are completely protected from Bid or Bak/Bax-mediated apoptosis by Bcl-xL.
Fig. 2.
Bcl-xL KO mice with Bak or Bax KO background. (A) Offspring from mating bak+/− bcl-xflox/flox AlbCre mice and bak+/− bcl-xflox/flox mice were sacrificed at 6 weeks after birth. Serum ALT levels and western blot of whole liver lysate for the expression of Bcl-xL and Bak are shown. N = 14 mice per group. * and **P < 0.05 versus the other three groups. (B) Offspring from mating bax+/− bcl-xflox/flox AlbCre mice and bax+/− bcl-xflox/flox mice were sacrificed at 6 weeks after birth. Serum ALT levels and western blot of whole liver lysate for the expression of Bcl-xL and Bax are shown. N = 15 mice per group. * and **P < 0.05 versus the other two Bcl-xL+/+ groups. (C, D, and E) Offspring from mating bak−/− baxflox/+ bcl-xflox/flox AlbCre mice and bak−/− baxflox/+ bcl-xflox/flox mice were sacrificed at 6 weeks after birth. Bax+/+ stands for −baxflox/flox without AlbCre, and Bax−/− stands for baxflox/flox with AlbCre. N = 8 or 10 mice per group. Serum ALT levels and western blot of whole liver lysate for the expression of Bcl-xL, Bak, and Bax are shown (C). *P < 0.05 versus Bak−/− Bax+/+ Bcl-xL+/+ and Bak−/− Bax−/− Bcl-xL−/− groups; **P < 0.05 versus Bak−/− Bax−/− Bcl-xL−/− group. Statistics of TUNEL-positive cells (D). * and **P < 0.05 versus Bak−/− Bax+/+ Bcl-xL+/+ and Bak−/− Bax−/− Bcl-xL−/− groups. Serum caspase-3/7 activity (E). * and **P < 0.05 versus Bak−/− Bax+/+ Bcl-xL+/+ and Bak−/− Bax−/− Bcl-xL−/− groups.
Bcl-xL Interacts with Cytosolic Bax and Mitochondrial Bak in the Liver
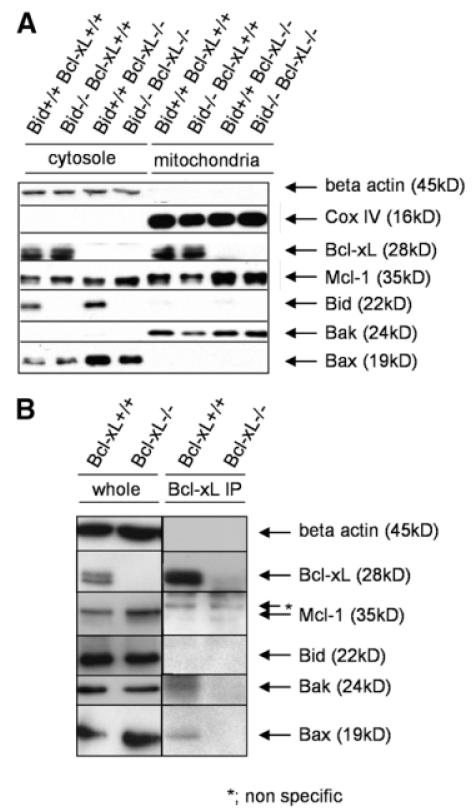
To examine the expression of a variety of Bcl-2–related molecules in the liver, cytosolic and mitochondrial fractions from liver lysate were subjected to western blot analysis (Fig. 3A). Anti-apoptotic Bcl-2 proteins, Bcl-xL and Mcl-1, were expressed at both the mitochondria and the cytosol. In contrast, Bak and Bax were exclusively expressed at the mitochondria and the cytosol, respectively. Full-length Bid was expressed mainly in the cytosol. To examine whether Bcl-xL physically interacts with those Bcl-2–related proteins, liver lysate was immunoprecipitated with Bcl-xL and identified using corresponding antibodies (Fig. 3B). At least a part of Bcl-xL was bound to Bak and Bax, but not to Mcl-1 or full-length Bid.
Fig. 3.
Expression of Bcl-2-related molecules in the liver and their association with Bcl-xL. Bcl-xL+/+ stands for bcl-xflox/flox without AlbCre, and Bcl-xL−/− stands for bcl-xflox/flox with AlbCre. (A) Western blot after cellular fractionations of the liver lysate. Loading amounts of cytosolic and mitochondrial fractions were adjusted to be equivalent for the starting liver samples. (B) Western blot after anti-Bcl-xL immunoprecipitation. Whole cellular lysate and immunoprecipitates with anti-Bcl-xL were verified with the indicated antibodies. Samples from Bcl-xL−/− mice were included as a negative control.
tBid, But Not Full-Length Bid, Displaces Bak and Bax from Bcl-xL by Binding to Bcl-xL
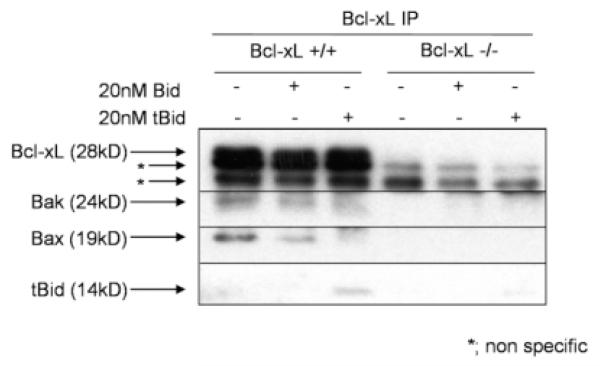
Bcl-2–like molecules have been shown to be capable of binding Bak or Bax, and through this interaction, to neutralize each activity.17 Conversely, other research showed that Bcl-xL does not have to bind Bax-like molecules to protect against cell death.18 To examine the impact of tBid on the association between Bcl-xL and Bak or Bax, we added tBid to the liver lysate and examined the interaction of each Bcl-2–related protein with Bcl-xL by immunoprecipitation. Addition of 500 nM tBid abolished the association between Bcl-xL and Bak or Bax (Supporting Fig. 4). Simultaneously, Bcl-xL binding of tBid was observed. Addition of 20 nM tBid also abolished, if not completely, the association between Bcl-xL and Bak or Bax (Fig. 4). In contrast, adding the same concentration of full-length Bid had little effect on Bcl-xL binding of Bak or Bax (Fig. 4). These results indicated that tBid can bind to Bcl-xL and suggest that tBid binding of Bcl-xL unleashes Bak or Bax from Bcl-xL.
Fig. 4.
tBid binds to Bcl-xL and displaces Bak or Bax from Bcl-xL. Liver lysate from bcl-xflox/flox without AlbCre (Bcl-xL+/+) and bcl-xflox/flox with AlbCre (Bcl-xL−/−) were incubated with or without 20 nM recombinant tBid or recombinant full-length Bid at 37°C for 20 minutes. After immunoprecipitation with Bcl-xL, immunoprecipitates are verified with indicated antibodies. Immunoprecipitated lysate from Bcl-xL−/− mice was loaded as a negative control.
A Small But Significant Level of tBid Is Detected in the Healthy Liver
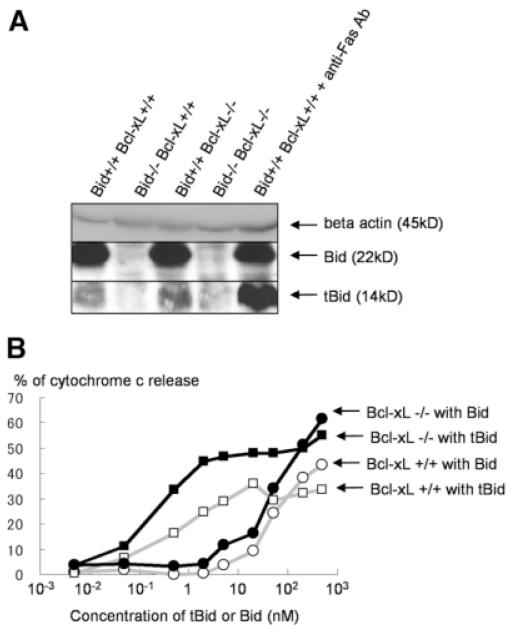
Genetic evidence that Bid is required for Bak/Bax-dependent apoptosis caused by Bcl-xL deficiency and biochemical evidence that full-length Bid is inactive for displacing Bak or Bax from Bcl-xL together suggest that tBid is produced in wild-type liver. To confirm this, we performed western blot analysis using antibody that can detect tBid (Fig. 5A). Liver lysate from Bid KO mice served as a negative control, whereas that from wild-type mice injected with anti-Fas antibody served as a positive control. A significant level of tBid was detected in wild-type liver, although the amount was smaller than in Fas-stimulated mice, which displayed massive live cell apoptosis.
Fig. 5.
A small amount of tBid is expressed in wild-type liver and is sufficient for producing cytochrome c release from Bcl-xL–deficient mitochondria. (A) Western blot of liver lysate for Bid and tBid expression. Lysate from wild-type (Bid+/+ Bcl-xL+/+) mice 1 hour after intravenous injection of 10 μg anti-Fas antibody (clone Jo2) and from Bid−/− mice were included as a positive and a negative control of tBid, respectively. (B) Mitochondrial release of cytochrome c to tBid. Mitochondria were isolated from Bcl-xL-deficient or wild-type liver and incubated with recombinant tBid or recombinant full-length Bid at various concentrations for 30 minutes. Similar results were obtained in three times repeated experiments.
Bcl-xL–Deficient Mitochondria Are Susceptible to a Trace Amount of tBid
To examine the impact of a small amount of tBid on Bcl-xL–deficient mitochondria, tBid or full-length Bid at various concentrations was incubated with mitochondria isolated from Bcl-xL–deficient liver or wild-type liver (Fig. 5B). In agreement with previous reports,19 wild-type mitochondria efficiently released cytochrome c on exposure to tBid. Full-length Bid was far less effective at releasing cytochrome c. Importantly, Bcl-xL–deficient mitochondria were capable of releasing cytochrome c on exposure to a smaller amount of tBid than wild-type mitochondria. This agrees with the in vivo findings that Bcl-xL–deficient hepatocytes, but not wild-type hepatocytes, underwent apoptosis with a trace amount of tBid.
Administration of ABT-737 Produces ALT Elevation in Wild-Type Mice But to a Lesser Extent in Bid KO Mice
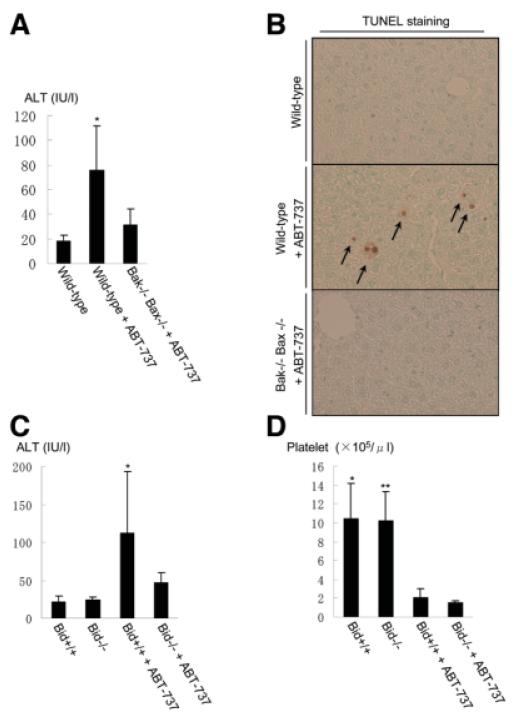
Bcl-2–like molecules have been receiving attention as a target for inducing apoptosis, especially in cancer cells.20 A variety of BH3 mimetics that interact with the hydrophobic groove of anti-apoptotic Bcl-2 proteins has been developed. They inhibit binding of anti-apoptotic Bcl-2–like molecules with BH3-only proteins and presumably with Bak and Bax. ABT-737, a prototype of this class of agents, was designed to mimic the BH3-only protein Bad and can inhibit the function of Bcl-2, Bcl-xL, or Bcl-w but not that of Mcl-1.21 Our data on Bcl-xL KO mice raised the possibility that pharmacological inhibition of Bcl-xL may cause hepatocyte apoptosis. To examine this possibility, we injected ABT-737 and examined the liver injury. As expected, the levels of ALT were clearly elevated in wild-type mice (Fig. 6A). TUNEL staining of the liver section showed apoptosis in hepatocytes scattered in the liver lobule (Fig. 6B). Importantly, no significant elevation of serum ALT levels was observed with a Bak/Bax double-KO background. The data indicated that genetic and pharmacological ablation of Bcl-xL led to a similar apoptosis phenotype in the liver.
Fig. 6.
ABT-737 administration in wild-type, Bak/Bax double-KO, and Bid KO mice. (A and B) Wild-type mice or hepatocyte-specific Bak/Bax double-KO mice were challenged with intraperitoneal injection of ABT-737 at 100 mg/kg or vehicle alone and sacrificed 16 hours later. Serum ALT levels (A) and representative pictures of TUNEL staining in the liver (B) are shown. N = 5 or more than 5 mice per group. *P < 0.05 versus the other two groups. (C and D) Wild-type mice or Bid KO mice were challenged with intraperitoneal injection of ABT-737 at 100 mg/kg or vehicle alone and sacrificed 16 hours later. Serum ALT levels (C) and circulating platelet counts (D) were determined. N = 5 or more than 5 mice per group. *P < 0.05 versus the other three groups for (C); * and **P < 0.05 versus the other two groups, with ABT-737 for (D).
To examine the impact of Bid in ABT-737–induced hepatocyte apoptosis, ABT-737 was administered to wild-type mice and Bid KO mice. Elevation of serum ALT levels was ameliorated with a Bid KO background (Fig. 6C). It has been well established that administration of ABT-737 led to acute thrombocytopenia.22 This was explained by the fact that Bcl-xL is a critical apoptosis antagonist in platelets.10 In our experiment, the counts of circulating platelets declined significantly in the wild-type mice (Fig. 6D), which is in the agreement with previous studies.10 Interestingly, a similar degree of thrombocytopenia was observed even in Bid KO mice, suggesting that Bid does not play a significant role in regulating platelet homeostasis, unlike in hepatocytes. The data imply that the impact of Bid in the Bcl-2 network in healthy cells is cell-type specific.
Discussion
One of the important findings of the current study is that the BH3-only protein Bid is an essential molecule for apoptosis of differentiated hepatocytes caused by Bcl-xL deficiency. This is surprising, because differentiated hepatocytes are generally considered to be quiescent cells. Organ homeostasis may be ensured in two ways: one is through turnover of cells, and the other is by the quiescence of matured cells. Typical examples for the former are hematopoietic organs, intestine and skin, whereas those for the latter are a variety of solid organs, such as the liver, lung, pancreas, heart, and brain. Because hematopoietic cells die at particular time points to maintain host homeostasis, it would not be surprising that their life span may be controlled by a variety of death signals. Indeed, Bim KO mice have excess hematopoietic cells, particularly lymphocytes, suggesting that Bim strictly controls homeostasis of hematopoietic cells.23 In contrast, healthy cells in the solid organs are usually considered to not suffer from apoptotic stimuli. Although interaction between core Bcl-2 proteins and BH3-only proteins is important for understanding apoptosis regulation, little work has been done by generating mice simultaneously deficient in molecules of both groups. To the best of our knowledge, the only example clearly using this approach is a study on Bim/Bcl-2 double-KO mice that showed that growth retardation, skin abnormality, and lymphoid cell reduction found in Bcl-2 KO mice were ameliorated with a Bim-deficient background.24 This suggested that lymphoid cells constitutively sense Bim-mediated killing signals, and, without Bcl-2, decrease in number. The current study is the first demonstration that parenchymal cells in a solid organ such as differentiated hepatocytes also suffer from Bid-mediated BH3 stress.
Bid is ubiquitously expressed in many cell types. Generally, Bid is inactive for death induction and is activated on proteolytic cleavage by caspase-8 or other proteases. In the current study, we found that not only full-length Bid but also tBid could be detected in wild-type liver. Administration of tBid, but not that of full-length Bid, at 20 nM in wild-type liver lysate or mitochondria was sufficient for unleashing Bak or Bax from Bcl-xL and releasing cytochrome c. Conversely, a lesser amount of tBid (for example, at 2 nM) was sufficient for inducing cytochrome c release from Bcl-xL–deficient mitochondria. These results are consistent with the idea that a small amount of tBid produced in the liver could activate cytochrome c release and apoptosis in hepatocytes of the Bcl-xL–deficient mice. What mechanisms are involved in the production of tBid from full-length Bid in the healthy liver is not known yet. Our results suggest that Myd88 and TNF-α may not be involved in the activation of tBid under physiological conditions. However, other ligation of death receptors such as Fas, and TNF-related apoptosis-inducing ligand receptor, can cause caspase-8 activation followed by Bid cleavage.15,25 Bile salts, which are consistently produced in and secreted from hepatocytes, are capable of inducing hepatocyte apoptosis through Fas activation.26 Natural killer cells are predominant lymphocytes accumulating in the liver and constitutively express TNF-related apoptosis-inducing ligand.27 Further study is needed to examine what kinds of stresses activate the Bid pathway in a physiological setting.
Adult differentiated hepatocytes express at least two anti-apoptotic Bcl-2 proteins, Bcl-xL and Mcl-1, but not prototype Bcl-2.8 Recently, Vick et al.28 reported that hepatocyte-specific Mcl-1 KO mice developed naturally occurring apoptosis in hepatocytes. We also independently generated hepatocyte-specific Mcl-1 KO mice and obtained an apoptosis phenotype that could not be distinguished from that of hepatocyte-specific Bcl-xL KO mice.29 Thus, Mcl-1, like Bcl-xL, plays a critical role in maintaining integrity of differentiated hepatocytes. There are two major models regarding how BH3-only proteins mediate Bak/Bax-dependent apoptosis: a direct model and an indirect model.30 From the viewpoint of the indirect model, our data would mean a small amount of tBid is sequestered by Bcl-xL and Mcl-1 and, without Bcl-xL, is sufficient for neutralizing Mcl-1 to promote apoptosis. Conversely, from the viewpoint of the direct model, both Bcl-xL and Mcl-1 are needed to completely sequester a small amount of tBid, and without Bcl-xL, unleashed tBid would directly activate Bak and Bax. In the current study, we observed that tBid when administered in liver lysate could bind to Bcl-xL. This observation seems to agree with the indirect model, although we could not exclude the possibility of the direct model. Further study will be needed by developing Bid/Bcl-xL/Mcl-1 KO mice to examine the underlying mechanisms of how activated Bid regulates the mitochondrial pathway of apoptosis in the liver.
Malignant tumors frequently overexpress one or more members of the anti-apoptotic Bcl-2 family, which confers the resistance of tumor cells to apoptosis.31,32 Recently, small molecules targeting specific anti-apoptotic Bcl-2 family proteins have been developed for treatment of cancer therapy.33,34 The underlying concept of this strategy is the difference in addiction to anti-apoptotic Bcl-2 family proteins between normal cells and transformed cells. In general, normal cells are not considered to suffer from apoptotic stimuli or to have activated BH3-only proteins. In contrast, transformed cells suffer from a variety of apoptotic stimuli such as genotoxic p53 activation and environmental stresses, and possess activated BH3-only molecules. If a single anti-apoptotic Bcl-2 protein is neutralized by a small molecule, it could release BH3-only molecules, which then neutralize other anti-apoptotic Bcl-2 proteins or directly activate Bax-like molecules, leading to cell death. However, the current study clearly indicated that normal hepatocytes could be under activation of Bid, raising concern that hepatocyte injury may be produced if Bcl-xL function is completely knocked down. Indeed, we have shown that administration of a high dose of ABT-737, which is an antagonist for Bcl-xL/Bcl-2, not for Mcl-1,21 induced Bak/Bax-dependent hepatocyte apoptosis in wild-type mice but to a lesser extent in Bid KO mice. Therefore, special caution should be paid to hepatotoxicity when systemically administering a high dose of Bcl-xL–targeting molecules, because hepatocytes are suffering from Bid-mediated stresses.
In conclusion, we have demonstrated here that the BH3-only protein Bid is activated and antagonized by anti-apoptotic Bcl-2 family proteins under physiological conditions. BH3 stress or Bcl-2 addiction is not a unique characteristic of tumor cells. Even in healthy cells, cellular integrity is not controlled by a simple rheostat between Bax-like molecules and Bcl-2–like molecules. The current study reveals a previously unrecognized complicated network of Bcl-2 family proteins controlling the integrity of healthy cells. Dissection of the Bcl-2 network will be important for further understanding of liver pathophysiology.
Supplementary Material
Acknowledgment
The authors thank Abbott Laboratories for providing ABT-737.
Supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to T. Takehara).
Abbreviations
- ALT
alanine aminotransferase
- BH3
Bcl-2 homology domain 3
- KO
knockout
- tBid
truncated form of Bid
- TNF
tumor necrosis factor
- TUNEL
terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 2.Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- 3.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 4.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin XM. Bid, a BH3-only multi-functional molecule, is at the cross road of life and death. Gene. 2006;369:7–19. doi: 10.1016/j.gene.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 8.Takehara T, Tatsumi T, Suzuki T, Rucker EB, III, Hennighausen L, Jinushi M, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Shindler KS, Latham CB, Roth KA. Bax deficiency prevents the increased cell death of immature neurons in bcl-x-deficient mice. J Neurosci. 1997;17:3112–3119. doi: 10.1523/JNEUROSCI.17-09-03112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason KD, Carpinelli MR, Fletcher JI, Collonge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 11.Wagner KU, Claudio E, Rucker EB, 3rd, Riedlinger G, Broussard C, Schwartzberg PL, et al. Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development. 2000;127:4949–4958. doi: 10.1242/dev.127.22.4949. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ. Essential role of BAX, BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci U S A. 2005;102:11272–11277. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takehara T, Hayashi N, Tatsumi T, Kanto T, Mita E, Sasaki Y, et al. Interleukin 1beta protects mice from Fas-mediated hepatocyte apoptosis and death. Gastroenterology. 1999;117:661–668. doi: 10.1016/s0016-5085(99)70460-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 15.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 16.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. HEPATOLOGY. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 17.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1129–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Zhu Y, Dai S, White J, Peyerl F, Kappler JW, et al. Bcl-xl does not have to bind Bax to protect T cells from death. J Exp Med. 2006;203:2953–2961. doi: 10.1084/jem.20061151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TH, Zhao Y, Barber MJ, Kuharsky DK, Yin XM. Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore and Bax. J Biol Chem. 2000;275:39474–39481. doi: 10.1074/jbc.M003370200. [DOI] [PubMed] [Google Scholar]
- 20.Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 23.Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 24.Bouillet P, Cory S, Zhang LC, Strasser A, Adams JM. Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3-only antagonist Bim. Dev Cell. 2001;1:645–653. doi: 10.1016/s1534-5807(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 25.Tada-Yamada H, Oikawa S, Uchida A, Kawanishi S. TRAIL causes cleavage of bid by caspase-8 and loss of mitochondrial membrane potential resulting in apoptosis in BJAB cells. Biochem Biophys Res Commun. 1999;265:130–133. doi: 10.1006/bbrc.1999.1641. [DOI] [PubMed] [Google Scholar]
- 26.Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, et al. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137–145. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 28.Vick B, Weber A, Urbanik T, Maass T, Teufel A, Krammer PH, et al. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. HEPATOLOGY. 2009;49:627–636. doi: 10.1002/hep.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hikita H, Takehara T, Shimizu S, Kodama T, Li W, Miyagi T, et al. Mcl-1 and Bcl-xL cooperatively maintain integrity of hepatocytes in developing and adult murine liver. HEPATOLOGY. 2009 doi: 10.1002/hep.23126. doi:10.1002/hep.23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takehara T, Liu X, Fujimoto J, Friedman SL, Takahashi H. Expression and role of Bcl-xL in human hepatocellular carcinomas. HEPATOLOGY. 2001;34:55–61. doi: 10.1053/jhep.2001.25387. [DOI] [PubMed] [Google Scholar]
- 32.Takehara T, Takahashi H. Suppression of Bcl-xL deamidation in human hepatocellular carcinomas. Cancer Res. 2003;63:3054–3057. [PubMed] [Google Scholar]
- 33.Labi V, Grespi F, Baumgartner F, Villunger A. Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy? Cell Death Differ. 2008;15:977–987. doi: 10.1038/cdd.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mott JL, Gores GJ. Piercing the armor of hepatobiliary cancer: Bcl-2 homology domain 3 (BH3) mimetics and cell death. HEPATOLOGY. 2007;46:906–911. doi: 10.1002/hep.21812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.