Vaccines are one of our most successful tools for protecting the public's health. It seems simple: a pharmaceutical company develops a new vaccine, the U.S. Food and Drug Administration (FDA) licenses it, health-care providers give it to their patients, and we see disease disappear. But vaccination in the United States is much more complex and only made possible through a robust public-private partnership that begins with the development of the vaccine and continues long after it is used routinely. Along every step of the way, public health—at the national, state, and local levels—plays a fundamental role. The success of the pneumococcal conjugate vaccine (PCV) in preventing suffering, disability, and death is one example that illustrates the essential role of our nation's public health systems and workforce in protecting us from vaccine-preventable diseases (VPDs).
Streptococcus pneumoniae (pneumococcus) is a major cause of invasive disease, including meningitis, pneumonia, and bacteremia. In the absence of a pediatric vaccine, pneumococcus was a significant public health concern, causing approximately 63,000 cases of invasive pneumococcal disease and 6,100 deaths in the U.S. each year.1 Young children and older adults are especially vulnerable, and many children who develop pneumococcal meningitis have long-term complications such as deafness or seizures.
In 2000, a pediatric heptavalent PCV (PCV7) was licensed for use in the U.S. There are more than 90 strains of pneumococcal bacteria, and PCV7 provided protection against seven of them. Before PCV7 was introduced, these seven strains caused more than 80% of severe pneumococcal infections among children. 2
Most health-care providers in the U.S. look to public health for guidance in the use of vaccines. After a careful review of the evidence, including data about the burden of disease caused by pneumococcus, the effectiveness and safety of the vaccine, and the feasibility of incorporating it into the immunization program, the Advisory Committee on Immunization Practices (ACIP) made a recommendation for the routine use of PCV7 among children, 2 and the Centers for Disease Control and Prevention (CDC) incorporated it into the immunization schedule, which gives doctors specific recommendations for the use of vaccines across the life span.
Public health helps inform people about the vaccines they need and the risks and benefits of receiving vaccines. Parents consider many factors when deciding to vaccinate their children. Using science-based strategies, public health provided parents with information about the risks of pneumococcal disease and the benefits of the new vaccine. State and local public health experts worked with the health-care providers in their communities to make sure they had the tools they need to help their patients make informed decisions, from how to hold a child during a vaccination to responding to questions about the need for, and the safety of, the vaccine.
Vaccine supply interruptions are managed by public health to ensure that those most at risk can be protected first. Following the 2000 recommendation, the supply of PCV7 vaccine did not keep up with demand. 3 Because some children are at higher risk of disease and/or its complications than others, public health was called upon to help conserve vaccine and direct it to protect those children at highest risk. CDC issued interim vaccination recommendations to withhold vaccine from healthy children aged 2 years and older, and to defer some doses for healthy children who were younger than 2 years of age.4 Public health experts and systems at the state and local levels provided strategies for implementing the vaccination guidelines, such as using immunization registries to identify those children who need to be vaccinated and issuing reminders to parents to get them vaccinated.
Public health continued to monitor the supply of PCV7 and, in 2004, when the supply stabilized, public health terminated the interim recommendations so that the vaccine could be used according to the 2000 recommendation.5
Public health evaluates our national vaccine programs and policies. Post-licensure evaluation of vaccine performance is necessary to ensure that vaccines have the intended public health impact. In the first five years that PCV7 was used, the incidence of invasive pneumococcal disease in children fell by 94%.6 In addition to the protection provided to vaccinated children, the work done by our public health systems and experts demonstrated that the vaccine also significantly reduced transmission of pneumococcus to unvaccinated people, including adults. Public health data from Active Bacterial Core surveillance (ABCs) showed a more than 90% decrease in the rate of invasive pneumococcal disease caused by the vaccine serotype disease among people aged 5 years and older, with a dramatic decrease in disease among adults 65 years of age and older.6 In addition to impressive reductions in disease, economic analyses conducted by CDC showed that every $1 invested in PCV7 resulted in a cost savings of $1.50.7 This type of information informs our national strategies for preventing invasive pneumococcal disease, including the best investment of health-care resources.
Public health data are critical to the development of new and better vaccines. Over time, public health's pneumococcal surveillance showed that while there were significant reductions in disease caused by the pneumococcus serotypes prevented by PCV7, there were increases in invasive disease caused by non-vaccine serotypes. This finding prompted vaccine manufacturers to develop a new vaccine and, in 2010, the FDA licensed PCV13, which provides protection against six additional serotypes. In particular, PCV13 protects against serotype 19A, which was not covered in PCV7 and which had become the most common pneumococcal serotype. Another important benefit of PCV13 is that S. pneumoniae of serotype 19A is often resistant to antibiotics.
Following the recommendation for routine use of PCV13,8 public health systems and experts at the national, state, and local levels have worked with health-care providers to incorporate this new vaccine into the childhood schedule. This change included guidance for transitioning the use of PCV7 to PCV13, and monitoring vaccine coverage and the incidence of pneumococcal disease caused by the 13 strains in the new vaccine.9
A decade after licensure of the first generation of PCV, the story continues. In 2011, PCV13 was licensed for use among adults aged 50 years and older. In June 2012, the ACIP voted to recommend that adults at very high risk due to immunocompromising conditions be vaccinated with PCV13. While PCV13 may prove to be an effective tool for protecting adults from pneumococcal disease, the ACIP is awaiting more data on vaccine efficacy and more information about the indirect protection vaccinating children provides to unvaccinated adults before making a recommendation for routine use of PCV13 in adults.10 Our public health systems and experts will continue to track the use of PCV13 in the U.S., monitor the vaccine's effectiveness and safety, and gauge its impact on pneumococcal disease among all age groups. This information is essential to inform sound national strategies to prevent pneumococcal disease and guide decisions for investing health-care resources.
Public health infrastructure is fundamental to the provision and execution of public health services at all levels. A strong infrastructure provides the capacity to prepare for and respond to both acute (emergency) and chronic (ongoing) threats to the nation's health. Infrastructure is the foundation for planning, delivering, and evaluating public health.
INTRODUCTION
The achievements of our national immunization program are impressive. Today, we have recommendations for the routine use of vaccines to prevent 17 VPDs. We enjoy record-high immunization coverage rates, most VPDs are at or near record lows, and a majority of diseases are showing a 90% or higher decline in reported cases when compared with the pre-vaccine era.11 Immunization continues to be one of the most impactful and cost-effective public health interventions. For each birth cohort vaccinated against 13 diseases in accordance with the routine childhood immunization schedule, $13.6 billion in direct medical costs and 42,000 lives are saved, and 20 million cases of disease are prevented.7
The success of our national childhood immunization program rests on a robust public-private partnership. Public health, with unique roles at the national, tribal, state, and local levels, has built the foundation of an immunization system that ensures that our immunization policies and programs promote equitable access to safe and effective vaccines, regardless of whether or not a vaccine is publicly or privately purchased, particularly for the pediatric population.
Purchasing and administering vaccine is only a small piece of the entire enterprise, however, as illustrated by the aforementioned pneumococcal story. A strong immunization system also requires a stable infrastructure from the federal to the local levels that includes a highly trained immunization workforce, disease surveillance experts and systems, scientific support for developing immunization policies, systems for monitoring and assuring vaccine safety, and mechanisms to monitor vaccine coverage rates.
In the past decade, we have also seen several changes that present both new opportunities and challenges to the immunization system. The number of lifesaving vaccines available has increased dramatically, while the cost of vaccines has also risen. The passage of the Affordable Care Act (ACA)12,13 has the potential to provide unprecedented access to vaccines by increasing the number of people insured and, thus, covered for vaccination, as well as removing copayments for these people, thereby incentivizing administration and receipt of the vaccines. As expanded health coverage is fully realized, greater numbers of children and adults will gain access to vaccines through public and private health insurance. However, as we have learned over the years, insurance coverage alone is not enough to ensure disease control or high vaccination coverage rates. It will be necessary to maintain a strong public health infrastructure to support the U.S. vaccination program. Current vaccine financing strategies, including those offered now by the ACA, do not address the fundamental resource needs to support the immunization infrastructure. With these many changes since the enactment of the Section 317 Immunization Program, the National Vaccine Advisory Committee (NVAC) feels that this is an ideal and indeed critical time to reexamine and strengthen the immunization infrastructure.
THE SECTION 317 IMMUNIZATION PROGRAM
A key resource in support of establishing and maintaining the public health immunization infrastructure is the contribution of the Section 317 Immunization Program.14 The Section 317 Program, administered by CDC, was enacted in 1962 through the Vaccine Assistance Act, or Section 317 of the Public Health Service Act. During its 50-year history, the Section 317 Program has played a critical role in helping to achieve national immunization goals by addressing unmet needs and supporting efforts to plan, develop, and maintain an immunization infrastructure necessary to ensure high vaccination coverage levels and low incidence of VPDs. A profound strength of the program is its flexibility, allowing it to respond efficiently and effectively to state-specific priorities and unexpected outbreaks of disease. Initially designed to purchase polio, diphtheria, pertussis, tetanus, and smallpox vaccines, with measles vaccines added in 1965, the Section 317 Program has evolved with the introduction of new vaccines and immunization recommendations and the changing health-care landscape to become much more than a vaccine purchase program. However, the Section 317 Program is a discretionary program; therefore, funding is set through the annual appropriations process and is not guaranteed from year to year.
As with all discretionary programs, the President submits the budget request each year to Congress for the following fiscal year, as required by the Budget and Accounting Act of 1921.15 The President's budget request outlines the administration's intended spending and is developed through an iterative process in which agencies and operating divisions present proposed funding levels for consideration, with the Office of Management and Budget setting a final spending level that is included in the President's budget request. Ultimately, discretionary spending is determined through negotiations between the House and Senate Appropriations Committees and their various subcommittees. They may or may not use the levels requested in the President's annual budget.
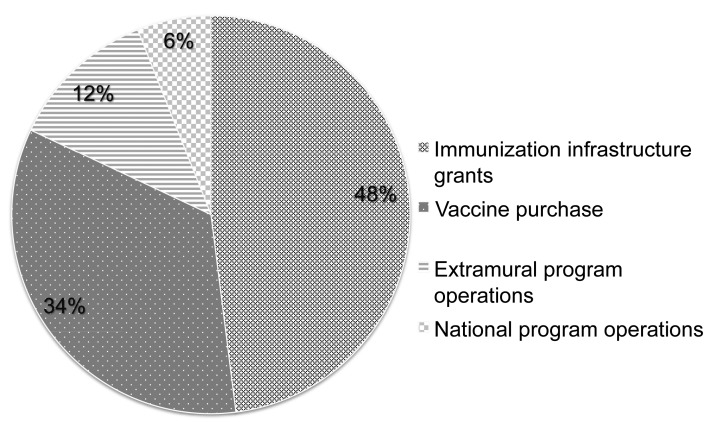
The Section 317 Program helps protect all Americans from VPDs by providing the backbone of the U.S. immunization program—regardless of the payer for the vaccine given—by ensuring that vaccines are accessible, safe, effective, and used most successfully to protect the nation's health. The Section 317 Program provides the majority of federal support for national, state, and local immunization systems and the workforce necessary to implement a comprehensive immunization program (Figure 1) through:
Figure 1.
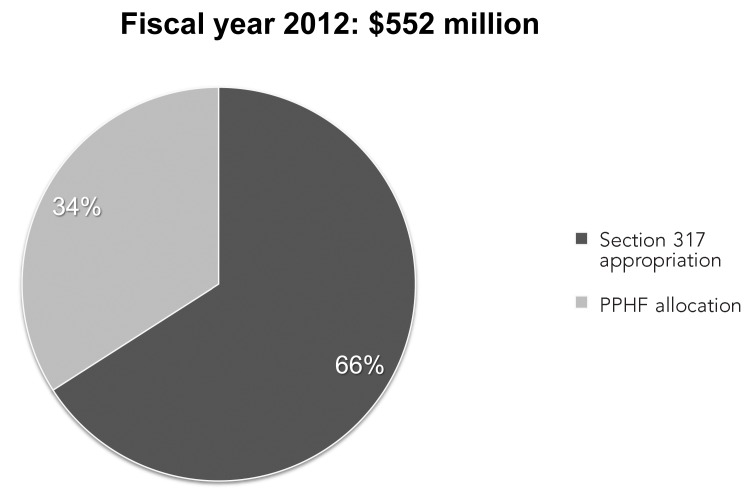
Section 317 Immunization Program, fiscal year 2012: Section 317 appropriation and Prevention and Public Health Funds ($552 million)
Immunization infrastructure grants: funds awarded to 64 immunization programs (all 50 states, the District of Columbia, five large cities, five U.S. territories, and three Pacific freely associated states) to support immunization workforce and systems at the state and local levels to recruit and educate networks of immunization providers, provide continual quality assurance, promote public awareness of new and expanded vaccine recommendations, manage vaccine shortages, and prepare for and respond to vaccine--preventable outbreaks.
Vaccine purchase: federally purchased vaccines allocated to the 64 immunization programs to protect non-Vaccines for Children (VFC) Program-eligible populations with routinely recommended vaccines, and to meet urgent needs such as responding to VPD outbreaks. Unlike VFC Program vaccines, for which there are very specific eligibility criteria, Section 317 vaccines can be used to rapidly protect all members of a community when VPD outbreaks occur.
Extramural program operations: contributes to the systems and workforce that conduct disease surveillance, assess vaccination coverage, perform post-marketing evaluation of vaccine effectiveness and safety, develop and implement immunization information technologies, support centralized vaccine ordering and distribution systems, and create and implement public awareness campaigns and resources, as well as provider education and tools.
National program operations: provides national public health expertise in VPDs that supports national, state, and local vaccination program efforts, including expertise in epidemiology and surveillance, laboratory methods and science, immunology, immunization policy, health communications science, vaccine management, and program implementation.
During the past decade, appropriations to the Section 317 Program have stagnated at levels that are insufficient to maintain this important work. Most recently, as the annual appropriation to the Section 317 Program has eroded, gaps in support for these important activities have been funded through the Prevention and Public Health Fund (PPHF), which was established through the ACA. The PPHF has proven a valuable resource for strengthening immunization systems and workforce at the national, state, and local levels. However, the instability of this funding source poses risks as it funds a larger proportion of the Section 317 Program (Figure 2).
Figure 2.
Federal immunization funding: Section 317 Immunization Program appropriation and Prevention and Public Health Funds
PPHF = Prevention and Public Health Fund
THE NATIONAL VACCINE ADVISORY COMMITTEE
The NVAC is a federal advisory committee, established in 1987, that provides vaccine and immunization advice and policy recommendations to the Director of the National Vaccine Program (NVP). The Assistant Secretary for Health (ASH) has been designated by the Secretary of Health and Human Services (HHS) as the Director of the NVP.14 The NVAC recommends ways to achieve optimal prevention of human infectious diseases through immunization and to achieve optimal prevention against adverse reactions to vaccines. Specifically, the NVAC was chartered, through the Public Health Service Act, with four main responsibilities:
Study and recommend ways to encourage the availability of an adequate supply of safe and effective vaccination products in the U.S.
Recommend research priorities and other measures that should be taken to enhance the safety and efficacy of vaccines.
Advise the ASH on the implementation of the NVP's responsibilities and the National Vaccine Plan, a coordinated, strategic framework established to achieve the vision of the NVP.
Identify the most important areas of government and nongovernment cooperation that should be considered in implementing the NVP's responsibilities and the National Vaccine Plan.16
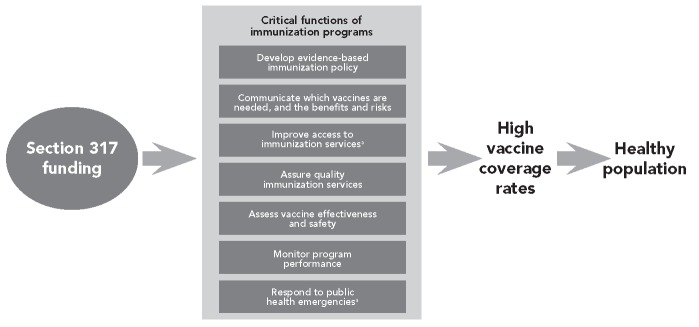
In 2011, the NVAC identified the need to describe the nation's public health immunization infrastructure and to explore policy options to assure the stability of that infrastructure through the Section 317 Program in the changing health-care landscape. Increases in Section 317 funding have been positively and significantly associated with increases in vaccination coverage.17 A Working Group on Immunization Infrastructure was appointed by the chairman of the NVAC to describe critical functions of immunization programs at the national, state, tribal, and local levels, and included representatives from federal agencies, professional organizations, consumer groups, individuals in vaccine research or with manufacturing experience, state and local health departments, and academic centers. This report contains NVAC recommendations based on the Working Group's findings and conclusions. The overall goal of the report is to describe the essential public health functions supported by the Section 317 Program (Figure 3) and to examine and recommend strategies that maintain these functions, while encouraging public health at the national, state, and local levels to adapt and evolve to meet these new challenges and opportunities toward protecting all Americans from VPDs.
Figure 3.
Role of Section 317 Immunization Program funding in preventing infectious disease
aSection 317 funds can be used to purchase vaccine.
DEVELOPING EVIDENCE-BASED IMMUNIZATION POLICY
In the U.S., the ACIP advises CDC on national vaccine policy for preventing infectious diseases in the civilian population. Once adopted by CDC, the Committee's recommendations establish the standard of practice for preventing VPDs and, as of September 2010, determine the vaccines that must be covered without cost to vaccine recipients by private health insurance plans.18
Comprising 15 voting members with expertise in medicine and public health along with a consumer representative, the Committee holds public meetings three times a year to make its vaccine recommendations. The members consider the approved indications for vaccines, principles of equity and social justice, and the evidence base for use of a given vaccine among the U.S. civilian populations. The immunization systems and expertise that are supported by the Section 317 Program make substantial contributions to the evidence base upon which the ACIP deliberates in making its recommendations. They do so by providing data about the burden of disease that can be prevented, about the safety and efficacy of the vaccine, and by supplying economic analyses (including cost-effectiveness data) in addition to information about other factors, such as how an ACIP recommendation can be implemented by the health-care system in conjunction with other recommended vaccines. The Committee's recommendations provide guidance on the population to be vaccinated; the appropriate route, dose, and frequency of vaccine administration; and information about contraindications and precautions, as well as recognized adverse events. Once the Committee's recommendations are accepted by the CDC Director, they are published in the Morbidity and Mortality Weekly Report.
Each year, the individual vaccination recommendations are summarized in immunization schedules for children, adolescents, and adults. The immunization schedule provides a roadmap for health-care providers about all of the vaccines recommended for their patients. To reduce confusion and proliferation of different recommendations, public health collaborates in developing its schedules with the medical associations whose members provide vaccines, including the American Academy of Pediatrics, the American Academy of Family Physicians, the American College of Physicians, and the American College of Obstetricians and Gynecologists.
The ACIP continues to review the safety and effectiveness of vaccines even after the vaccines are recommended, and adjustments may be made as more data become available. New data are reviewed in the context of the risks of adverse effects and the benefits provided by the vaccine. For example, when polio was more prevalent globally, the U.S. primarily used oral polio vaccine (OPV) to protect Americans from the risk of imported cases because it was perceived to be more effective at reducing the transmission of disease, even though it was associated with a risk of vaccine-associated poliomyelitis of one in 750,000 first doses.19 As global polio control improved and the risk of polio importations into the U.S. decreased, the risk-benefit of use of OPV was reevaluated. In 2000, the ACIP recommended that OPV be replaced with the inactivated polio vaccine, which has no risk of vaccine-associated paralytic disease.
Immunization policies are also made at the state and local levels, and many states have immunization advisory committees that provide advice. State and local policies can significantly impact the health of their jurisdictions, and public health systems and expertise supported by the Section 317 Program provide important evidence and data to inform those decisions. State and local policies are most often focused on vaccination requirements for day care and school entry, and on vaccinating personnel and patients in health-care settings. Immunization requirements reinforce clinical recommendations for vaccination and are an important tool for improving and maintaining high vaccination coverage. For example, state and local vaccination requirements for school entry have been shown to increase vaccination coverage among elementary schoolchildren, and more recently have been shown to increase coverage for some adolescent vaccinations.20
COMMUNICATING VACCINE RECOMMENDATIONS AND THEIR BENEFITS AND RISKS
In the U.S., we enjoy the benefits of vaccination recommendations across the life span, from infancy to old age. Public health plays an important role at the national, tribal, state, and local levels in making the public aware of the vaccines that are recommended for them. Communication specialists apply communication science and best practices to create materials and tools to improve the public's awareness about the vaccines that are available to and recommended for them. The Section 317 Program contributes to this science base and supports efforts to deliver information about the risks of VPDs, the benefits of immunization, and any known side effects of vaccination using various formats, channels, and trusted spokespeople who resonate with the target audience's values and beliefs.
By protecting myself, I am protecting her
Section 317 has contributed to the public health communications research that has found that different groups view the benefits of vaccination differently. Using these findings, materials have been developed that focus on the benefits that a particular group values most. For example, older adults, who are at increased risk of seasonal influenza, value their ability to protect their loved ones more so than protecting themselves from influenza. Based on these findings, public health promotes the influenza vaccination to seniors as the best way to protect themselves and those they love from the flu.
Informed patients make the best decisions for themselves and their families. As with any medical intervention, most patients have questions about vaccines—why they are needed, if they are effective, and if they are safe—and health-care providers are an important and trusted source for answers to these questions. Public health works with medical associations and other partners to develop resources and tools for health-care providers and their patients to inform their decisions about vaccines. The Section 317 Program supports national, state, and local efforts to educate health-care providers through multiple formats and venues about evidence-based strategies and techniques for answering questions about vaccine effectiveness, safety, and the diseases they prevent, as well as advising the public on the vaccines they need for themselves and their children.
IMPROVING ACCESS TO IMMUNIZATION SERVICES
In the U.S., immunization services, especially for children, are provided primarily in the medical home. However, vaccines are also offered in many other settings, such as public health clinics and community health centers, and complementary venues such as pharmacies, retail clinics, and schools. With the expansion of routinely recommended vaccines for adolescents and adults, medical specialties other than pediatrics and family medicine play an increasingly important role in providing access to immunization services.
The Section 317 Program plays an important role in supporting the immunization systems and workforce that recruit and train robust networks of vaccine providers by building strong partnerships at the national, state, tribal, and local levels to ensure that vaccines are accessible, regardless of the individual's insurance coverage. Public health works with associations representing public and private health-care providers, vaccine manufacturers, and health insurance plans to identify strategies to recruit and train networks of vaccine providers so that there is adequate access to VFC Program21 providers in every community, and access to in-network providers for those with insurance. It is important to recognize that while the VFC Program provides vaccine at no cost to eligible children and adolescents, the significant achievements of the VFC Program in improving vaccination coverage and eliminating disparities would not be possible without the immunization infrastructure supported by the Section 317 Program.22 Public health promotes the use of proven strategies to improve vaccination coverage among children, adolescents, and adults through resource materials, trainings, and visits to clinical sites. The Section 317 Program has been an essential resource for developing, evaluating, and promoting evidence-based public health tools,23 which include:
AFIX (assessment, feedback, incentives, eXchange), which incorporates four key strategies proven reliable to improve providers' immunization service delivery and raise vaccination coverage levels;
Standing orders for vaccinations, which stipulate that all people meeting certain eligibility requirements should be vaccinated, thus eliminating the need for individual physician's orders for each patient; and
Reminder and recall systems that alert health-care providers and patients that the patient is due or overdue for vaccination. Immunization information systems (IISs) and electronic health records (EHRs) can facilitate automation of reminder-recall systems.
ASSURING THE QUALITY OF IMMUNIZATION SERVICES
Quality assurance for vaccine delivery and immunization practice is an important public health function. State and local public health systems and workforces that are supported in part by the Section 317 Program give health-care providers ongoing training and technical assistance to support their vaccination programs. Public health experts visit clinical offices to assure appropriate vaccine storage and handling practices, and to identify opportunities to improve vaccination coverage among their patients. However, the resources required to establish public-private collaborations to fully support and improve practice for immunization across the life span are currently not sufficient and, as a result, efforts in adult immunization have lagged behind those in pediatrics.24 Thus, while the Section 317 Program is authorized to support adult immunization efforts, historically the funds appropriated have been inadequate to do so.
Vaccines need to be stored in the right equipment at the right temperature to ensure the most protection. The Section 317 Program supports efforts to improve storage and handling practices at the provider office. For example, in California, the Section 317 Program supports the California Department of Public Health's effort to provide training and resources for health-care providers to help them effectively store and manage their vaccine inventory. This maintenance includes easily accessible Web-based materials,25 such as the EZIZ Online Immunization Skills Curriculum on storing vaccines, monitoring refrigerator temperatures, and monitoring freezer temperatures; and Vaccine Storage and Handling Job Aids for refrigerator and freezer setup, monitoring temperatures, transporting vaccines, and managing vaccine inventory.
State and local public health officials assist providers with response to vaccine administration errors (e.g., what to do when a vaccine is administered subcutaneously instead of intramuscularly) and management of vaccine stocks that have been exposed to temperature conditions outside the recommended range. These important public health functions are also partly funded through the Section 317 Program.
Finally, information technology plays an important role in immunization program management and implementation by improving the quality of immunization data and enhancing accountability and stewardship of public vaccine resources. IISs are now a critical part of immunization programs, ensuring that individuals get the vaccines they need when they need them. The Section 317 Program is a critical resource for assuring that immunization technologies support the integration of quality immunization services into clinical preventive care. The Section 317 Program contributes to the efforts to improve the interoperability of these public health systems with other health-care systems to enhance the completeness, timeliness, and exchange of data across systems. Interoperability of IISs with EHRs will make it easier for health-care providers to report and receive data about their patients' immunization status from state or local IISs, ultimately making the determination of a patient's vaccination needs routine. IISs can also provide vaccine inventory tracking and are now being integrated with the national vaccine ordering and tracking system for vaccines purchased from CDC vaccine contracts, linking information about vaccines administered with vaccine ordering and distribution data. Other efforts to advance improvements in data quality include the addition of two-dimensional barcoding to vaccine packaging, which will allow providers to scan vaccine information into patient records, reducing data input errors and improving data completeness. IISs are computerized systems that:
Record all shots on all age groups given by all providers in a geopolitical catchment area.
Have functions and features needed by an immunization program (e.g., vaccine inventory management and adverse event reporting).
Have interoperability with other health information systems, including EHRs.
Consolidate vaccine records into a single, electronic source that helps facilitate reminder-recall systems and clinician decision support to avoid missed and unnecessary vaccinations and to reduce wasted vaccine.
ASSESSING VACCINE EFFECTIVENESS AND SAFETY
Once a vaccine is recommended for routine use, it is essential that systems be in place to monitor the impact of the vaccine on the disease or the vaccine may not provide the level of protection needed to meet disease control objectives. For example, in the 1980s, outbreaks of measles occurred in schools that had high coverage with one dose of measles vaccine. Recognizing that a single dose of measles vaccine was not providing sufficient immunity, the ACIP recommended in 1989 that a second dose of measles vaccine be added to the routine immunization schedule. Unexpected changes in the epidemiology of disease may also follow vaccine introduction, such as following the introduction of PCV7, after which there was an increase in disease caused by non-vaccine serotypes that were resistant to some commonly used antibiotics.
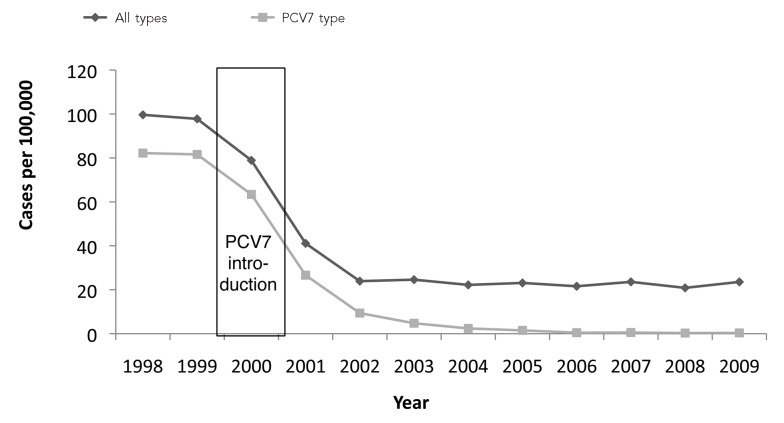
Through its support of extramural and national program operations, the Section 317 Program contributes to the public health systems and expertise that are fundamental to assuring that our national vaccine policies and programs have the intended impact. Public health implements several different approaches to monitor the epidemiology of VPDs. Some diseases are monitored through notifiable disease systems, which collect data at the national and state levels about diseases and conditions for which there is required reporting. For many conditions, laboratory-based diagnosis is essential to confirm the diagnosis, and public health laboratories at the federal and state levels provide essential support for case investigation. Some VPDs are monitored through population-based laboratory reporting systems, such as the ABCs, which provided important data on the impact of PCV7 vaccination of children in the U.S. (Figure 4) and is now providing the first evidence of the impact of the additional serotypes included in the PCV13 vaccine.
Figure 4.
Impact of PCV7 vaccine on invasive pneumococcal disease among children <5 years of age, 1998–2009a
aPilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010;201:32-41.
PCV = pneumococcal conjugate vaccine
Other VPDs that may not be routinely confirmed by laboratory testing are included in special sentinel surveillance projects. This approach has provided valuable information about the impact of rotavirus vaccines on acute gastroenteritis among young children in the U.S.
Specialized molecular testing provides critical information to help determine the source of disease transmission when epidemiologic linkages cannot be identified. For example, molecular typing data are essential to determine the source of measles and rubella cases in the U.S. and provide documentation of the elimination of endemic measles and rubella. Molecular testing also plays an important role in assessing vaccine safety. For example, genetic sequencing data have documented varicella vaccine strain virus within the cerebrospinal fluid of vaccinated individuals, supporting the hypothesis that varicella vaccine may rarely cause aseptic meningitis as a side effect of vaccination. In contrast, measles vaccine virus has not been isolated from the brain of people with subacute sclerosing panencephalitis, a rare and chronic progressive inflammation of the brain caused by persistent infection of measles virus; indeed, it has been shown with molecular typing that when isolated measles viruses were analyzed, they have always been consistent with wild-type measles instead of measles vaccine virus. Such testing is only available through public health laboratories. There is no private market for such testing; therefore, the capacity must remain in the public sector.
After a vaccine is licensed and used in the U.S., public health employs several approaches to monitor vaccine safety. The Section 317 Program helps support the national expertise in vaccine safety and the systems that identify and evaluate potential vaccine adverse reactions.
The Vaccine Adverse Event Reporting System (VAERS) is a national spontaneous reporting system that is jointly managed by CDC and the FDA. Certain reports are mandatory, but anyone can report any adverse event to VAERS. Although VAERS reports alone rarely can establish causality, and the limitations of the system are significant, the system has helped prompt investigations that ultimately identified serious adverse events. Safety is also assessed by special studies that are organized and funded by CDC, FDA, or the vaccine's manufacturer. Since 1992, the Vaccine Safety Datalink (VSD), a consortium of managed care organizations with access to electronic data files on both immunization and various health outcomes, has provided critical infrastructure for vaccine safety studies in the U.S. In recent years, VSD investigators have developed new methods for more rapid approaches to evaluate safety through ongoing review of accrued data for specified diagnoses during specified intervals following a specified vaccine. This approach, called rapid cycle analysis (RCA), has been used to evaluate the safety of a number of newer vaccines. For example, RCA identified an increased risk of febrile seizures following the combined measles-mumps-rubella-varicella vaccine among children aged 12–23 months compared with the separate administration of measles-mumps-rubella and varicella vaccines in this age group.
Data collected also allow the risks of adverse events following immunization to be reviewed in the context of the benefits provided by the vaccine. Data on adverse events may lead to recommendations for additional information to be provided prior to vaccination, changes in the vaccine recommendation, or even the withdrawal of recommendations for use of a vaccine.
Post-licensure monitoring of vaccine safety
Vaccine safety begins at product development before a vaccine is licensed and continues after licensure to make sure uncommon potential adverse events are identified. If a significant safety event occurs, measures are taken to respond, including suspending the use of and/or withdrawing a product from the market. Such an event occurred in the late 1990s.
In 1998, the first pediatric rotavirus vaccine (RotaShield®, Pfizer, New York, New York) was licensed to protect infants and young children from severe acute gastroenteritis (i.e., vomiting and severe diarrhea) caused by rotaviruses. This vaccine became linked to a condition called intussusception among vaccinated infants, which is a blockage or twisting of the intestines that sometimes requires surgery and can be fatal. Although five cases of intussusception were noted in pre-licensure studies of RotaShield, it was not clear that this incidence was in excess of what would be expected to occur naturally. This information was included in the product labeling when the vaccine was approved, and a code was established to allow specific reporting of this condition to VAERS. By May 21, 1999, 10 cases were identified among infants who had received RotaShield in the U.S.; six of these cases occurred within three to six days following vaccination. In June 1999, CDC initiated a multistate case-control study.26 By July 7, 1999, 15 cases among recipients of rotavirus vaccine had been reported to VAERS; of these, 12 had developed intussusception within one week of vaccination. Additional data from a manufacturer-sponsored post-licensure study and preliminary data from the CDC case-control study became available in early July 1999. On July 16, 1999, CDC announced a suspension of the use of RotaShield pending completion of ongoing studies.27 Both the CDC case-control study and a large linked database study were completed by October 1999 and confirmed an approximately 30-fold increase in risk of intussusception following the first dose of RotaShield. In October 1999, the manufacturer voluntarily withdrew the product from the market and the ACIP withdrew its recommendation for the use of RotaShield in the U.S.28
More recently, two new rotavirus vaccines (RotaTeq®, Merck Vaccines, West Point, Pennsylvania, licensed in 2006, and Rotarix®, GlaxoSmithKline, Philadelphia, Pennsylvania, licensed in 2008) have been licensed in the U.S. and are recommended for routine use in infants. Both vaccines were evaluated in large (more than 30,000 vaccinated infants) pre-licensure studies to evaluate the risk of intussusception, and no evidence of increased risk was found. Post-licensure studies in other countries have found about a fivefold increase of risk of intussusception following the first dose in some populations. Ongoing studies of RotaTeq in the VSD in the U.S. have found no evidence of an increase in risk; monitoring of both vaccines continues.
MONITORING PROGRAM PERFORMANCE
The performance of national, state, and local immunization programs is monitored through vaccine coverage assessments toward Healthy People 2020 goals.29 The public health systems and workforce funded through the Section 317 Program identify gaps in immunization coverage and guide program priorities using multiple approaches.
The National Immunization Survey (NIS), a large, random-digit-dial survey, is conducted at the national level and is used to obtain immunization coverage estimates for children aged 19–35 months. Provider-verified records are obtained and estimates are made both of national and state-specific coverage by vaccine and by several different vaccine series on an annual basis. Since 2006, the NIS has been expanded to collect data on vaccination of adolescents aged 13–17 years (NIS-Teen). NIS-Teen provides coverage estimates by vaccine and by state on an annual basis. Vaccine coverage among adults is monitored at the national level by the National Health Interview Survey (NHIS) and at the state level by the Behavioral Risk Factor Surveillance System (BRFSS). Other systems were developed during the 2009 H1N1 influenza pandemic to collect timely information on coverage. These systems include Internet panel surveys currently used to monitor influenza vaccine coverage among pregnant women and health-care workers, and work is ongoing to identify alternatives to telephone surveys, given their decreasing response rates.
In addition to these national efforts, the Section 317 Program also contributes to efforts at the state and local levels to monitor program performance. This contribution includes efforts to implement IISs that, in addition to quality assurance, can be used to monitor immunization coverage and identify communities with lower vaccine coverage. Through support from the Section 317 Program, states also monitor the impact of state vaccine requirement policies by monitoring vaccine coverage at school entry and exemption rates.
RESPONDING TO PUBLIC HEALTH EMERGENCIES
Immunization programs are regularly involved in responding to public health emergencies. These activities include managing vaccine supply interruptions and shortages and responding to disease outbreaks. The flexibility of the Section 317 Program to support the immunization workforce and systems necessary for responding to such emergencies is an important feature of the program.
Manufacturing vaccines is a complex process, and it is not uncommon for supply interruptions to occur, thereby causing vaccine shortages. When vaccine shortages occur, partners—including vaccine manufacturers, other public health officials, and medical professional societies—work with CDC to manage vaccine orders from both the private and public sectors and to control the distribution of available vaccine to assure equity between the two sectors. If available and projected vaccine supplies are not adequate to provide all recommended doses of the vaccine to the population for whom it is recommended, temporary changes in recommendations may be made to maximize protection while reducing demand to meet supply. During the Haemophilus influenzae type b (Hib) vaccine shortage in 2009–2010, the booster dose was temporarily halted except for certain children at increased risk of Hib disease. The Section 317 Program contributes to the communications systems that deliver important information about vaccine supply and encourage compliance with any shortage recommendations. This system includes a CDC Web page with timely and updated information about vaccine shortages.
Public health response to VPD outbreaks is largely conducted at the local and state levels. Some diseases such as polio and measles are no longer endemic in the U.S., so every suspected case requires timely and thorough investigation, including efforts to identify where the infection was acquired and settings in which further transmission may have occurred. Responding to any outbreak is a labor-intensive and costly effort that requires epidemiologists, laboratory support, and extensive fieldwork. The Section 317 Program -contributes to outbreak response by supporting the immunization systems and workforce at the state and local levels that actively search for cases, collect information needed to confirm the diagnosis, identify settings where disease transmission may be occurring, and follow up with all individuals who might have been exposed to implement control. The data from these investigations are essential tools in determining and guiding outbreak control strategies, such as quarantining exposed people, providing antimicrobial or immune globulin prophylaxis, canceling public events, and guiding vaccination efforts. Public health response may also involve mass immunization efforts, such as those done for meningitis, hepatitis A, and pertussis outbreaks in recent years. The Section 317 Program contributes to the workforce and vaccine for such efforts. The flexibility of the Section 317 Program to vaccinate adults is particularly important in outbreak response.
At the national level, the Section 317 Program supports efforts to document and communicate nationally about outbreak response and its impact, provide guidance on outbreak control, and, if invited by state health officials, initiate or enhance field investigations to support state and local public health responses.
The cost of a VPD outbreak: measles
The U.S. recently experienced an increase in measles—a highly contagious disease spread through the air when an infected person coughs or sneezes. Measles is so contagious that if one person is infected, nine out of 10 people with whom they have close contact who are not immune to measles will become infected.30 Before the measles vaccine was introduced in 1963, there were about three to four million cases of measles in the U.S. alone; essentially every child had the disease by the time they were 15 years of age. About 1,000 people suffered disabilities from the disease, such as deafness or permanent brain damage from encephalitis, and approximately 450 people died each year from measles. By 2000, naturally occurring cases of measles had been eliminated.31 During 2000–2008, there were approximately 50 measles cases each year that had come from other countries where measles was still endemic,32 with increasing numbers of importations into the U.S. as a result of increases in measles cases in countries visited by U.S. travelers, including some parts of Europe, Asia, the Pacific, and Africa.33
Outbreaks of measles and other VPDs can occur at any time in the U.S. In 2011, 17 measles outbreaks occurred, resulting in 222 cases of measles. Forty-four percent of the outbreak-associated cases were among people who chose not to be vaccinated, and 90% of cases were traced to other countries with lower immunization rates. The 2011 cases marked the highest number of cases in 15 years. The public health investment in staff and resources is significant in responding to these outbreaks. Each case of measles triggers intense public health response to prevent this highly contagious disease from spreading. Section 317 contributes to the public health expertise and workforce, systems, and strategies, such as vaccination, that are necessary to contain measles outbreaks. The following response examples highlight the investment in resources involved in supporting response efforts.
A public health response to measles in Salt Lake County, Utah, from March to April 2011 involved nine measles cases, but required officials to trace thousands of contacts, review immunization records of hospital workers and teachers, give post-exposure prophylaxis to nearly 400 people, and isolate 200 others. The estimated cost of this response was $300,000.
To stop a 14-person measles outbreak that began with one unvaccinated tourist visiting a U.S. hospital emergency room in Arizona in 2008, the Arizona Department of Health had to track down and interview 8,321 people, seven Tucson hospitals had to furlough staff members for a combined 15,120 work-hours, and two hospitals where patients were admitted spent $799,136 to contain the disease.
The 2008 measles outbreak in San Diego, California, exposed 839 people, including 11 unvaccinated children who developed the disease, and resulted in the hospitalization of an infant who was too young to be vaccinated. The total cost of response was $124,517. The net public-sector cost was $10,376 per case, and the average cost to the affected families was approximately $775 per child.
OPPORTUNITIES
In many ways, the potential of vaccines to reach and protect the health of the public has never been greater. We have record-high childhood immunization coverage rates and significant decreases in disease. Since 2000, 11 additional vaccines have been routinely recommended for children, adolescents, or adults along with numerous recommendations for vaccine use in expanded age groups, such as the universal recommendation for seasonal influenza vaccine, to protect Americans from serious and life-threatening diseases. Access to immunization services is expected to improve as health insurance reforms are fully implemented, and improvements in health information technologies have the potential to create efficiencies and improve health-care quality. Yet, with each of these opportunities come challenges that, if ignored, could undermine our success.
One challenge is a direct byproduct of our success. As we have significantly decreased the rates of most VPDs, the absence of disease has decreased public awareness about the importance of maintaining high vaccination coverage rates. The immunization infrastructure needs to be able to fully support efforts to provide the scientific evidence that underpins national immunization policies, including vaccine safety systems, and the development and dissemination of information about the benefits and potential side effects of vaccines within the context of the risks of the diseases they prevent.
New and expanded vaccine recommendations provide ever-increasing protection from serious and deadly infectious diseases. Along with this protection come increased costs and crowded and complex immunization schedules. There are now new populations recommended for vaccinations and new provider groups to reach and educate. For example, public health is strengthening partnerships with medical specialties, such as obstetricians and gynecologists to improve vaccination of pregnant women, and with complementary health-care venues, such as pharmacies and other retail clinics, to increase capacity and coverage for seasonal flu vaccination. The success of these partnerships in achieving national immunization goals relies on robust public health systems and a highly skilled public health workforce to provide outreach and education, improve collaboration among diverse providers, ensure proper vaccine storage and handling, support the use of effective immunization information technologies, and address disparities. While it has not been the only resource, the Section 317 Program has been and continues to be the core funding for these critical activities, as illustrated in the pertussis response of 2010–2012.
CASE STUDY: THE ROLE OF IMMUNIZATION INFRASTRUCTURE IN RESPONDING TO PERTUSSIS, 2010–2012
Pertussis, also known as whooping cough, is a respiratory infection that causes very prolonged coughing illness in most people, but can lead to pneumonia and fatal respiratory failure in infants. The disease can be cyclical, with peaks every three to five years, and the U.S. experienced a 50-year high in 2012. Most recently, large outbreaks in California and Washington State illustrate public health immunization infrastructure in action.
In 2010, California reported more than 9,000 cases of pertussis, with 10 infant deaths.34 Experiencing its worst outbreak of whooping cough in decades, Washington State declared an epidemic of pertussis on April 3, 2012. As of early August 2012, Washington State had nearly 3,50035 reported cases compared with 965 cases reported statewide for all of 2011. As of August 11, 2012, 46 states and the District of Columbia reported increases in disease compared with the same time period in 2011.36 A number of efforts are under way to control these outbreaks and prevent the spread of disease; these efforts rely upon the following critical immunization program functions, which are illustrated in Figure 5.
Figure 5.
Role of Section 317 Immunization Program funding in preventing infectious disease: pertussis case study, 2010–2012
aSection 317 funds can be used to purchase vaccine
ACIP = Advisory Committee on Immunization Practices
Tdap = tetanus-diphtheria-pertussis vaccine
CDC = Centers for Disease Control and Prevention
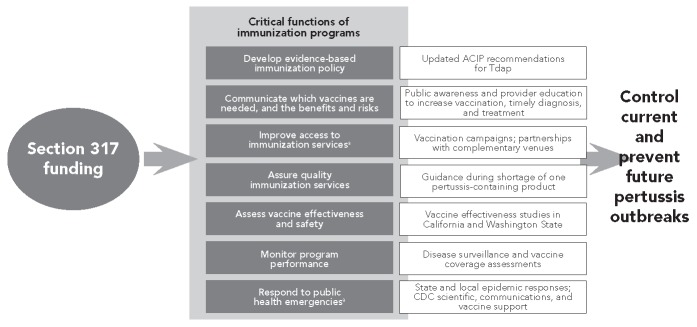
Response to public health emergencies
During the past two years, the Section 317 Program14 has provided critical support for the public health response to the increasing number of pertussis outbreaks. The immunization systems and workforce at the state and local levels have conducted epidemiologic studies, implemented targeted vaccination campaigns, and increased public awareness and provider education activities. At the federal level, CDC has provided scientific expertise in pertussis; science-based communication tools and resources for use nationwide, statewide, and locally; and Section 317-purchased vaccines for targeted campaigns. The ability of the Section 317 Program to respond to public health emergencies makes it a valuable asset in protecting the nation's health.
Communicating which vaccines are needed, and the benefits and risks
Messages about the pertussis outbreaks, the risks of serious complications and deaths among infants too young to be vaccinated, and tetanus-diphtheria-pertussis (Tdap) vaccine recommendations for adolescents and adults are delivered using a variety of technologies and venues. CDC made available science-based provider education and public awareness resources, including audio and video, social media, print materials, publications, and multimedia tools. In Washington State, the Department of Health focused its provider education efforts on diagnosing, treating, and preventing pertussis. Its public awareness efforts focused on the signs and symptoms of pertussis and vaccination recommendations, using multiple communication venues (e.g., messages on public transportation).
Improved access to immunization services
An important feature of the Section 317 Program is the flexibility in directing resources to meet priority needs, such as outbreak control, and to support public-private efforts to improve access to vaccination across the life span. During the 2010 pertussis epidemic in California, the Department of Public Health offered free Tdap vaccine to birthing hospitals and local health departments to vaccinate mothers and close contacts of newborns. In Washington State, targeted efforts substantially increased Tdap vaccination among adults, with a 140% increase in adult vaccination from March 25 to May 26, 2012, compared with the same time period in 2011, resulting in 82,453 vs. 34,171 doses administered in 2012 vs. 2011, respectively. Washington State was also able to use its Section 317 vaccine funding to allocate an additional 27,000 doses of Tdap vaccine to reach uninsured adults.
Developing an evidence-based immunization policy
Upon review of new data about the incidence of pertussis and the populations affected, the ACIP updated and expanded its recommendations for the use of Tdap vaccines among adolescents and adults to reduce the transmission of pertussis to infants.37
Assuring quality of immunization services
Vaccines need to be stored in the right equipment at the right temperature to ensure the most protection. The Section 317 Program supports efforts to help providers store, handle, and use vaccines for the best public health impact. For example, during an anticipated shortage of one pertussis-containing vaccine during the summer of 2012, CDC provided guidance on the use of the available pertussis-containing products to ensure their patients were fully vaccinated.38
Assessing vaccine effectiveness and safety
An effective outbreak response controls the outbreak and provides data and information to help prevent future outbreaks, including data and information that can lead to better and more effective vaccines and recommendations. CDC and Washington State are undertaking a number of efforts to evaluate strategies to prevent pertussis, including assessments of the effectiveness of maternal and caregiver vaccination in protecting infants, and the duration of protection provided by the vaccine given to adolescents. This information is valuable in informing and evaluating immunization recommendations.
Monitoring program performance
Vaccination coverage is monitored and reported through the NIS, the BRFSS, and the NHIS. This information has been used to identify gaps in vaccination coverage to inform targeted pertussis vaccination campaigns and increase public awareness and provider education efforts. It is also contributing to pertussis vaccine effectiveness studies.
CHALLENGES
While expanded coverage provisions of the ACA provide unprecedented access to immunizations, they also have the potential to place greater stresses on the existing immunization infrastructure. Although the health insurance reforms of the ACA, when fully implemented, will improve access to clinical preventive services by improving payment for vaccines and their administration, the essential public health functions that make vaccination possible are not addressed by the ACA.
Some communities may not currently have enough in-network providers to assure access to immunization services across the life span that are now afforded through the health insurance reforms of the ACA. The role of public health in recruiting and training robust networks of in-network vaccine providers will be essential in realizing the potential of the ACA health insurance reforms. However, this training will require public health to forge new and expanded partnerships with employers, health insurance plans, and health-care providers.
The ACA also increases the visibility and role of the ACIP recommendations in determining health-care coverage policies, making it more important than ever that the ACIP has the capacity to make timely and transparent recommendations. The public health systems and workforce that monitor disease, vaccine effectiveness, and safety will become even more essential in the new and evolving health-care landscape to support the work of the ACIP.
Improved health information technologies give us the opportunity to increase vaccination coverage rates as well as promote efficiency and improve quality of care. However, this field is rapidly changing and evolving and spans multiple sectors of the health-care delivery system. It is critical that the public health investments in health information technologies be strategic, coordinated, and standardized to the greatest extent possible to achieve efficiencies and improve quality of care. Expertise has already been developed and is held in the immunization workforce as described previously, but the demands on this knowledge base will grow immensely and must be supported.
Finally, the evolving health-care landscape may call into question the continued relevance of the Section 317 Program to the immunization enterprise. This questioning would be a severe error in judgment. For nearly 50 years, the Section 317 Program has been directed toward filling gaps in the national immunization program through its authorities to provide operations funding to states; to support vaccine-related epidemiology, laboratory, program, and communications efforts; and to purchase vaccines for children, adolescents, and adults. For the past 20 years, the contribution of the Section 317 Program in providing vaccines to underinsured children who are not eligible for VFC Program vaccines has been the most visible aspect of the program, leaving the vital contributions of the Section 317 Program to immunization infrastructure and the workforce largely unknown among decision makers and the public. While the Section 317 Program will continue to be an important resource in providing a vaccine safety net for uninsured Americans39 and a flexible resource for provision of vaccines when responding to disease outbreaks and urgent needs, it is the Section 317 systems and workforce described in this report that provide the backbone for the U.S. immunization program, regardless of the payer of the vaccine given. Educating decision makers and the public about the need for a strong immunization infrastructure may in fact be one of our greatest challenges and our greatest opportunities. If we fail, we risk the erosion of a sound and trusted immunization system that has contributed to historic improvements in public health. If we succeed, we have the potential to transition and strengthen our immunization infrastructure and performance to realize the full potential of vaccines across the life span for millions of Americans today and well into the future.
CONCLUSIONS AND RECOMMENDATIONS
Conclusion 1
Vaccination is one of our most important public health tools for directly protecting individuals from infectious diseases and indirectly protecting those who cannot be vaccinated, such as those with medical contraindications, those who are too young to be vaccinated, or those with compromised immune systems. This indirect effect, termed “herd or community immunity,” is achieved when high vaccination coverage rates stop the transmission of disease from person to person, so that those remaining unvaccinated are protected from exposure to the infectious agent. Achieving high population coverage rates cannot be accomplished with vaccine alone.
The Section 317 Program supports the public health systems and workforce at the local, state, and national levels that are essential to meeting national immunization goals for children, adolescents, and adults. Much more than a program that can purchase vaccines, the Section 317 Program has inherent flexibility that supports the critical immunization infrastructure to deliver vaccines into the arms of patients. Altogether, Section 317-funded resources are vital to achieving optimal levels of vaccination coverage. Vaccination acts as a firewall in the spread of disease, slowing and preventing the spread of disease to others, particularly to those who are unable to be vaccinated because they are too young, too old, or have compromised immune systems that prevent them from being vaccinated or for other reasons.
However, recent erosion of the Section 317 budget authority for core immunization activities and the use of other funding streams such as the PPHF to fill funding gaps pose a risk to the stability of the immunization workforce and systems at the national, state, and local levels. Although it is a valuable resource for filling public health needs, sources such as PPHF may be unstable or redirected based on other priorities.
Recommendation 1
The NVAC recommends that the Section 317 Program be sustained to assure a strong public health infrastructure necessary to achieve and sustain high vaccination coverage and low disease burden among the U.S. civilian population.
Conclusion 2
During the past years, appropriations for the Section 317 Program have not kept pace with the increasing demand. Estimates of the Section 317 funding necessary to assure a comprehensive immunization program at the state and national levels is a useful tool to fully inform policy and decision makers. Historically, the Section 317 report to Congress has been one important tool for updating the needs of the nation's immunization infrastructure at the local, state, and federal levels.
Recommendation 2a
CDC should present its professional judgment regarding the size and scope of the Section 317 Program necessary to support a comprehensive immunization program. This judgment should include program operations at the federal, state, tribal, and local levels, and vaccine purchase to provide a safety net and timely response to public health emergencies. CDC should present its professional judgment to the NVAC annually at its June meeting for deliberation and discussion.
Recommendation 2b
HHS should consider CDC's professional judgment for the Section 317 Program as an important input to its decision-making during the budget formulation process.
Conclusion 3
The highly decentralized and complex national immunization system is shaped by an increasing number of routinely recommended vaccines, changes in the health-care delivery system, and reductions in federal immunization resources at the national and state levels. The need to sustain high levels of immunization coverage under these circumstances requires adapting immunization programs to operate in ways that meet these new challenges and opportunities.
Recommendation 3
The NVAC recommends that federal, state, tribal, and local public health should seek efficiency and innovation to achieve Healthy People 2020 targets and ensure high immunization levels across all age spans. Examples of such efficiencies include, but are not limited to, improved vaccine ordering, supply management, storage, and handling, such as through the use of vaccine barcodes. Examples of innovation include, but are not limited to, implementation and use of IISs and EHRs; innovative communication strategies; providing vaccines as an in-network provider for the receipt of vaccine in public health clinics; and expanding vaccination sites, such as schools, workplaces, and pharmacies.
The NVAC supports current innovations in operations and encourages continued innovation. The NVAC recommends that HHS through the National Vaccine Program Office hold a public meeting of experts to examine and explore contributions toward efficiency and innovation at state and local health departments.


Footnotes
Some of the contributors are employees of the U.S. Department of Health and Human Services. Although they served as contributors on the basis of their areas of expertise, the views expressed in this article are those of the National Vaccine Advisory Committee. The positions expressed and the recommendations made in this article do not necessarily represent those of the U.S. government or of departmental employees who contributed.
REFERENCES
- 1.Schuchat A, Hilger T, Zell E, Farley MM, Reingold A, Harrison L, et al. Active bacterial core surveillance of the emerging infections program network. Emerg Infect Dis. 2001;7:92–9. doi: 10.3201/eid0701.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49(RR-9):1–38. [PubMed] [Google Scholar]
- 3.Notice to readers: decreased availability of pneumococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2001;50(36):783–4. [Google Scholar]
- 4.Notice to readers: updated recommendations on the use of pneumococcal conjugate vaccine in a setting of vaccine shortage—Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2001;50(50):1140–2. [Google Scholar]
- 5.Notice to readers: pneumococcal conjugate vaccine shortage resolved. MMWR Morb Mortal Wkly Rep. 2004;53(36):851–2. [Google Scholar]
- 6.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 7.Zhou F, Shefer A, Wenger J, Messonnier M, Wang LY, Lopez A, et al. Economic evaluation of the routine childhood immunization program in the United States, 2009. Abstract presented at the Pediatric Academic Societies' Annual Meeting; 2012 Apr 28-May 1; Boston. [DOI] [PubMed] [Google Scholar]
- 8.Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):258–61. [PubMed] [Google Scholar]
- 9.Invasive pneumococcal disease and 13-valent pneumococcal conjugate vaccine (PCV13) coverage among children aged ≤59 months—selected U.S. regions, 2010-2011 [published erratum appears in MMWR Morb Mortal Wkly Rep 2011;60(44):1530] MMWR Morb Mortal Wkly Rep. 2011;60(43):1477–81. [PubMed] [Google Scholar]
- 10.Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR Morb Mortal Wkly Rep. 2012;61(21):394–5. [PubMed] [Google Scholar]
- 11.Roush SW, Murphy TV Vaccine-Preventable Disease Table Working Group. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA. 2007;298:2155–63. doi: 10.1001/jama.298.18.2155. [DOI] [PubMed] [Google Scholar]
- 12. Pub. L. No. 111-48, 124 Stat. 119 (2010).
- 13. Pub. L. No. 111-52, 124 Stat. 1029 (2010).
- 14. Title XXI of the Public Health Service Act (Pub. L. No. 99-660) (§2105) (42 U.S.C. 300aa-5).
- 15. Pub. L. No. 67-13, 42 Stat 20, 31 U.S.C. 11.
- 16.Shen AK, Spinner JR, Salmon DA, Gellin BG. Strengthening the U.S. vaccine and immunization enterprise: the role of the National Vaccine Advisory Committee. Public Health Rep. 2011;126:4–8. doi: 10.1177/003335491112600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rein DB, Honeycutt AA, Rojas-Smith L, Hersey C. Impact of the CDC's Section 317 Immunization Grants Program funding on childhood vaccination coverage. Am J Public Health. 2006;96:1548–53. doi: 10.2105/AJPH.2005.078451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Health and Human Services (US). Recommended preventive services. [cited 2012 Oct 25]. Available from: URL: http://www.healthcare.gov/law/resources/regulations/prevention/recommendations.html.
- 19.Poliomyelitis prevention in the United States: updated recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49(RR-5):1–22. [PubMed] [Google Scholar]
- 20.Bugenske E, Stokley S, Kennedy A, Dorell C. Middle school vaccination requirements and adolescent vaccination coverage. Pediatrics. 2012;129:1056–63. doi: 10.1542/peds.2011-2641. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (US). Vaccines for Children Program (VFC) [cited 2012 Aug 13]. Available from: URL: http://www.cdc.gov/vaccines/programs/vfc/index.html.
- 22.National, state, and urban area vaccination coverage among children aged 19–35 months—United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55(36):988–93. [PubMed] [Google Scholar]
- 23.Guide to Community Preventive Services. Vaccinations to prevent diseases: universally recommended vaccinations. [cited 2012 Oct 25]; Available from: URL: http://www.thecommunityguide.org/vaccines/universally/index.html. [Google Scholar]
- 24.National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee. Public Health Rep. 2012;127(Suppl 1):1–42. doi: 10.1177/00333549121270s101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.California Department of Public Health. Vaccine storage and handling. [cited 2012 Oct 25]. Available from: URL: http://www.cdph.ca.gov/programs/immunize/Pages/VaccineStorageandHandling.aspx.
- 26.Kramarz P, France EK, Destefano F, Black SB, Shinefield H, Ward JI, et al. Population-based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J. 2001;20:410–6. doi: 10.1097/00006454-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Intussusception among recipients of rotavirus vaccine—United States, 1998-1999. MMWR Morb Mortal Wkly Rep. 1999;48(27):577–81. [PubMed] [Google Scholar]
- 28.Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48(43):1007. [PubMed] [Google Scholar]
- 29.Department of Health and Human Services (US). Healthy People 2020. [cited 2012 Sep 21]. Available from: URL: http://www.healthypeople.gov/2020/default.aspx.
- 30.Centers for Disease Control and Prevention (US). Transmission of measles. [cited 2012 Aug 13]. Available from: URL: http://www.cdc.gov/measles/about/transmission.html.
- 31.Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16-17 March 2000. J Infect Dis. 2004;189(Suppl 1):S43–7. doi: 10.1086/377696. [DOI] [PubMed] [Google Scholar]
- 32.Parker Fiebelkorn A, Redd SB, Gallagher K, Rota PA, Rota J, Bellini W, et al. Measles in the United States during the postelimination era. J Infect Dis. 2010;202:1520–8. doi: 10.1086/656914. [DOI] [PubMed] [Google Scholar]
- 33.Measles—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(15):253–7. [PubMed] [Google Scholar]
- 34.California Department of Public Health. Pertussis (whooping cough) [cited 2012 Sep 16]. Available from: URL: http://www.cdph.ca.gov/HealthInfo/discond/Pages/Pertussis.aspx.
- 35.Washington State Department of Health. Pertussis epidemic 2012. [cited 2012 Aug 21]. Available from: URL: http://www.doh.wa.gov/YouandYourFamily/IllnessandDisease/WhoopingCough.aspx.
- 36.Centers for Disease Control and Prevention (US). Pertussis (whooping cough): outbreaks. [cited 2012 Sep 15]. Available from: URL: http://www.cdc.gov/pertussis/outbreaks.html.
- 37.Centers for Disease Control and Prevention (US). Vaccines – immunizations: publications: ACIP recommendations. [cited 2012 Sep 15]. Available from: URL: http://www.cdc.gov/vaccines/pubs/ACIP-list.htm#tdappreg.
- 38.Centers for Disease Control and Prevention (US). Vaccines and preventable diseases: current vaccine shortages – delays. [cited 2012 Sep 18]. Available from: URL: http://www.cdc.gov/vaccines/vac-gen/shortages/default.htm#note1.
- 39.Congress of the United States Congressional Budget Office (US). Estimates for the insurance coverage provisions of the Affordable Care Act updated for the recent Supreme Court decision. [cited 2012 Oct 25]. Available from: URL: http://www.cbo.gov/publication/43472.