Abstract
Isoflavones are phenolic compounds widely distributed in plants and found in a high percentage in soybeans. They have important biological properties and are regarded as potential chemopreventive agents. The aim of this study was to verify the preventive effect of two soy isoflavones (genistein and daidzein) by a micronucleus assay, analysis of GST activity, and real-time RT-PCR analysis of GSTa2 gene expression. Mutagens of direct (doxorubicin) and indirect (2-aminoanthracene) DNA damage were used. Hepatoma cells (HTC) were treated with genistein or daidzein for 26 h at noncytotoxic concentrations; 10 μM when alone, and 0.1, 1.0 and 10 μM when combined with genotoxic agents. The micronucleus test demonstrated that both isoflavones alone had no genotoxic effect. Genistein showed antimutagenic effects at 10 μM with both direct and indirect DNA damage agents. On phase II enzyme regulation, the current study indicated an increase in total cytoplasmic GST activity in response to genistein and daidzein at 10 μM supplementation. However, the mRNA levels of GSTa2 isozymes were not differentially modulated by genistein or daidzein. The results point to an in vitro antimutagenic activity of genistein against direct and indirect DNA damage-induced mutagenicity.
Keywords: Hepatoma cell line, Antimutagenicity, Genistein, Daidzein, Phase II enzyme
Introduction
The intake of soy isoflavones (genistein and daidzein) has been associated with a reduced risk of cancer particularly estrogen-related cancers, such as breast cancer, heart disease, osteoporosis, and menopause symptoms (Linseisen et al. 2004; Klein and King 2007). Genistein and daidzein have received considerable attention for their chemopreventive properties against cancer initiation and development. Both isoflavones belong to the chemical group of flavonoids, which have little to no toxicity and have a long history of human consumption, making them excellent candidates for chemopreventive agents (Moon et al. 2006).
Cancer chemoprevention refers to the use of natural or synthetic compounds that are able to inhibit, delay, or reverse the multi-step process of carcinogenesis. The protective action of these agents is explained as a combination of various proposed mechanisms involving molecular association with carcinogens, the modulation of enzymes involved in biotransformation reactions (such as cytochrome p450 and glutathione S-transferase), anti-oxidant, anti-inflammatory, anti-angiogenesis, and immunomodulatory actions, apoptosis induction, cell-cycle arrest, cell-differentiation induction, antimicrobial effects, tyrosine kinase inhibition, and others (Akiyama et al. 1987; Chen et al. 2003; Foti et al. 2005; Moon et al. 2006; Rufer and Kulling 2006; Chodon et al. 2007; Choi and Kim 2008; Naithani et al. 2008).
Previous studies on the genotoxic or antigenotoxic effects of genistein are controversial. Genistein was able to induce micronuclei and DNA strand breaks in Chinese hamster lung fibroblasts (V79 cells) (Kulling and Metzler 1997), chromosomal structural aberrations in human lymphocytes (Kulling et al. 1999), and micronuclei in mouse splenocytes and V79 cells (Record et al. 1995; Di Virgilio et al. 2004). There is little evidence for genotoxic activity associated with daidzein, as there was only a slight increase in micronucleus formation in V79 cells treated with 75 μM daidzein (Di Virgilio et al. 2004). Genistein and daidzein have also been reported as antigenotoxic compounds (Polívková et al. 2006; Pugalendhi et al. 2009).
The glutathione S-transferases (GSTs) represent a major group of detoxification enzymes that work by conjugating xenobiotics to reduced glutathione to facilitate dissolution in the aqueous cellular and extracellular media. Some GSTs are free in the cytoplasm (alpha, mu, pi, sigma, theta, zheta, and omega), and some are bound to cellular membranes (microsomal GST, and leukotriene C4 synthetase). Xenobiotics are able to increase the expression of certain GST genes (alpha, mu, and pi isoenzymes) by interaction with the following: (a) the antioxidant-responsive element (ARE); (b) the xenobiotic-responsive element (XRE); GST P enhancer l (GPE); or (c) the glucocorticoid-responsive element (GRE) (Hayes and Pulford 1995; Singh and Michael 2009).
The present study evaluated the antiproliferative, mutagenic, and antimutagenic potential of genistein and daidzein on hepatoma cells from Rattus norvegicus (HTC) in vitro. For the induction of DNA lesions, we used one direct agent (doxorubicin, DXR) and one indirect agent dependent on metabolic activation via cytochrome P450s (2-aminoanthracene, 2AA). The DNA lesions were assessed by the frequency of micronucleus formation, and the participation of GST in the chemopreventive effects was evaluated by total cytoplasmic GST activity and GSTalpha2 (GSTa2) gene expression.
Materials and methods
Chemical agents
For this study, doxorubicin (CAS no. 25316-40-9) (DXR, Pharmacia) was used as a direct genotoxic agent and 2-aminoanthracene (CAS no. 613-13-8) (2-AA, Acros Organics) as an indirect genotoxic agent. DXR was generously supplied by the University Hospital of North Parana, Londrina—Paraná—Brazil. DXR was dissolved in PBS, and 2-AA was dissolved in dimethyl sulfoxide (DMSO, Mallinckrodt Chemicals). Genistein (CAS no. 446-72-0) and Daidzein (CAS no. 486-66-8) were obtained from Acros Organics and dissolved in dimethyl sulfoxide (DMSO) to prepare the appropriate concentrations. The final DMSO concentration in the culture medium did not exceed 1 %.
HTC cell culture conditions
HTC cells from a Rattus norvegicus hepatoma were obtained from the Cell Bank of Rio de Janeiro (RJCB—Brazil). The cells were grown as monolayer cultures in 25 cm2 culture flasks in DMEM/F12 (Gibco) with the addition of 10 % fetal bovine serum (FBS, Gibco). The cultures were incubated in a humidified incubator at 37 °C and 5 % CO2.
MTT cytotoxicity assay
The cytotoxic effect of the isoflavones, genistein and daidzein on the rat HTC cell line was determined by the 3-(4-5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay based on the protocol described by Mosmann (1983). Briefly, cells were plated into 96-well tissue culture dishes at a density of 2.5 × 104 cells/well in 100 μL of medium. The cells were cultured for 24 h at 37 °C and 5 % CO2 to adhere to the well. The supernatant was removed and replaced with fresh medium without FBS, containing genistein or daidzein at concentrations of 0.1, 1, 10, 50, and 100 μM. After 4 h, 150 μL of culture medium plus MTT (0.5 mg/mL) was added to each well. The MTT solution was carefully decanted off and formazan was extracted from the cells with 200 μL of DMSO in each well. Color was measured with a 96-well ELISA plate reader at 550 nm. Data were plotted as relative cell survival rate (%) = (Absorbance Test/Absorbance control) × 100. MTT assays were performed in triplicate.
Cell proliferation kinetics
HTC cells (5 × 104 cells/flask) were seeded in 10 cm2 tissue flasks and cultured for 24, 48, 72 and 96 h supplemented with genistein or daidzein at 10 μM. The number of cells were counted every 24 h. Cells were trypsinized and counted using a Neubauer hemocytometer. Cell viability was evaluated by trypan blue staining. The population doubling time was calculated using the algorithm provided by http://www.doubling-time.com. Experiments were carried out in triplicate.
The micronucleus assay with a cytokinesis block (MNCtB)
Exponentially growing HTCs were seeded at a density of 106 cells/flask in 25 cm2 tissue flasks and incubated for 24 h before treatment allowing cell adhesion. After this period, the cells were washed with PBS and treated for 26 h with fresh medium containing cytochalasin B at a final concentration of 3.0 μg/mL and the chemicals continued in the respective treatment protocols. For the genotoxicity evaluation protocol, the cells were treated with genistein or daidzein at 10 μM. For antigenotoxicity, cells were treated with genistein or daidzein at 0.1, 1 or 10 μM along with either DXR at 0.2 μM or 2-AA at 13 μM. One thousand binucleated cells with well-preserved cytoplasm were scored on coded slides in three experimental repetitions using a microscope at 400 × magnification, which resulted in the analysis of 1,000 cells per treatment. The criteria for the identification of binucleated cells and micronuclei were described by Fenech (2000). The capacity of genistein or daidzein to reduce the DNA damage induced by DXR or 2-AA was calculated according to Waters et al. (1990) using the following formula:
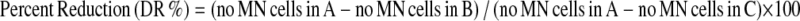 |
where, A = DNA damage-inducing agent, B = associated treatment and C = negative control.
For cell cycle analysis, 500 cells per treatment group were scored for the presence of one, two, or more than two nuclei and the nuclear division index (NDI) was calculated as described by Fenech (2000). Experiments were performed in triplicate.
GST activity
Total cytoplasmic glutathione S-transferase (GST) activity was measured according to the protocol described by Houghton et al. (2007). This method is based on the GST-catalyzed reaction between GSH and 1-Chloro-2,4-dinitrobenzene (CDNB, Sigma), which acts as an electrophilic substrate for GSTs. HTC cells (106) were seeded in 25 cm2 culture flasks with 5 ml of complete culture medium. After this period, cells were treated with genistein or daidzein at 10 μM concentration for 12 or 24 h. After incubation, the cells were trypsinized and kept in an ice bath. The cells were disrupted using 3 cycles of freezing at −80 °C followed by vortexing. To guarantee 100 % cell disruption, the cells were evaluated with trypan blue staining. The cell lysates were centrifuged at 90 × g for 5 min at 4 °C, and the cytosolic solutions were obtained from the supernatant. For measurement of the enzymatic activity, the cytosolic solution was pre-incubated for 5 min at 30 °C (Houghton et al. 2007). For each measurement, 100 μl of the reaction mixture (GSH + CDNB at final concentrations of 8.3 mM GSH and 3.3 mM CDNB) was added. A kinetic analysis was performed in which the absorbance of the mixture at 340 nm was measured every minute for 5 min using a Libra S32 spectrophotometer (Biochrom) coupled to a temperature regulator TE-2005 (Tecnal). To calculate the GST activity, the following formula was used: GST activity = Slope (ΔAbs/ml)/9.6 mM−1cm−1 × protein concentration (mg/ml). The activity was expressed as nmol/min/mg of protein, where 1 enzyme unit is the amount of enzyme required to conjugate 1,000 nmol/min/mg at 30 °C. The protein concentration was measured by the Bradford method (Bradford 1976). Each enzymatic measurement was performed in duplicate, and the average of the results obtained was used in the calculation of GST activity. The experiments were performed in triplicate.
Real-time quantitative RT-PCR
The study of GSTa2 mRNA was chosen based on the study by Wiegand et al. (2009), which showed a significant increase in the expression levels of this enzyme in Wistar rat liver tissue. The HTC cells (106/flask) were treated with genistein or daidzein at 10 μM for 12 h. Total RNA was prepared from genistein or daidzein-treated HTC cells using Trizol-LS® reagent (Invitrogen Life Technologies) according to the manufacturer’s protocol. RNA samples were incubated with DNase (1U) (CAS 18068-015, Invitrogen Life Technologies). The quantity of RNA was determined by spectrophotometer analysis (BioPhotometer—Eppendorf), and the integrity by eletrophoresis in a 1 % agarose gel. The cDNA synthesis was carried out in reactions containing 1 μg total RNA (20 μl), 10 pmol/μL oligo dT primer (1μL), 10 mM dNTPs (2μL), RNAseOUT (0.1μL) and reverse transcriptase M-MLV (1μL) (Invitrogen Life Technologies). The real-time RT-PCR was performed using Platinum® SYBR Green qPCR SuperMix-UDG (Invitrogen Life Technologies). In each reaction, 0.4 μM of primer (forward and reverse) and 2 μL of template cDNA was added to make a final volume of 20 μL. The reactions were performed in PTC 200 DNA Engine Cycler using a Chromo4 Detection System (MJ Research). The primers utilized in these experiments are Rattus norvegicus glutathione S-transferase (GST) and β-actin as shown in Table 1.
Table 1.
The primer pairs used in this study for PCR amplification
The reaction conditions were as follows: 95 °C for 3 min, 40 cycles (95 °C for 30 s, 60 °C for 30 s, 72 °C for 20 s), 95 °C for 10 s and 40 °C for 1 min., followed by melting curve analysis temperature between 50 and 90 °C (at 0.5 °C every 5 s). The data were normalized by using the β-actin internal standard gene. The relative differences between HTC cell control (DMSO) and treatment groups (genistein or daidzein) were calculated according to the method describe by Pfaffl (2001).
Each experimental protocol was performed in triplicate in two independent experiments. Data are expressed as mean values ± standard derivation (SD).
Statistical analyses
The MTT assay, the micronucleus assay, and the GST activity data were analyzed by analysis of variance (ANOVA) followed by Tukey’s test. The cell proliferation kinetic was estimated by exponential regression as described by Weisstein http://mathworld.wolfram.com/LeastSquaresFittingExponential.html. Point to point analysis was performed by analysis of variance (ANOVA) followed by Dunnett’s test.
For RT-PCR, REST (Relative Expression Software Tool—384, REST-384©—version 2) (Pfaffl et al. 2002) software was used. For the mRNA induction or repression studies, only expression levels above twofold with statistically significant differences were seen as relevant.
Results
MTT cytotoxicity assay
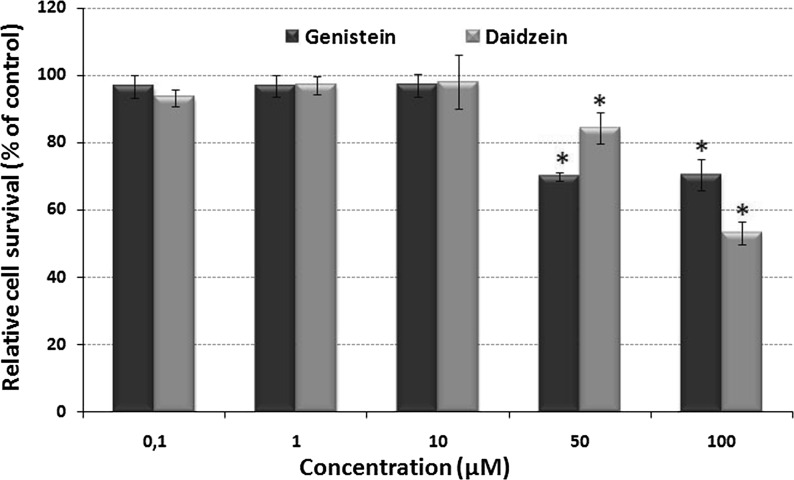
Figure 1 shows the effect of various concentrations of genistein or daidzein on the relative cell survival percentage of HTC cells after 24 h. Survival of vehicle-treated control groups, not exposed to isoflavones or DNA damage inductors, was defined as 100 % survival. Concentrations ranging from 0.1 to 10 μM showed poor inhibition of HTC growth. However, at the higher concentrations of 50 and 100 μM, a marked inhibition occurred for genistein and daidzein in comparison to the control.
Fig. 1.
Relative HTC cell survival determined by the cytotoxicity assay. Cells were treated with different concentrations of genistein or daidzein (0.1–100 μM), and the cytotoxicity was determined using the MTT assay for 24 h. Data are represented as the mean ± SD of triplicate experiments. The asterisk (*) indicates a significant difference (p < 0.01) between the group treated and the group control. The statistic analysis was performed using the absorbance data
Cell proliferation kinetics
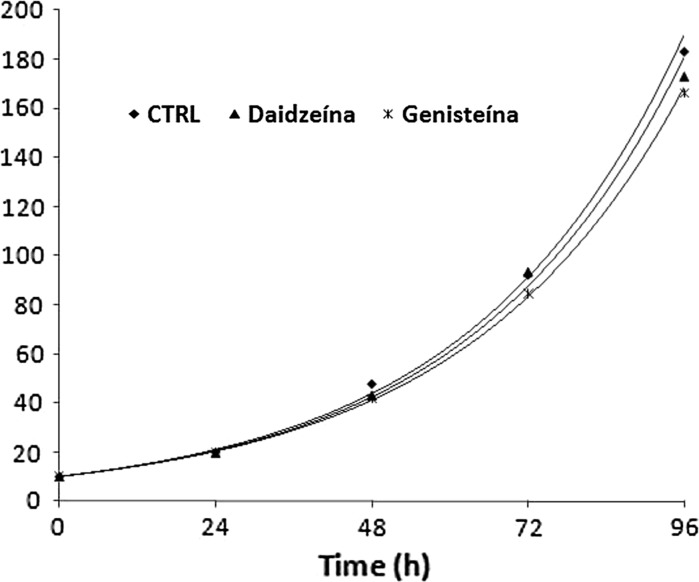
The effect of genistein and daidzein on the growth kinetics of HTC cells is shown in Fig. 2. Cells were incubated with genistein or daidzein at 10 μM. Cell counting was used to measure cell proliferation. Both isoflavones displayed similar growth rates compared with control treatment (Fig. 2). The R2 of >0.97 in these models suggesting a successful regression on proliferation data. The doubling time for control, genistein and daidzein was estimated to be 23.17, 23.5 and 23.2 h, respectively.
Fig. 2.
Cell proliferation kinetics for genistein and daidzein (10 μM) on HTC cell
The micronucleus assay
In Tables 2 and 3, the total micronuclei formation, means, standard deviation and damage reduction percentage in HTC cells are presented. No significant difference in the induction of micronuclei was observed between the groups treated with genistein or daidzein and the negative control (p > 0.05). These findings indicate the absence of a genotoxic effect of genistein or daidzein at the concentrations tested. Direct or indirect DNA damage agents, used as positive controls, demonstrated the sensitivity of the micronucleus assay and yielded a clear positive response at the concentrations used. The results also show that genistein (p > 0.001) at a 10 μM concentration in combination with DXR or 2AA significantly reduced the frequency of micronuclei (Tables 2 and 3). This reduction was 38.59 and 71.43 % in the micronucleus assay for DXR and 2AA, respectively. However, no significant difference in the frequency of micronuclei was observed for cultures treated with low genistein or daidzein concentrations (0.1 and 1 μM) that were associated with DNA damage inductors, and daidzein 10 μM showed no protective effect. The nuclear division indices (NDI) observed demonstrate that genistein and daidzein did not influence the cell cycle kinetics between treatments.
Table 2.
Evaluation of mutagenicity and antimutagenicity against doxorubicin for genistein or daidzein on HTC cells, determined by micronucleus assay
| Treatments | Mean ± SD | DR % | NDI |
|---|---|---|---|
| Control | 8.33 ± 0.57b | 1.86 | |
| DXR | 65.66 ± 0.57a*** | 1.84 | |
| Genistein 10 μM | 8.33 ± 0.57a | 1.87 | |
| Daidzein 10 μM | 9.33 ± 0.57a | 1.9 | |
| Genistein 0.1 μM + DXR | 61.66 ± 3.21b | 6.43 | 1.89 |
| Genistein 1 μM + DXR | 56.00 ± 10.44b | 16.37 | 1.85 |
| Genistein 10 μM + DXR | 43.33 ± 2.88b*** | 38.59 | 1.83 |
| Daidzein 0.1 μM + DXR | 62.00 ± 2.64b | −0.01 | 1.88 |
| Daidzein 1 μM + DXR | 59.66 ± 4.72b | −1.76 | 1.85 |
| Daidzein 10 μM + DXR | 65.00 ± 5.00b | 0.58 | 1.85 |
DR % percent reduction, NDI nuclear division index, DXR doxorubicin (0.2 μM)
*** Statistically significant difference
aCompared statiscally to the control
bCompared statiscally to DXR. Values are expressed as the mean of micronucleus in 1,000 analysed cells ± SD
Table 3.
Evaluation of mutagenicity and antimutagenicity against 2-aminoanthracene for genistein or daidzein on HTC cells, determined by micronucleus assay
| Treatments | Mean ± SD | DR % | NDI |
|---|---|---|---|
| Control | 9.00 ± 1.00b | 1.78 | |
| 2AA | 30.00 ± 3.00a*** | 1.82 | |
| Genistein 10 μM | 10.33 ± 1.53a | 1.76 | |
| Daidzein 10 μM | 11.33 ± 2.08a | 1.78 | |
| Genistein 0.1 μM + 2AA | 30.00 ± 1.00b | 0.00 | 1.80 |
| Genistein 1 μM + 2AA | 29.00 ± 1.00b | 4.76 | 1.78 |
| Genistein 10 μM + 2AA | 15.00 ± 3.60b*** | 71.43 | 1.75 |
| Daidzein 0.1 μM + 2AA | 30.66 ± 0.57b | −3.17 | 1.80 |
| Daidzein 1 μM + 2AA | 30.00 ± 1.00b | 0.00 | 1.80 |
| Daidzein 10 μM + 2AA | 27.33 ± 2.08b | 12.70 | 1.78 |
DR % percent reduction, NDI nuclear division index, 2AA 2-amino-anthracene (13 μM)
*** Statistically significant difference
aCompared statiscally to the control
bCompared statiscally to 2AA. Values are expressed as the mean of micronucleus in 1,000 analysed cells ± SD
GST activity
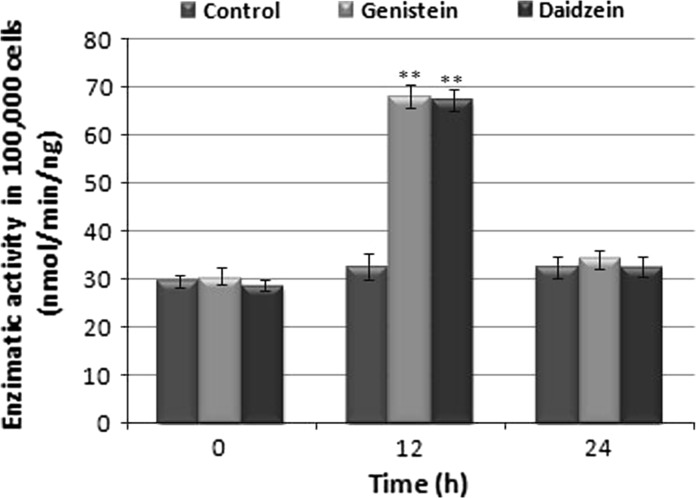
Figure 3 shows activities of GST, a phase II xenobiotic-metabolizing enzyme, in the HTC cell lineage. The activities of phase II enzymes were significantly increased in cells treated with genistein and daidzein for 12 h compared to control. This effect was not observed with cells treated for 24 h (Fig. 3).
Fig. 3.
Measurement of GST in HTC cells subjected to treatment with 10 μM genistein and daidzein, for 12 h and 24 h. The asterisks (**) represent significant differences compared to the enzyme dosage with respect to control at the respective time (p < 0.001)
GST gene expression
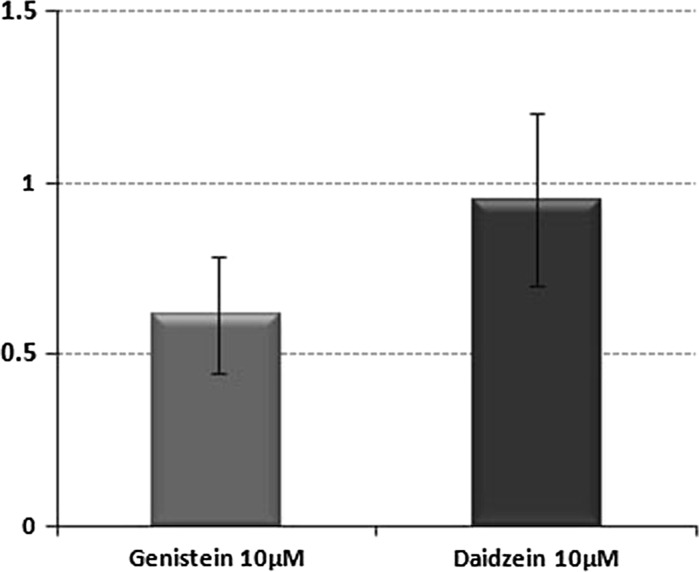
As observed in Fig. 4, the exposure of HTC cells to 10 μM of genistein and daidzein for 12 h did not affect GSTa2 expression, as shown by real time PCR. Data are expressed as fold change between treatment and control, which is the difference between the normalized RNA expression level (ΔCt, where Ct is the threshold cycle) of the gene in the control group and that of the gene in the treatment group.
Fig. 4.
Fold change of GSTa2 mRNA in HTC cells after 12 h exposure to genistein or daidzein. Data represent the mean ± SD of two experiments
Discussion
HTC cell lineage is a drug-metabolizing cell line that has been used for the direct and indirect identification of antigenotoxic agents (Bellini et al. 2006; Oliveira et al. 2006; Marcarini et al. 2011). We investigated the protective effects of genistein and daidzein on DNA damage induced by doxorubicin (a direct-acting mutagen) and 2-aminoanthracene (an indirect-acting mutagen) on HTC cells by using the micronucleus test. We first examined the cytotoxic effect of genistein and daidzein at concentrations ranging from 0.1 to 100 μM for 24 h to avoid false positives in the genotoxicity test. The highest noncytotoxic doses (10 μM) of genistein and daidzein were further evaluated at 96 h to explore the cell proliferation kinetics. Our results of the MTT assay show that genistein and daidzein have similar cytotoxic effects on HTC cells. Only the highest concentrations of both (50 and 100 μM) showed cytotoxic effect. Both genistein and daidzein at 10 μM did not alter the proliferation rate of HTC cells at 96 h. Control, genistein, and daidzein showed similar doubling times (22.7, 23.4 and 23.2 h, respectively), excluding the possibility of time-dependent effects. The concentrations of 0.1, 1, and 10 μM of genistein and daidzein were selected for subsequent assays.
For the tested concentrations of genistein and daidzein, we did not observe mutagenic effects on HTC cells because the number of micronuclei did not differ significantly from the controls. Many studies reported that genistein is able to induce DNA damage, though its mechanism is not known. It has been shown that genistein, daidzein and equol (a metabolite of daidzein) induced micronucleus formation in V79 cell lines. Genistein showed a clastogenic effect, and equol showed an aneugenic effect. Also, genistein has shown a pronounced genotoxic effect compared to daidzein (Di Virgilio et al. 2004). Genistein, but not daidzein, induced DNA strand breaks, and micronuclei in Chinese hamster V79 cells (Kulling and Metzler 1997), chromosomal structural aberrations in human lymphocytes (Kulling et al. 1999), and in vitro formation of micronuclei in mouse splenocytes (Record et al. 1995). The required concentrations for genotoxicity described in these studies ranged from 5 to 25 μM. However, when mice or humans are exposed to higher levels of dietary isoflavones, genetic damage is not observed (Record et al. 1995; Miltyk et al. 2003).
In contrast to these in vitro studies, we observed antimutagenicity against DXR or 2AA for genistein. This is in agreement with in vivo studies that have reported antimutagenic effects of genistein against direct and indirect mutagens and carcinogens in a bone marrow micronucleus test. (Polívková et al. 2006, Pugalendhi et al. 2009). Antigenotoxic compounds can directly interact with genotoxins, inactivating them chemically (scavenging) or enzymatically (inhibiting the metabolic activation of pro-mutagenic compounds or inducing phase II enzymes) (Antunes and Araujo 2000).
The use of DXR as an antitumor drug is due to its direct action on DNA. Different processes are responsible for the biological effects of DXR, such as through interactions with DNA topoisomerase II and the generation of free radicals and subsequent oxidative stress (Quiles et al. 2002). The proposed mechanism for antimutagenicity of genistein is related to its antioxidant properties. Genistein is a known antioxidant (Foti et al. 2005; Lee et al. 2004; Hernandez-Montes et al. 2006) and has been reported to inhibit oxidative stress and DNA damage induced by methylglyoxal (a reactive dicarbonyl compound) in human mononuclear cells (Wu and Chan 2007). Another possible mechanism for the reduction of DNA damage by genistein is the stimulation of DNA repair. It was recently reported that genistein up-regulated the expression of genes involved in DNA damage repair in prostate tumor cells (Bhamre et al. 2010).
The genotoxic effect of 2AA requires metabolic activation in order to exert their genotoxic effects. The main activating enzymes include CYPs isoforms (cytochrome P450), which convert hydrophobic compounds in reactive hydrophilic compounds (Jemnitz et al. 2004). On the other hand, phase II enzymes such as glutathione-S-transferase (GSTs), UDP-glucuronyl transferase (UGT) and NAD(P)H quinine oxidoreductase (QR) catalyze conjugation reactions (Pool-Zobel et al. 2005). Thus, phase I enzymes often result in bioactivation compared to inactivation resulting from phase II. According to Manson et al. (1997) the result of exposure to an environmental toxin in terms of acute or chronic toxicity largely depends on the balance between these two processes. To better understand the antimutagenic mechanism of genistein on indirect mutagens, we focused on the metabolic activation of phase II enzymes.
It is important to note that the concentration of genistein (10 μM) which showed to be antimutagenic in the present study was beyond the concentrations of genistein or daidzein normally obtained in plasma of humans consuming isoflavone containing diets (less than 1–5 μM) (Maubach et al. 2003; Safford et al. 2003; Wiseman et al. 2004). However, intake of an aglycone isoflavone supplement (containing 450 mg genistein, 300 mg daidzein, and other isoflavones daily for 6 months) in a group of 53 prostate cancer patients displayed high plasma levels of genistein (39.85µM) and daidzein (45.59 µM) (deVere White et al. 2010). Moreover, data in humans and animals showed that concentrations of flavonoids in the endocrine-responsive tissues including liver, mammary gland, ovary, prostate, and uterus may exceed plasma levels several fold (Gardner et al. 2009; Chang et al. 2000; Janning et al. 2000). Therefore, a concentration of 10 uM of genistein and daidzein was used that showed to be nontoxic for HTC cells for subsequent experiments of GST activity and gene expression. We observed a time-dependent increase of total cytoplasmic GST activity for both isoflavones (increase was observed only for 12 h of treatment, normalizing by 24 h), but mRNA levels of GSTa2 isoenzymes were not differentially modulated by either genistein or daidzein after 12 h of treatment. These results show that genistein and daidzein are able to increase GST activity by up-regulation of GST isoforms other than GSTa2. The increase of total cytoplasmic GST activity found in our study may be responsible for the reduction of 2AA-mediated DNA damage by genistein. GST could increase the activity of the detoxification system, which may facilitate the elimination and inactivation of the active mutagens formed by metabolism of 2AA. However, this mechanism only partially explained the protective role of genistein because daidzein similarly induced GST activity in HTC cells, but did not show an antigenotoxic effect. For in vivo assays, Wiegand et al. (2009) showed that GST activity was not affected by feeding a genistein-supplemented diet to rats, while mRNA levels of GSTa2 isoenzymes were significantly increased (plasma genistein concentration was 7.6 ± 1.0 μM).
From the chemical point of view, genistein and daidzein have strong similarities, except for the 5′-hydroxyl group; thus, they may perform their functions via similar pathways. Our results showed that genistein and daidzein similarly induce GST activity. However, genistein and daidzein exhibited different abilities to protect HTC cells against DNA damage by DXR or 2AA. Previous studies have shown that the number and position of the hydroxyl group of the A ring effectively increased the radical scavenging activity of genistein (Choi et al. 2009). Thus, it is clear that several mechanisms may be involved in this antimutagenic process by genistein. Further investigation is needed to elucidate the mechanisms of cellular responses to soybean isoflavone exposure.
Acknowledgments
The authors thank National Council of Scientific and Technological Development (CNPq), Coordination for the Improvement of Superior Level (CAPES) and Araucaria Foundation for the Support of Scientific and Technological Development of Parana—Brazil.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Itoh SWN, Shibuya N, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Antunes LMG, Araujo MCP. Mutagenicidade e antimutagenicidade dos principais corantes para alimentos. Rev Nutr. 2000;13:81–88. doi: 10.1590/S1415-52732000000200002. [DOI] [Google Scholar]
- Bellini MF, Angeli JPF, Matuo R, Terezan AP, Ribeiro LR, Mantovani MS. Antigenotoxicity of Agaricus blazei mushroom organic and aqueous extracts in chromosomal aberration and cytokinesis block micronucleus assays in CHO-k1 and HTC cells. Toxicol in Vitro. 2006;20:355–360. doi: 10.1016/j.tiv.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Bhamre S, Sahoo D, Tibshirani R, Dill DL, Brooks JD. Gene expression changes induced by genistein in the prostate cancer cell line LNCaP. Open Prost Cancer J. 2010;3:86–98. [Google Scholar]
- Bradford MM. A rapid sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding”. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang HC, Churchwell MI, Delclos KB, Newbold RR, Doerge DR. Mass spectrometric determination of Genistein tissue distribution in diet-exposed Sprague-Dawley rats. J Nutr. 2000;130:1963–1970. doi: 10.1093/jn/130.8.1963. [DOI] [PubMed] [Google Scholar]
- Chen WF, Huang MH, Tzang CH, Yang M, Wonga MS. Inhibitory actions of genistein in human breast cancer (MCF-7) cells. Biochim Biophys Acta. 2003;1638:187–196. doi: 10.1016/S0925-4439(03)00082-6. [DOI] [PubMed] [Google Scholar]
- Chodon D, Ramamurty N, Sakthisekaran D. A Preliminary studies on induction of apoptosis by genistein on HepG2 cell line. Toxicol in Vitro. 2007;2:887–889. doi: 10.1016/j.tiv.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Choi EJ, Kim GH. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine. 2008;15:683–690. doi: 10.1016/j.phymed.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Choi JN, Dockyu K, Hyung KC, Kyung MY, Jiyoung K, Choong HL (2009) 2'-hydroxylation of genistein enhanced antioxidant and antiproliferative activities in MCF-7 human breast cancer cells. J Microbiol Biotechnol 19(11):1348–1354 [DOI] [PubMed]
- deVere White RW, Tsodikov A, Stapp EC, Soares SE, Fujii H, Hackman RM. Effects of a high dose, aglycone-rich soy extract on prostate-specific antigen and serum isoflavone concentrations in men with localized prostate cancer. Nutr Cancer. 2010;62:1036–1043. doi: 10.1080/01635581.2010.492085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio AL, Iwami K, Wätjen W, Kahl R, Degen GH. Genotoxicity of isoflavones genistein, daidzein and equol in V79 cells. Toxicol Lett. 2004;151:151–162. doi: 10.1016/j.toxlet.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/S0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Foti P, Erba D, Riso P, Spadafranca A, Criscuoli F, Testolin G. Comparison between daidzein and genistein antioxidant activity in primary and cancer lymphocytes. Arch Biochem Biophys. 2005;433:421–427. doi: 10.1016/j.abb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Gardner CD, Oelrich B, Liu JP, Feldman D, Franke AA, Brooks JD. Prostatic soy isoflavone concentrations exceed serum levels after dietary supplementation. Prostate. 2009;69:719–726. doi: 10.1002/pros.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione-S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance part I. Crit Rev Biochem Mol Biol. 1995;30:445–520. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Hernandez-Montes E, Pollard SE, Vauzour D, Jofre-Montseny L, Rota C, Rimbach G, Weinberg PD, Spencer PE. Activation of glutathione peroxidase via Nrf1 mediates genistein’s protection against oxidative endothelial cell injury. Biochem Biophys Res Commun. 2006;346:851–859. doi: 10.1016/j.bbrc.2006.05.197. [DOI] [PubMed] [Google Scholar]
- Houghton P, Fang R, Techatanawat I, Teventon G, Hylands PJ, Lee CC. The sulphorhodamine (SRB) assay and other approaches to testing plants extracts and derived compounds for activities related to reputed anticancer activity. Methods. 2007;42:377–387. doi: 10.1016/j.ymeth.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Janning P, Schuhmacher US, Upmeier A, Diel P, Michna H, Degen GH, Bolt HM. Toxicokinetics of the phytoestrogen daidzein in female DA/Han rats. Arch Toxicol. 2000;74:421–430. doi: 10.1007/s002040000149. [DOI] [PubMed] [Google Scholar]
- Jemnitz K, Geza V, Torok G, Toth E, Vereczkey L. Comparative study in the Ames test of benzo[a]pyrene and 2-aminoanthracene metabolic activation using rat hepatic S9 and hepatocytes following in vivo or in vitro induction. Mutagenesis. 2004;19:245–250. doi: 10.1093/mutage/geh026. [DOI] [PubMed] [Google Scholar]
- Klein CB, King AA. Genistein genotoxicity: critical considerations of in vitro exposure dose. Toxicol Appl Pharmacol. 2007;224:1–11. doi: 10.1016/j.taap.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Kulling SE, Metzler M. Induction of micronuclei, DNA strand breaks and HPRT mutations in cultured Chinese hamster V79 cells by the phytoestrogen coumoestrol. Food Chem Toxicol. 1997;35:605–613. doi: 10.1016/S0278-6915(97)00022-7. [DOI] [PubMed] [Google Scholar]
- Kulling SE, Rosenberg B, Jacobs E, Metzler M. The phytoestrogens coumoestrol and genistein induce structural chromosomal aberrations in cultured human peripheral blood lymphocytes. Arch Toxicol. 1999;73:50–54. doi: 10.1007/s002040050585. [DOI] [PubMed] [Google Scholar]
- Lee R, Kim YJ, Lee YJ, Chung WH. The selective effect of genistein on the toxicity of bleomycin in normal lymphocytes and HL-60 cells. Toxicology. 2004;195:87–95. doi: 10.1016/j.tox.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Linseisen J, Piller R, Hermann S, Chang-Claude J. Dietary phytoestrogen intake and premenopausal breast cancer risk in a German case-control study. Int J Cancer. 2004;110:284–290. doi: 10.1002/ijc.20119. [DOI] [PubMed] [Google Scholar]
- Manson MM, Ball HWL, Barrett MC, Clark Hl, Judah DJ, Williamson G, Neal GE (1997) Mechanism of action of dietary chemoprotective agents in rat liver: induction of phase I and II drug metabolizing enzymes. Carcinogenesis 18:1729–1738 [DOI] [PubMed]
- Marcarini JC, Tsuboy MSF, Luiz RC, Ribeiro LR, Hoffmann-Campo CB, Mantovani MS. Investigation of cytotoxic, apoptosis-inducing, genotoxic and protective effects of the flavonoid rutin in HTC hepatic cells. Exp Toxicol Pathol. 2011;63:459–465. doi: 10.1016/j.etp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Maubach J, Bracke ME, Heyerick A, Depypere HT, Serreyn RF, Mareel MM, De Keukeleire D. Quantitation of soy-derived phytoestrogens in human breast tissue and biological fluids by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:137–144. doi: 10.1016/S1570-0232(02)00789-4. [DOI] [PubMed] [Google Scholar]
- Miltyk W, Craciunescu CN, Fischer L, Jeffcoat RA, Koch MA, Lopaczynski W, Mahone C, Jeffcoat RA, Crowell J, Paglieri J, Zeisel SH. Lack of significant genotoxicity of purified soy isoflavones (genistein, daidzein, and glycitein) in 20 patients with prostate cancer. Am J Clin Nutr. 2003;77:875–882. doi: 10.1093/ajcn/77.4.875. [DOI] [PubMed] [Google Scholar]
- Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol in Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid Colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–56. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Naithani R, Huma LC, Moriarty RM, Mccormick DL, Mehta RG. Comprehensive review of cancer chemopreventive agents evaluated in experimental carcinogenesis models and clinical trials. Curr Med Chem. 2008;15:1044–1071. doi: 10.2174/092986708784221403. [DOI] [PubMed] [Google Scholar]
- Oliveira RJ, Ribeiro LR, Silva AF, Matuo F, Mantovani MS. Evaluation of antimutagenic activity and mechanisms of action of b-glucan from barley, in CHO-k1 and HTC cell lines using the micronucleus test. Toxicol in Vitro. 2006;20:1225–1233. doi: 10.1016/j.tiv.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polívková Z, Langová M, Šmerák P, Bártová J, Bárta I. Antimutagenic Effect of Genistein. Czech J Food Sci. 2006;24:119–126. [Google Scholar]
- Pool-Zobel B, Veeriah S, Bohmer FD (2005) Modulation of xenobiotic metabolizing enzymes by anticarcinogens – focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat Res 591(1–2):74–92 [DOI] [PubMed]
- Pugalendhi P, Manoharan S, Panjamurthy K, Balakrishnan S, Nirmal MR. Antigenotoxic effect of genistein against 7,12-dimethylbenz[a]anthracene induced genotoxicity in bone marrow cells of female Wistar rats. Pharmacol Rep. 2009;61:296–303. doi: 10.1016/s1734-1140(09)70035-0. [DOI] [PubMed] [Google Scholar]
- Quiles JL, Huertas JR, Battino M, Mataix J, Ramirez-Tortosa M. Antioxidant nutrients and adriamycin toxicity. Toxicology. 2002;180:79–95. doi: 10.1016/S0300-483X(02)00383-9. [DOI] [PubMed] [Google Scholar]
- Record IR, Jannes M, Dreosti IE, King RA. Induction of Micronucleus Formation in Mouse Splenocytes by the Soy Isoflavone Genistein In Vitro but not In Vivo. Food Chem Toxicol. 1995;33:919–922. doi: 10.1016/0278-6915(95)00062-7. [DOI] [PubMed] [Google Scholar]
- Rufer CE, Kulling SE. Antioxidant Activity of Isoflavones and Their Major Metabolites Using Different in Vitro Assays. J Agric Food Chem. 2006;54:2926–2931. doi: 10.1021/jf053112o. [DOI] [PubMed] [Google Scholar]
- Safford B, Dickens A, Halleron N, Briggs D, Carthew P, Baker V. A model to estimate the oestrogen receptor mediated effects from exposure to soy isoflavones in food. Regul Toxicol Pharmacol. 2003;38:196–209. doi: 10.1016/S0273-2300(03)00091-6. [DOI] [PubMed] [Google Scholar]
- Singh MS, Michael M. Role of xenobiotic metabolic enzymes in cancer epidemiology. Methods Mol Biol. 2009;472:243–264. doi: 10.1007/978-1-60327-492-0_10. [DOI] [PubMed] [Google Scholar]
- Waters MD, Brady AL, Stack HF, Brockman HE. Antimutagenic profiles for some model compounds. Mutat Res. 1990;238:57–85. doi: 10.1016/0165-1110(90)90039-E. [DOI] [PubMed] [Google Scholar]
- Weisstein EW Least Squares Fitting–Exponential.” From MathWorld–A Wolfram Web Resource. http://mathworld.wolfram.com/LeastSquaresFittingExponential.html. Acess 07/02/2011
- Wiegand H, Wagner AE, Boesch-Saadatmandi C, Kruse HP, Kulling S, Rimbach G. Effect of Dietary Genistein on Phase II and Antioxidant Enzymes in Rat Liver. Cancer Genomics Proteomics. 2009;6:85–92. [PubMed] [Google Scholar]
- Wiseman H, Casey K, Bowey EA, Duffy R, Davies M, Rowland IR, et al. Influence of 10 wk soy consumption on plasma con-centrations and excretion of isoflavonoids and on gut microflora in healthy adults. Am J Clin Nutr. 2004;80:692–699. doi: 10.1093/ajcn/80.3.692. [DOI] [PubMed] [Google Scholar]
- Wu HJ, Chan WH. Genistein protects methylglyoxal-induced oxidative DNA damage and cell injury in human mononuclear cells. Toxicol in Vitro. 2007;21:335–342. doi: 10.1016/j.tiv.2006.09.002. [DOI] [PubMed] [Google Scholar]