Abstract
Three-dimensional cultivation of human cells is promising especially for long-term maintenance of specific functions and mimicking the in vivo tissue environment. However, direct viability assessment is very difficult in such systems. Commonly applied indirect methods such as glucose consumption, albumin or urea production are greatly affected by culture conditions, stress and time of cultivation and do not reflect the real time viability of the cells. In this study we established a real-time in situ viability assay namely; resazurin assay, in a 3D hollow-fiber bioreactor using human liver cells. Resazurin assay is based on the conversion of resazurin to a fluorescent dye by cytoplasmatic and mitochondrial enzymes. We show that the resazurin reagent in concentrations used in this study is non-toxic and could be rapidly removed out of the system. Moreover, we observed that dead cells do not affect the results of the assay. We optimized the assay on HepG2 cells and tested it with primary human hepatocytes. Moreover, we maintained primary human hepatocytes in the 3D bioreactor system in serum-free conditions and also assessed viability before and after the exposure to amiodarone using the resazurin assay. We show that this approach is applicable during long-term cultivation of cells in bioreactors under different conditions and can moreover be applied to pharmacological studies, e.g. investigation of chronic drug effects in such 3D bioreactors.
Keywords: 3D bioreactor, Primary hepatocytes, In situ viability assay, Drug testing, Resazurin
Introduction
The cell viability is routinely assessed during maintenance and testing of cell culture systems. A palette of established and validated assays based on diverse endpoints is available. Examples include assays such as the MTT and WST-1 assays which are based on the metabolic activity, Trypan-Blue exclusion method which relies on membrane integrity and Sulforhodamine B and crystal violet assays that quantify protein content, among many others. Other non-invasive methods such as measurement of respiration in 2D multiwell plates equipped with oxygen sensors have been reported to show high correlations to other endpoint assays (Noor et al. 2009). The resazurin assay, well-known under the trade name Alamar Blue®, is based on the reduction of the non-fluorescent substrate resazurin into the fluorescent dye resorufin by mitochondrial and cytosolic enzymes. The reduced form of the fluorescent dye is soluble and the cells can be further cultivated after the assay (O’Brien et al. 2000).
We recently reported long term maintenance and chronic toxicity assessment in 2D cultures with enriched medium (Mueller et al. 2012). Although 2D cultivation systems are easy to maintain and handle, these do not reflect the three-dimensional in vivo tissue environment. Alternative cell culture systems are based on 3D cell culture techniques mimicking the microenvironment of tissues or organs and allow long term maintenance of the cells. These cultivation systems include gel-based systems imitating extracellular matrix (Sodunke et al. 2007), spheroids (Abu-Absi et al. 2002; Mueller et al. 2011a), micro scaffolds (Bokhari et al. 2007) or various types of hollow fiber bioreactors (Schmitmeier et al. 2006; Schmelzer et al. 2010). Hollow fiber 3D bioreactors were applied as bioartificial liver for clinical applications (Gerlach et al. 2003) and were down-scaled for analytical purposes. Liver cells form three-dimensional aggregates around the hollow-fibers including the formation of liver-specific structures (Zeilinger et al. 2004). It was shown that the 3D bioreactors are suitable for long-term functional maintenance of primary human hepatocytes indicating high potential of these systems particularly for the assessment of chronic drug-induced effects (Mueller et al. 2011b). One main disadvantage of this hollow-fiber bioreactor is its black-box character, since direct cell counting methods as well as microscopic observation of cells is not possible. So far, only indirect methods such as monitoring of substrate consumption rates are reported for viability assessment. A real-time in situ viability assay would be very helpful for evaluating cellular state over extended periods of culture. Gloeckner et al. (2001) applied the resazurin assay to a miniaturized 3D hollow-fiber bioreactor monitoring proliferation of human leukemic cell lines. Using suspension cell lines, they found a higher sensitivity of their method compared to indirect methods such as monitoring of glucose consumption.
In the presented study, we tested this method for adherent cells for the viability assessment of the HepG2 cell line as well as of primary human hepatocytes in a 3D-hollow fiber bioreactor system. Moreover, we determined the concentration of the reagent that can be safely used in the bioreactor without causing toxicity and at the same time giving a measurable signal. We also assessed the retention of the reagent inside the bioreactor and determined the time needed to wash out the reagent from the system. Using a control, we show that dead cells or released enzymes do not affect the established assay. In case of primary human hepatocytes, the cells were exposed to a clinically relevant concentration of amiodarone for 4 days. Viability was assessed in the early and late phase of the cultivation (i.e. before and after drug exposure).
Although we used human liver cells, other cell types like kidney cells (Iwahori et al. 2005) or cardiac cells (Hosseinkhani et al. 2010) could also be maintained in a 3D bioreactor system. In all cases, viability assessment is fundamental. The real time in situ assay described in our study could be extended to these other cell types in various 3D bioreactor settings. This would additionally support studies on cell physiology and phenotype and can be also applied to long term drug toxicity assessment studies.
Materials and methods
Culture medium and cells
The human hepatoblastoma cell line, HepG2, was obtained from the German collection of microorganisms and cell cultures (DSMZ, Braunschweig, Germany). Cells were routinely maintained in Williams Medium E (PAN Biotec, Aidenbach, Germany) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and 10 % fetal calf serum (FCS; PAN Biotec, Aidenbach, Germany) at 37 °C in a cell incubator (Memmert GmbH, Schwabach, Germany) at 95 % relative humidity with 5 % CO2 supply.
Primary human hepatocytes (PHH) were isolated from resected liver tissues from patients with primary and secondary tumors. Tissue collection was done according to the institutional guidelines and with the patient’s written consent. Isolation and purification of non-tumor cells was performed as described previously (Mueller et al. 2011b).
In the 3D bioreactor system, cells were maintained in Heparmed Vito 143 (Biochrom AG, Berlin, Germany) medium supplemented with insulin (20 IU/l), transferrin (5 mg/l) and glucagon (3 μg/l), all from Biochrom AG.
3D bioreactor system
The 3D bioreactors were purchased from Stem Cell Systems GmbH (Berlin, Germany) (Fig. 1b). They consist of three interwoven hollow fiber capillary bundles that form four different compartments which are integrated into a polyurethane housing as recently described (Mueller et al. 2011b).
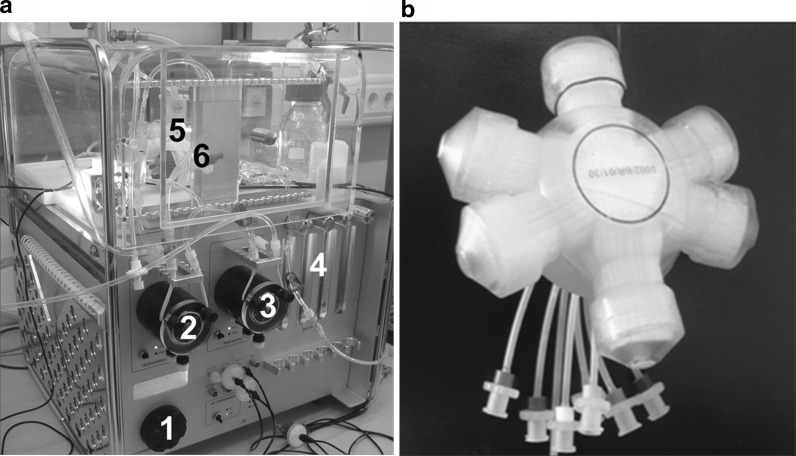
Fig. 1.
a Perfusion system for 3D bioreactor: pump control (1), recirculation pump (2), feed pump (3), gas rotameters (4), bioreactor (5) and sample port (6). b 3D bioreactor with tubing for medium in- and outflow as well as for cell inoculation
The bioreactor is kept in a chamber maintained at a temperature of 37.5 °C and is connected to a perfusion system (Fig. 1a). The perfusion system includes a recirculation pump for medium perfusion, a feed pump for fresh medium supply and a flow regulation for gas supply. Recirculation flow rate was adjusted to 7 ml/min with a feed rate of 1.5–2 ml/h. The system can be easily kept in recirculation mode when the feed pump is switched off allowing recirculation of the medium in the system, e.g. during assays. Bioreactor and tubing system were assembled under sterile conditions on a laminar flow workbench. After assembly, the bioreactor was connected to the perfusion system.
In case of the HepG2 cells, 3× or 6 × 107 viable cells were re-suspended in 7 ml Heparmed Vito medium supplemented with 2.5 % FCS and inoculated into the 3D bioreactor. Whereas in case of primary human hepatocytes (PHH), 108 cells were inoculated using Heparmed Vito medium supplemented with 2.5 % FCS. At day 10, the system was adjusted to serum-free medium.
Assessment of the sensitivity of resazurin
Resazurin reagent was purchased as CellTiter-Blue® Cell Viability Assay from Promega GmbH (Mannheim, Germany). For the assessment of applicable resazurin concentrations giving sufficient signals, 5 × 104 HepG2 cells per well were seeded in a 96-well plate in Williams Medium E (WME) with 10 % FCS. After cell adherence overnight, the cells were washed and 200 μl of serum-free medium was added. Different resazurin concentrations, ranging from 0.1 to 20 % in triplicates were added. The plate was incubated at 37 °C and relative fluorescence unit (RFU) was measured every hour (in total 6 h) at ex/em 540/590 nm using a Fluoroskan Ascent CF fluorescence reader (Thermo Labsystems, Vantaa, Finland). Medium samples without cells were used as background controls.
Assessment of resazurin toxicity
A concentration–response curve of the resazurin reagent was generated to determine its toxicity. 5 × 104 HepG2 cells were seeded per well in a 96-well plate in Williams Medium E containing 10 % FCS and incubated over night for cell adherence. Afterwards, cells were washed twice and serum-free medium was added. The cells were exposed to different concentrations of resazurin, ranging from 1 to 100 % (v/v) culture medium in triplicates. After 24 h, supernatant was aspirated off and cells were washed twice with PBS. Two hundred μl medium and resazurin reagent (20 % v/v) was added to each well. The cells were incubated for 5 h in the incubator. Fluorescence was measured as described above. Untreated cells were used as negative control (100 % viability) while cells treated with 20 % dimethylsulfoxide (DMSO) as positive (dead) control. Medium samples without cells were used as background controls.
Resazurin assay in 3D bioreactor
Resazurin reagent was slowly injected into the bioreactor system via sample port, resulting in a final concentration of 2 % (v/v). A 1 ml syringe with Luer-Lok connections was used for injection. Recirculation mode without medium feeding was adjusted. Samples were taken using two 1 ml syringes at the same time, adapted to a 3-way stopcock using Luer-Lok connections. First, about 1 ml of dead volume was drawn using one syringe. After that, 0.5 ml of sample was collected with the other syringe and dead volume was put back into the system. Using this sampling technique, accurate sampling was guaranteed. Fifty μl samples were transferred into 96-well plates. Fluorescence was measured at ex/em 540/590 nm. Medium samples without cells were used as background controls.
Investigation of artifacts due to extracellular components
Two hundred μl of medium and effluent (waste) sample taken from the 3D bioreactor were transferred into a 96-well plate. Forty μl of resazurin reagent were added and the plate was incubated at 37 °C in a cell incubator for 24 h. Fluorescence was measured at ex/em 540/590 nm. As positive control, a sample of the resazurin assay within the 3D HepG2 bioreactor (incubation time 6 h) was used.
Assay validation in 3D bioreactor system
HepG2 cells were inoculated into a 3D bioreactor. At cultivation day 3, viability was assessed by resazurin assay. The detergent Triton X-100 was then injected into the system at a concentration of 2 % (v/v). The system was kept in recirculation mode for 24 h and the cell viability was again assessed by resazurin assay.
Resazurin assay for viability assessment in 3D bioreactor of PHH during drug exposure
Amiodarone was injected slowly in the 3D bioreactor system, in a similar way to resazurin, preventing oscillating over concentration. End concentration was 1.2 μM. Resazurin assay was performed in the early and late phase of cultivation (i.e. before and after drug exposure) to investigate effects of the drug.
Results
Assessment of the sensitivity of resazurin
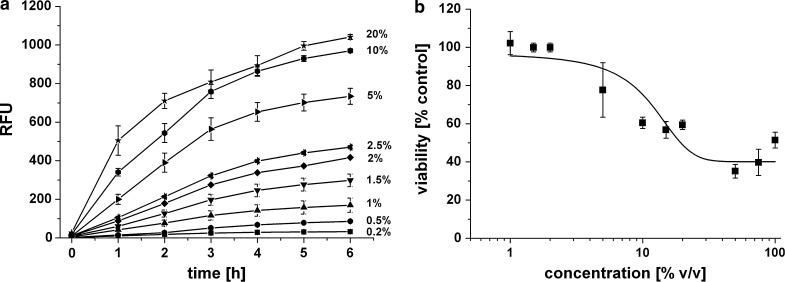
To determine the linearity between reagent concentration and increase in fluorescence, different resazurin concentrations ranging from 0.5 to 20 % were added to HepG2 cells in 96-well plates and fluorescence was measured over 6 h as shown in Fig. 2a. All tested resazurin concentrations showed a significant increase of fluorescence during 6 h of incubation. The highest correlation coefficients were found at concentrations between 1 and 2.5 % (v/v). The higher concentrations [≥5 % (v/v)] showed a linear range at the first hours of incubation, whereas saturation was observed after 4 h. Even the lowest concentration of resazurin reagent [(0.2 % v/v)] showed significant increase of fluorescence over time.
Fig. 2.
a Fluorescence signal intensity of different resazurin concentrations tested on HepG2 cells. 5 × 104 cells were seeded in 96-well plate format and incubated overnight. Different resazurin concentrations were added, ranging from 0.2 % - 20 % (v/v) as indicated. Fluorescence was measured over 6 h. Error bars indicate ± standard deviations (n = 3). RFU = relative fluorescence units. b Concentration–response curve for resazurin reagent giving percentage viability of HepG2 cells after 24 h. Cell viability was assessed relative to untreated control. Concentration of resazurin reagent in the medium (v/v) is given in percentage of culture medium. Error bars indicate ± standard deviations (n = 3)
Assessment of resazurin toxicity
For optimization of resazurin assay for its use in the 3D bioreactor, dose–response was assessed using HepG2 cells. As shown in Fig. 2b, cells exposed to higher concentrations of resazurin (i.e. >10 % v/v) for 24 h showed a lower viability as compared to control cells. However, lower resazurin concentrations (≤2 % v/v) had no effect on cell viability.
Resazurin assay in 3D bioreactor
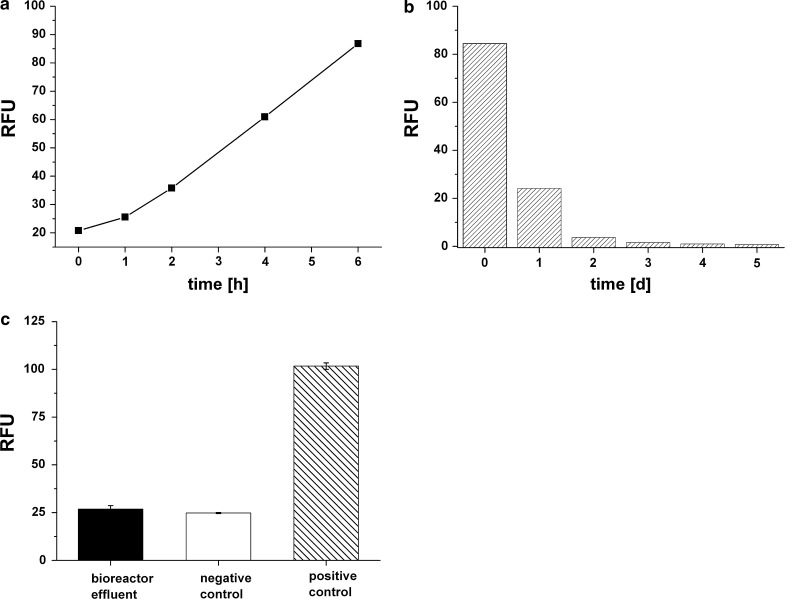
Based on the above results, we chose 2 % (v/v) resazurin concentration for viability assessment in the 3D bioreactor system. A direct injection of the Alamar Blue dye would lead to an oscillation of the concentration. To prevent this, reagent injection was carried out slowly via the sample port of the tubing system and slight mixing within the injection syringe was performed. The resazurin assay was performed at day 3 after inoculation of 3 × 107 HepG2 cells (Fig. 3a). At the start of the assay, only background fluorescence which was 21 (±1) RFU was observed. Over 6 h, linear increase of fluorescence was detected (Fig. 3a), with regression coefficients of 0.992 between RFU and time. After 6 h, the highest fluorescence was observed (102 RFU) and the slope of the straight line was 12.9.
Fig. 3.
a Resazurin assay on HepG2 cells in the 3D bioreactor system at cultivation day 3 for 6 h (3 × 107 inoculated cells) b Fluorescence measurements on effluent samples after resazurin application to determine the removal of the reagent product from the 3D bioreactor. Samples were transferred into 96-well plates and intrinsic fluorescence was measured at ex/em at 540 nm and 590 nm. Day 0 represents the day of the actual resazurin assay after medium change, day 1–5 after the resazurin assay. These samples were effluent samples collected every 24 h. The dye was washed out with a flow rate of 2 ml/h. RFU = relative fluorescence units. c Fluorescence measurements on effluent sample of 3D bioreactor and fresh medium to exclude possible artifacts due to extracellular components. Samples were transferred into 96-well plates and incubated with resazurin reagent (20 % v/v) at 37 °C for 24 h. Fluorescence was measured at ex/em at 540 nm and 590 nm. Negative control represents fresh medium incubated with resazurin reagent, positive control represents a sample of the resazurin assay within the 3D bioreactor at time point 6 h. Error bars indicate ± standard deviations (n = 3). RFU = relative fluorescence units
Investigation of artifacts due to extracellular components
Removal of the fluorescent product out of the 3D bioreactor system should occur as fast as possible to prevent any interference in other studies or assays. Effluent samples after wash out of the resazurin assay were collected and intrinsic fluorescence was measured. It was observed that the product could be washed out of the system just by feeding one reactor volume of the fresh medium and turning the system into feed mode, whereby 2 ml/h fresh medium was added. Effluent flowed out with the same rate. As depicted in Fig. 3b, only negligible fluorescence was observed at day 3 while at day 4 all resazurin assay product was washed out and removed from the bioreactor system.
As mentioned before, resazurin reagent is reduced to a fluorescent dye by both, mitochondrial and cytosolic enzymes. Possible artifacts due to extracellular components would lead to false positive values. To exclude this, both effluent sample of the 3D bioreactor, which has been in contact with the cells before, and fresh medium as negative control were incubated with resazurin reagent for 24 h. As shown in Fig. 3c, both effluent sample as well as fresh medium (negative control) show only intrinsic reagent fluorescence (26 ± 1).
Assay validation in 3D bioreactor system
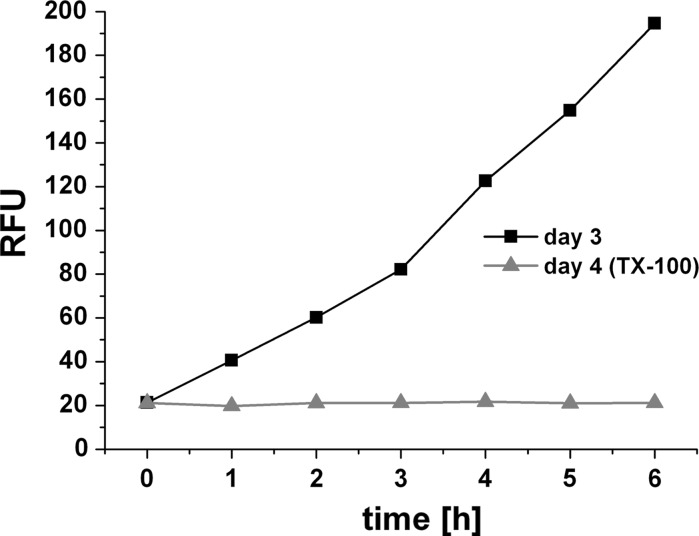
We tested whether the established assay showed a dependence on the inoculated cell number and if a positive (dead) control would create signal noise or affect the results of the assay. Therefore, we inoculated 6 × 107 HepG2 cells into the 3D bioreactor system and performed the first resazurin assay at day 3 after inoculation (Fig. 4). Again, a linear increase in fluorescence over time was measured in the recirculation samples (R2 = 0.978). Fluorescence after 6 h assay time was 195 RFU. At day 4, we injected the detergent Triton X (2 % v/v) and kept the system in recirculation mode. We again assessed viability by resazurin assay as shown in Fig. 4. We saw a stable background signal of 21 ± 0.6 RFU during 6 h assay time indicating null viability.
Fig. 4.
Resazurin assay on HepG2 cells (6 × 107 inoculated cells) in 3D bioreactor for 6 h at cultivation day 3 (closed square) and upon incubation with Triton X-100 as positive (dead) control (closed triangle)
Resazurin assay for viability assessment in 3D bioreactor of PHH during drug exposure
After optimization with HepG2 cells we proceeded with PHH in the 3D bioreactor. For primary human hepatocytes, viability was assessed in the early phase after adjusting serum-free conditions (Fig. 5a). Amiodarone was then injected into the 3D bioreactor system as described above. After exposure, amiodarone was washed-out by feeding fresh drug-free medium. Viability was again assessed in the late cultivation phase using the described resazurin method (Fig. 5b).
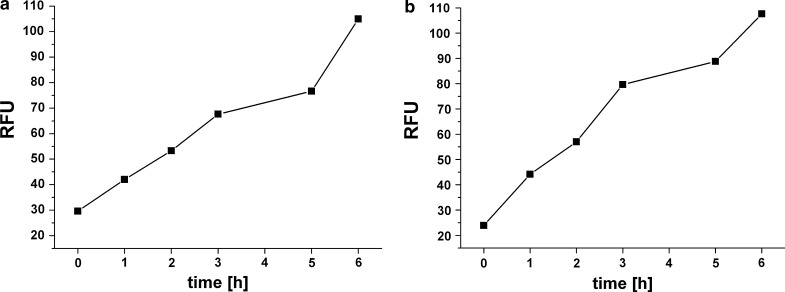
Fig. 5.
Resazurin assay on primary human hepatocytes (108 inoculated cells) in 3D bioreactor system at a day 10 b day 27. Resazurin concentration was 2 % (v/v). Samples were taken over 6 h, transferred into 96-well plates and fluorescence was measured at ex/em 540 and 590 nm. RFU relative fluorescence units
At both time points, increase of fluorescence was observed within the assay time. Fluorescence value at assay start was 27 ± 4. After 6 h, RFU was 105 (R2 = 0.955) for the first assay in the early (day 11) and 108 (R2 = 0.959) for the second assay in the late cultivation phase (day 27) as shown in table 1.
Table 1.
Resazurin assay in the 3D bioreactor system
| Primary human hepatocytes | ||
|---|---|---|
| Early phase (day 10) | Late phase (day 27) | |
| RFU after 6 h | 105 | 108 |
| Linearity (R2) | 0.955 | 0.959 |
| Slope | 11.3 | 13.0 |
Table shows relative assay values after 6 h, as well as linearity and slope of fluorescence curve over time. For primary human hepatocytes, two assays were performed in the early and late cultivation phase, i.e. before and after drug exposure
Discussion
A variety of assays are used for viability assessment in two-dimensional (2D) cultures. The 2D systems and assays are adequate and sufficient for most in vitro applications. Nevertheless, 2D monolayer cultures differ in their architecture, in the micro environment and in the phenotype from the in vivo tissue. Various 3D bioreactors offer an alternative for maintenance of cells in a tissue like environment. This is especially important for long term cultivation while maintaining the phenotype and the functionality of the test system. The possibility of long term cultivation has an impact on the testing of compounds in preclinical screening as well as in safety pharmacology for e.g. chronic liver toxicity assessments. As a prerequisite to any study, cell viability must be assessed over extended culture periods without disturbing the cells. As such a non-invasive and real time assay is required. We recently demonstrated long-term cultivation of primary human hepatocytes in a 3D bioreactor system (Mueller et al. 2011b) for 3 weeks. We used biochemical parameters such as galactose consumption and lactate production as well as production of the liver-specific markers albumin and urea for an indirect estimation of cellular viability since a direct, microscopic observation of the cells is not possible in this bioreactor. We, however, modified the system by incorporating an on-line respiration measurement device allowing the monitoring of cell viability. However, a simple and technically less demanding assay for viability assessment would be very useful.
In this study, we used a resazurin based viability assay, to directly monitor cell viability within the 3D bioreactor. This method allows direct in situ real time monitoring of viability of cells despite the black-box character of the bioreactor. Based on the results on 2D monolayer system using HepG2, we chose a concentration of 2 % (v/v) of resazurin since this concentration had no effects on the viability of the cells and gave a linear signal for 6 h.
We assessed the viability of HepG2 cells (3 × 107 cells inoculated) maintained in the 3D bioreactor at day 3 of inoculation. Linear increase of the fluorescence signal was detected during 6 h of incubation time. It was further demonstrated that easy wash-out of the product is possible without perturbing the cells. The short incubation time is moreover helpful since the whole assay can be carried out within a few hours. Reduction of the substrate by medium components or released cellular enzymes due to cell death, which would lead to false-positive values was also excluded experimentally. It was concluded that the reagent reduction was exclusively caused by living cells in the 3D bioreactor.
As further controls, we tested the established resazurin assay in a 3D bioreactor inoculated with 6 × 107 HepG2 cells. This is double the number of inoculated HepG2 cells in the above bioreactor. The highest fluorescence value observed upon 6 h assay time was 195 RFU, which is 1.9 fold higher as in the first experiment. Therefore, we conclude that the established assay show a significant dependence on the inoculated cell number. Due to the required material and the high costs of bioreactors, it is not possible to establish calibration curves using a range of different inoculated cell numbers. However, the clearly observed cell number dependence show the potential and applicability of the assay, e.g. for physiological and toxicity studies. Furthermore, we tested if dead cells would influence the results of the assay by injecting the detergent Triton X-100. After recirculating overnight, resazurin assay was again performed and showed only background fluorescence indicating that all cells were killed by incubation with Triton X-100. Based on this, we proved that a lot of dead cells or released enzymes do not interfere with the assay.
A step further, we investigated another 3D bioreactor with primary human hepatocytes. In this system, we started the cultivation using 2.5 % fetal calf serum and adjusted to serum-free conditions at day 10. Use of serum limits pharmacological and “-omics” relevant studies due to the non-defined composition, batch-to batch variations and high endogenous protein content of the serum. In our previous study, we showed the serum-free cultivation of PHH in these 3D bioreactors for 11 days. Serum-free cultivation of PHH is of particular interest since it was reported that FCS induces dedifferentiation and therefore loss of functionality of these cells (Tuschl et al. 2009).
After adjusting to serum-free conditions, we continuously exposed the PHH to therapeutic concentration of amiodarone for 4 days. The system was washed over 3 days. In the late cultivation phase (day 27), viability was again assessed using resazurin assay. By comparing fluorescence values at the end of both assays (105 RFU and 108 RFU) it was observed that viability was maintained even after drug exposure in serum-free conditions. Therefore, we could conclude that the exposure to clinically relevant concentrations of amiodarone did not affect cell viability in this experiment. This also proves the non-invasiveness of the assay. However, future studies should include determination of free drug concentration within the 3D bioreactor system since drug binding to the capillaries inside the bioreactor could occur. This may partly explain why we did not observe any toxic effects of amiodarone in our system.
In addition, future studies focusing on the proliferation profiles of HepG2 in the 3D bioreactor over time would be very useful to improve the functionality of the HepG2 cells. Some studies have shown a more differentiated cellular phenotype for HepG2 cells when grown in a three-dimensional manner (Altmann et al. 2008; Lan and Starly 2011).
We show that the resazurin based assay is applicable to 3D bioreactor systems using adherent cells. This real time in situ assay will be of tremendous importance in monitoring long term cultivation of cells in such bioreactors. This will also facilitate the monitoring of long term drug/test compound induced effects on the cells. The established assay in presented 3D bioreactor would further support future cellular physiological as well as pharmacological studies in terms of the assessment of drug effects on cellular metabolome, fluxome, proteome or peptidome, where accurate assessment of viability is essential for qualitative and quantitative analyses. Moreover, toxic effects of drugs can be assessed and the assay could also be applied to any other cell type in 3D bioreactors, supporting different research fields.
Acknowledgments
The study was carried out as a part of the Project “3D-In vitro Model for Hepatic Drug Toxicity” (Project numbers 01GG0732; 01GG0733) funded by the Bundesministerium für Bildung und Forschung (BMBF), Germany.
References
- Abu-Absi SF, Friend JR, Hansen LK, Hu WS. Structural polarity and functional bile canaliculi in rat hepatocyte spheroids. Exp Cell Res. 2002;274:56–67. doi: 10.1006/excr.2001.5467. [DOI] [PubMed] [Google Scholar]
- Altmann B, Giselbrecht S, Weibezahn KF, Welle A, Gottwald E. The three-dimensional cultivation of the carcinoma cell line HepG2 in a perfused chip system leads to a more differentiated phenotype of the cells compared to monolayer culture. Biomed Mater. 2008;3:034120. doi: 10.1088/1748-6041/3/3/034120. [DOI] [PubMed] [Google Scholar]
- Bokhari M, Carnachan RJ, Cameron NR, Przyborski SA. Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J Anat. 2007;211:567–576. doi: 10.1111/j.1469-7580.2007.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach JC, Mutig K, Sauer IM, Schrade P, Efimova E, Mieder T, Naumann G, Grunwald A, Pless G, Mas A, Bachmann S, Neuhaus P, Zeilinger K. Use of primary human liver cells originating from discarded grafts in a bioreactor for liver support therapy and the prospects of culturing adult liver stem cells in bioreactors: a morphologic study. Transplantation. 2003;76:781–786. doi: 10.1097/01.TP.0000083319.36931.32. [DOI] [PubMed] [Google Scholar]
- Gloeckner H, Jonuleit T, Lemke HD. Monitoring of cell viability and cell growth in a hollow-fiber bioreactor by use of the dye Alamar Blue. J Immunol Methods. 2001;252:131–138. doi: 10.1016/S0022-1759(01)00347-7. [DOI] [PubMed] [Google Scholar]
- Hosseinkhani H, Hosseinkhani M, Hattori S, Matsuoka R, Kawaguchi N (2010) Micro and nano-scale in vitro 3D culture system for cardiac stem cells. J Biomed Mater Res A 94:1–8 [DOI] [PubMed]
- Iwahori T, Matsuno N, Johjima Y, Konno O, Akashi I, Nakamura Y, Hama K, Iwamoto H, Uchiyama M, Ashizawa T, Nagao T. Radial flow bioreactor for the creation of bioartificial liver and kidney. Transplant Proc. 2005;37:212–214. doi: 10.1016/j.transproceed.2005.01.080. [DOI] [PubMed] [Google Scholar]
- Lan SF, Starly B. Alginate based 3D hydrogels as an in vitro co-culture model platform for the toxicity screening of new chemical entities. Toxicol Appl Pharmacol. 2011;256:62–72. doi: 10.1016/j.taap.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Mueller D, Koetemann A, Noor F. Organotypic cultures of HepG2 cells for in vitro toxicity studies. J Bioeng Biomed Sci S. 2011;2:002. [Google Scholar]
- Mueller D, Tascher G, Muller-Vieira U, Knobeloch D, Nuessler AK, Zeilinger K, Heinzle E, Noor F (2011b) In-depth physiological characterization of primary human hepatocytes in a 3D hollow-fiber bioreactor. J Tissue Eng Regen Med 5:e207–218 [DOI] [PubMed]
- Mueller D, Muller-Vieira U, Biemel KM, Tascher G, Nussler AK, Noor F. Biotransformation of diclofenac and effects on the metabolome of primary human hepatocytes upon repeated dose exposure. Eur J Pharm Sci. 2012;45:716–724. doi: 10.1016/j.ejps.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Noor F, Niklas J, Muller-Vieira U, Heinzle E. An integrated approach to improved toxicity prediction for the safety assessment during preclinical drug development using Hep G2 cells. Toxicol Appl Pharmacol. 2009;237:221–231. doi: 10.1016/j.taap.2009.03.011. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- Schmelzer E, Triolo F, Turner ME, Thompson RL, Zeilinger K, Reid LM, Gridelli B, Gerlach JC (2010) Three-Dimensional perfusion bioreactor culture supports differentiation of human fetal liver cells. Tissue Eng Part A 16:2007–2016 [DOI] [PubMed]
- Schmitmeier S, Langsch A, Jasmund I, Bader A. Development and characterization of a small-scale bioreactor based on a bioartificial hepatic culture model for predictive pharmacological in vitro screenings. Biotechnol Bioeng. 2006;95:1198–1206. doi: 10.1002/bit.21089. [DOI] [PubMed] [Google Scholar]
- Sodunke TR, Turner KK, Caldwell SA, McBride KW, Reginato MJ, Noh HM. Micropatterns of Matrigel for three-dimensional epithelial cultures. Biomaterials. 2007;28:4006–4016. doi: 10.1016/j.biomaterials.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Tuschl G, Hrach J, Walter Y, Hewitt PG, Mueller SO. Serum-free collagen sandwich cultures of adult rat hepatocytes maintain liver-like properties long term: a valuable model for in vitro toxicity and drug–drug interaction studies. Chem Biol Interact. 2009;181:124–137. doi: 10.1016/j.cbi.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Zeilinger K, Holland G, Sauer IM, Efimova E, Kardassis D, Obermayer N, Liu M, Neuhaus P, Gerlach JC. Time course of primary liver cell reorganization in three-dimensional high-density bioreactors for extracorporeal liver support: an immunohistochemical and ultrastructural study. Tissue Eng. 2004;10:1113–1124. doi: 10.1089/ten.2004.10.1113. [DOI] [PubMed] [Google Scholar]