Abstract
Bone marrow derived stem cells (BMSC) have paved way to clinical approaches for its utilization in a variety of diseases due to its ease of isolation combined with its multilineage differentiation capacity. However, the applicability of BMSC is not successful due to the lesser number of nucleated cells obtained from large samples. Hence, culture expansion of BMSC is a prerequisite, as high numbers of stem cells are needed to meet the standards of clinical advancement. There are attempts on optimizing culture condition for large scale production of BMSC. It was believed that, prolonged culture of BMSC is difficult since they tend to lose their characteristics and differentiation potential. Hence, our study aims to determine whether BMSCs could retain its proliferative and differentiation capacity in prolonged in vitro culture by a comparative study on extensive culturing of BMSC with the following four media, DMEM LG (DMEM-Low Glucose), DMEM KO (DMEM-Knock Out), Alpha MEM (Alpha Minimal Essential Medium), DMEM F 12. We found that two samples among the three cultured tend to lose their property in long term culturing. Besides, we also found that DMEM LG and Alpha MEM were the optimal media for in vitro culturing of BMSC. Overall, it was concluded that BMSC can be cultured until passage 15 without losing its characteristics. However, its potency beyond passage 15 has to be further elucidated for utilization of the ex vivo expanded BMSC for subsequent cellular therapies.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-012-9471-0) contains supplementary material, which is available to authorized users.
Keywords: Bone marrow, Long term culture, Mesenchymal stem cells, Proliferation, Differentiation
Introduction
Regardless of the discovery of stem cells from various tissues and body fluids (Gargett et al. 2009; In't Anker et al. 2003; Jones et al. 2004; Seo et al. 2004), stem cells derived from bone marrow had been potentially considered as a primeval source for treating a wide horizon of diseases (Deda et al. 2008; Kumar et al. 2009). A disclosure of the existence of bone marrow derived stem cells (BMSC) and its significant research interest from its prehistoric origin has opened way to their large application in clinical approaches. Despite being a preliminary source, bone marrow derived stem cells were not ideal in attempting curative therapies. The first and foremost of all disadvantages put forward is the low frequency of nucleated cells obtained from large quantity of sample (Rebelatto et al. 2008). The second important disadvantage of BMSC is that the proliferation and differentiation capacity of MSC declines with age, reducing their therapeutic potential (Stolzing et al. 2008). Additionally, low frequency of mesenchymal stem cell creates a threat for cell migration and engraftment (Kuethe et al. 2004; Wagner et al. 2005; Hendrikx et al. 2006).
Hence, extensive expansion of BMSC ex vivo becomes a prerequisite to obviate the aforesaid difficulties to obtain sufficient cell number required for human cell based therapies. The use of MSCs for clinical approaches in many fields of medicine first requires the bio-safety of these ex vivo expanded cells and it has to be carefully investigated through appropriate and sensitive tests. Furthermore, as MSCs possess a greater propensity for ex vivo expansion, the International Society for Cellular Therapy (ISCT) reported that extensively passaged cells may be controlled for a normal karyotype to reduce the probability of chromosomal abnormalities, including potentially transforming events (Dominici et al. 2006). There have been attempts in lieu of these aforesaid features in optimizing culture conditions for BMSC that could be beneficial for clinical and therapeutic applications. Despite these attempts, there are several uncertainties related to the malignant transformation of adult human BMSC in extensive proliferative condition. Literary evidence reported that BMSC are not susceptible to malignant transformation and remain appropriate for therapeutic approaches (Bernardo et al. 2007). However, there are reports suggesting loss of stem cell characteristics, increase in telomerase activity and malignant transformation under prolonged culturing of BMSC (Pal et al. 2008; Miura et al. 2006).
Furthermore, there is paucity of reports on optimization of culture media for prolonged culturing of BMSC. Consequently, optimization of culture condition, retention of proliferative and differentiation ability, maintenance of normal karyotype are most crucial in order to overcome these challenges for their use in clinical trials. Hence, the aim of this study is to unravel the existing uncertainties by potentially ascertaining whether bone marrow could proliferate and differentiate in prolonged culturing in vitro using different media: DMEM LG, Alpha MEM, DMEM F12 and DMEM KO. Furthermore, this research aims to optimize the appropriate media for prolonged culture of bone marrow derived mesenchymal stem cells.
Materials and methods
Bone marrow donors
Bone marrow cells were harvested from patients undergoing experimental cell therapy for spinal cord injury according to the reviewed and accepted protocol from Lifeline multispecialty hospital, Chennai, India. Written informed consent was obtained prior to sample collection in accordance with the requirements of the ethical committee. 20 ml of the sample was aspirated from the iliac crest region of 3 subjects (n = 3, 2 men and a woman) of age group 23–50 with a mean BMI of 24.7 ± 2.6 kg/m2. The samples were processed within 2 h.
Cell isolation and culture
Mononuclear cells were isolated from bone marrow aspirate by density gradient centrifugation using Ficoll Paque™. Erythrocytes present in the cell fraction were lysed using 0.7 % NH4Cl solution for 5 min at room temperature. The cells were subjected to further centrifugation and the pellet recovered was resuspended in PBS. Single cell suspension was further enumerated and evaluated for its cell viability using Trypan Blue staining. Cells were plated at a density of 3.4 × 104/cm2 in T-25 flasks (Nunc, Roskilde, Denmark) and cultured in four different media: DMEM-LG (Invitrogen, Bangalore, India), α-MEM (Invitrogen), DMEM-F12 (Invitrogen), and in DMEM–KO (Invitrogen), each was supplemented with 10 % FBS (Invitrogen) and 1 % antibiotic–antimycotic solution (Invitrogen). The cells were maintained for 2–4 days before first medium change. Standard culture conditions of 37 °C, 5 % CO2 and 95 % humidity were maintained and 70–80 % confluency was obtained. The primary culture was subcultured until passage 20 with medium change twice every week.
Flowcytometric characterization
About 1 × 106 cells were treated with fluorochrome conjugated antibodies such as CD 34-PE, CD 31-FITC, HLA-DR-PERCP, CD 44-FITC, CD 73-PE, CD 29-PE, CD 166-PE, CD 54-PERCP (BD Biosciences, Gurgaon, India); CD 90-PERCP, CD 105-APC, CD 117-APC and CD 49d-PE (e-Biosciences, San Diego, CA, USA). The cells were labelled by incubating in dark for 20 min at 37 °C. The incubated cells were washed thrice with wash flow buffer (phosphate buffer supplemented with 2 % (v/v), FBS (Sigma Aldrich, Bangalore, India) and 0.1 % (w/v) sodium azide, NaN3 (Sigma Aldrich) and resuspended in BD FACS flow. BD FACS-DIVA Software was used for data acquisition and analysis.
Growth curve analysis
The growth curves were plotted to evaluate the growth characteristics of the isolated cell population in four different media (DMEM-LG, α-MEM, DMEM-F12 and in DMEM –KO, each supplemented with 10 % FBS and 1 % antibiotic–antimycotic solution). The growth characterization of bone marrow derived MSCs at P3 and P20 was carried out in a 12 well plate in duplicates with a seeding density of 3 × 105 cells per plate at day 0. The cells were harvested by trypsinization (0.25 %Trypsin–EDTA), viability was tested by trypan blue exclusion method (viability > 90 %) and enumerated. The growth of the cells was calculated until day 10 on a daily basis. As a result, the cells obtained at each day until day 10 were plotted on a log-linear scale. The growth curve symbolizes the exponential raise from day 1 till day 5 and existence of a lengthy stationary phase, with a very small lag phase.
Evaluation of population doubling time (PDT)
The population doubling time was calculated from the growth curve obtained. At the end of the exponential phase of the log-linear scale of growth curve, the population doubling time of the cells was calculated using the formula:
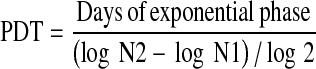 |
where, N1 was the number of cells at the beginning of the exponential growth and N2 was the number of cells at the end of the exponential growth.
Multilineage differentiation
BMSCs were differentiated into osteogenic and adipogenic lineages to confirm their multilineage differentiation potential. The cultured BMSCs of 70–80 % confluence were isolated at passage 3 and passage 20 and induced into osteogenic and adipogenic lineages using specific induction media.
Osteogenic differentiation
Sub-confluent cultures (70–80 %) of P3 and P20 cells were isolated by trypsinization (0.25 % Trypsin–EDTA solution) and approximately 3 × 104 cells were seeded into culture flasks and cultured in the growth medium at optimal culture condition. At 80–90 % confluence the growth medium was replaced with four different osteogenic induction media (DMEM-LG, α-MEM, DMEM-F12 and DMEM–KO each of which was supplemented with 10 % FBS, 1 % antibiotic, 0.1 μM dexamethasone (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich) and 2 mM ascorbic acid (Sigma-Aldrich)). Osteogenic differentiation was evident from day 15 with the nodule formation and refractile calcium deposit formation in the cultured cells, which were further confirmed by von Kossa and Alizarin Red staining.
Von Kossa staining was performed on 10 % formalin fixed cells. The cells were then treated with 1 % silver nitrate (Sigma-Aldrich) and illuminated with UV for 45 min. The illuminated cells were then washed and treated with 5 % sodium thiophosphate (Sigma-Aldrich). The treated cells were rinsed in distilled water and stained using Nuclear Fast Red (Sigma-Aldrich) for 1 min. The stained cells were then washed and imaged. Osteoblast calcium deposits were also stained using alizarin red. Alizarin red staining was done on 70 % ethanol fixed cells. The fixed cells were then washed and stained with alizarin red stain (Sigma-Aldrich) working solution and incubated for 30 min at room temperature. The stained specimens were then washed and imaged under microscope.
Adipogenic differentiation
Adipogenic differentiation was induced on an 80–90 % confluent culture by replacing the growth medium with adipogenic induction media (DMEM-LG, α-MEM, DMEM-F12 and DMEM–KO, each of which was supplemented with 10 % FBS, 1 % antibiotic, 1 μM dexamethasone (Sigma-Aldrich), 0.5 mM isobutyl methyl xanthine (Sigma-Aldrich), 10 μg insulin (Sigma-Aldrich) and 200 μM indomethacin (Sigma-Aldrich). Lipid vacuole accumulation occurs within 2 weeks and was evident from day 12 of culture in adipogenic medium. It was further confirmed by Oil-Red-O staining.
The adipocytes obtained upon differentiation were fixed using 10 % formalin. The fixed cells were washed with 60 % isopropanol and dried. The dried cells were then treated with 1 ml of Oil-O-Red (Sigma-Aldrich) working solution and incubated for 10 min. The stained cells were washed and imaged under microscope using DigiProView software.
Karyotyping
The BMSCs cultured in DMEM LG and DMEM KO were karyotyped at late passage to verify the maintenance of chromosomal normality. Standard Giemsa staining procedure was performed; the chromosome preparations were obtained from 70 to 80 % confluent cells. The cells were treated with Colcemid solution (KaryoMax-Colcemid, Invitrogen) to stop microtubule formation. The mitotic arrested cells were then harvested using 0.25 % trypsin–EDTA. The extracted cells were then immersed in 75 mmol/l KCl for 30 min at room temperature and were obtained by centrifugation. The supernatant was replaced with fixative (methanol: acetic acid, 3:1) and the suspension was spread over slides for observation and imaging. At least 20 metaphase spreads were analyzed. The karyotypes were imaged using Nikon-Eclipse-90i microscope (Nikon) using cytovision software.
Statistical analysis
All quantitative data are represented as mean ± standard error mean (SEM). The statistical analysis was carried out using the software, statistical package for social science, SPSS 15.0 (SPSS Inc., Chicago, IL, USA). The statistical significance was assessed by One Way Analysis of Variance (ANOVA) along with Duncan multiple range test. In all comparisons, p value <0.01 and <0.05 was considered statistically significant.
Results
Cell culture
The bone marrow derived stem cells (3.4 × 104 cells) were isolated and expanded in four different media (DMEM-LG, DMEM-F12, DMEM–KO and α-MEM) based on their adherence and found to maintain fibroblastic morphology throughout extensive culture (until P20) (Fig. 1a, b). However, it was found that only one sample out of the three could reach P20. Remaining samples could not grow beyond P15.
Fig. 1.
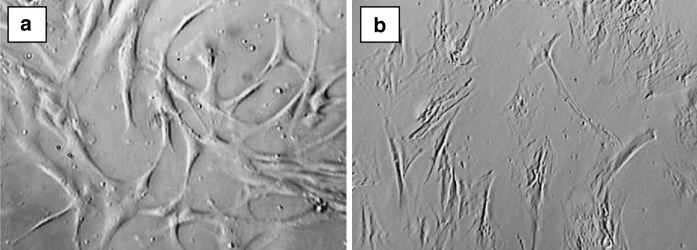
Morphology of cultured human bone marrow derived MSC. The morphology of cultured human Bone marrow derived MSCs at early passage-P3 (a) and late passage-P20 (b)
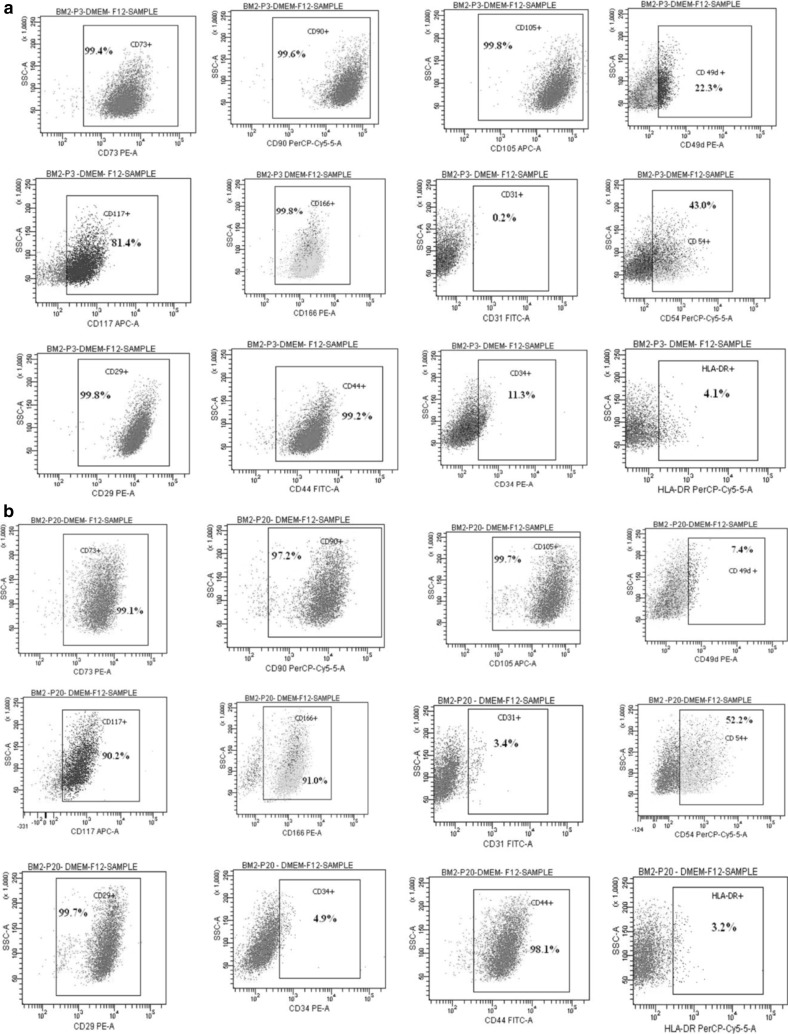
Immunophenotyping
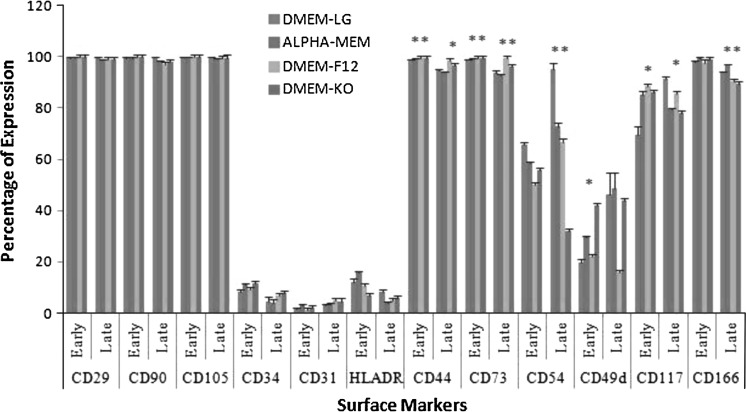
From the immunophenotypic analysis of early-P3 (Fig. 2a) and late-P20 passage (Fig. 2b) characteristics, we found that the mesenchymal stem cell markers (CD 90, CD 105, and CD 73) and certain cell adhesion molecules (CD 29, CD 44 and CD 166) were remarkably expressed throughout prolonged culture. On the other hand, the markers such as CD 34 and HLADR remained sparse, thereby providing evidence for retention of expression profile even in prolonged culture. However, a variation was observed throughout culturing in the expression profile of CD 117, CD 54 and CD 49d with respect to different media (Table S1). The statistical significance of the cell surface marker expression was computed at both early (P3) and late (P15) passages (Fig. 3).
Fig. 2.
Immunophenotypic analysis of cell surface markers of bone marrow derived MSC. Immunophenotypic expression profile of cell surface markers: CD73, CD90, CD105, CD49d, CD117, CD166, CD31, CD54, CD29, CD34, CD44 and HLA-DR of bone marrow derived MSC analysed using flowcytometry at early passage-P3 (a) and late passage-P20 (b)
Fig. 3.
Statistical comparison of cell surface marker expression. Early passage (P3); late passage (P15); statistical significance: * p < 0.05, ** p < 0.01
Multi-lineage differentiation
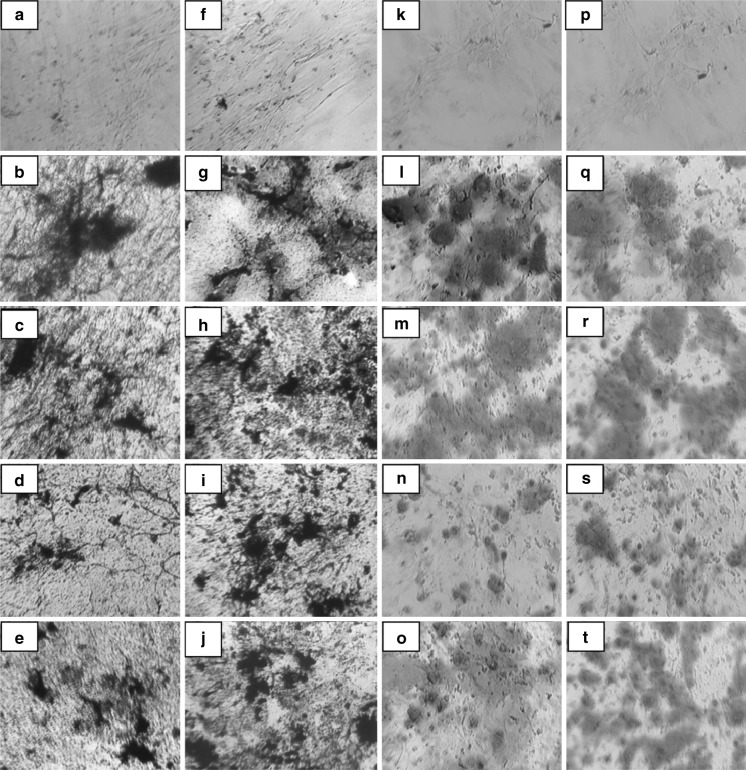
The plasticity of adherent MSCs derived from bone marrow was assessed by osteogenic and adipogenic differentiation of both early (P3) and late (P20) passages in the aforesaid media. Osteogenic differentiation was confirmed by staining the intracellular calcium deposits using von Kossa (Fig. 4a–j) and Alizarin red (Fig. 4k–t) staining on day 21. Adipocyte differentiation was evident from the 12th day by the appearance of intracellular lipid vacuoles and was further confirmed by Oil Red O (Fig. 5a–j) staining on day 18. Non- induced MSCs served as control for staining.
Fig. 4.
Osteogenic differentiation of bone marrow derived MSC. Confirmation of osteogenesis using von Kossa staining at early-P3 (a–e) and late passages-P20 (f–j) control: a, f; DMEM-LG: b, g; ALPHA-MEM: c, h; DMEM-F12: d, i; DMEM-KO: e, j) Alizarin Red staining at early-P3 (k–o) and late passages-P20 (p–t) control: k, p; DMEM-LG: l, q; ALPHA-MEM: m, r; DMEM-F12: n, s; DMEM-KO: o, t
Fig. 5.
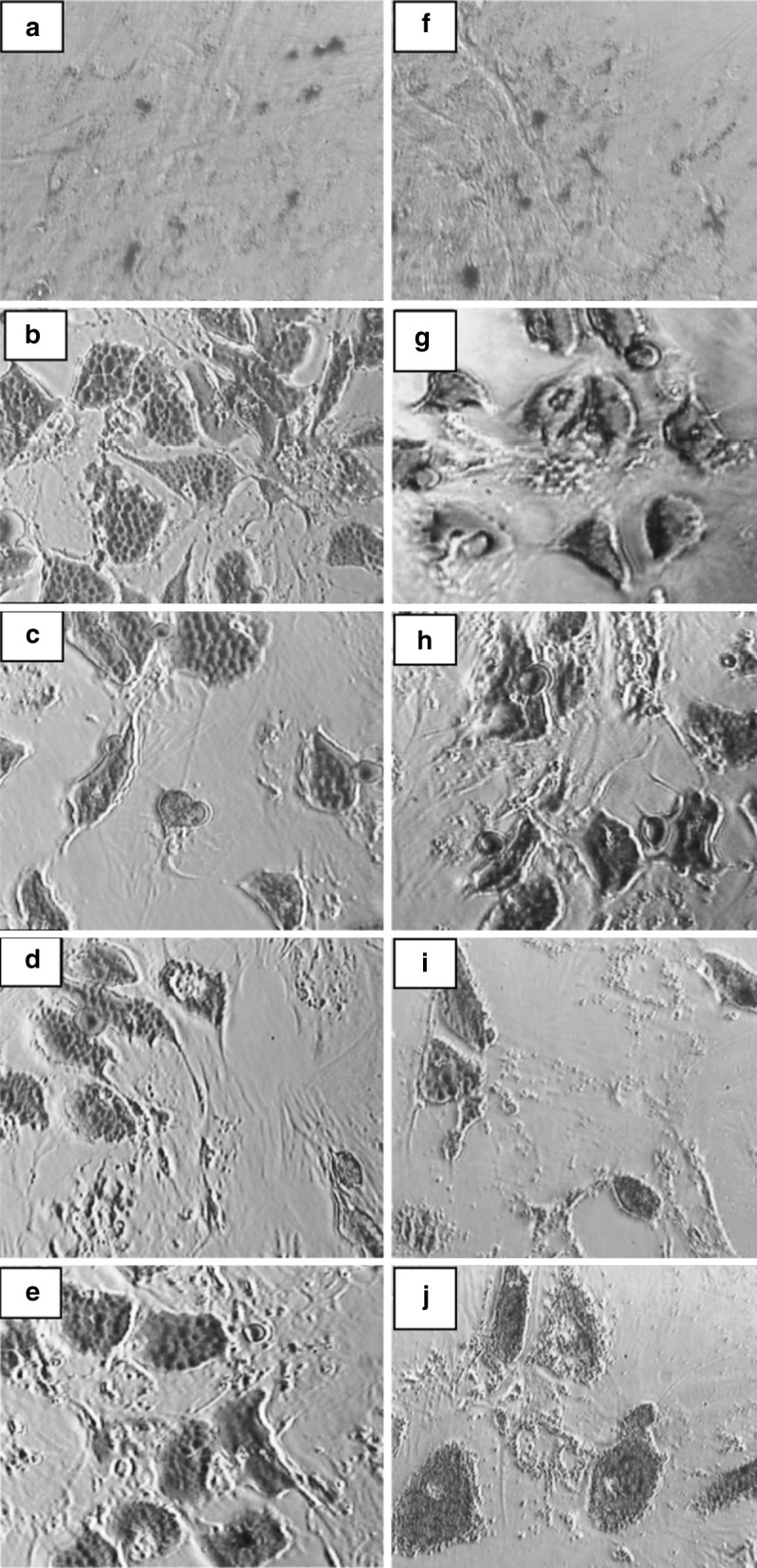
Adipogenic differentiation of bone marrow derived MSC. Confirmation of adipogenesis using Oil Red O staining at early-P3 (a–e) and late passages-P20 (f–j) control: a, f; DMEM-LG: b, g; ALPHA-MEM: c, h; DMEM-F12: d, i; DMEM-KO: e, j)
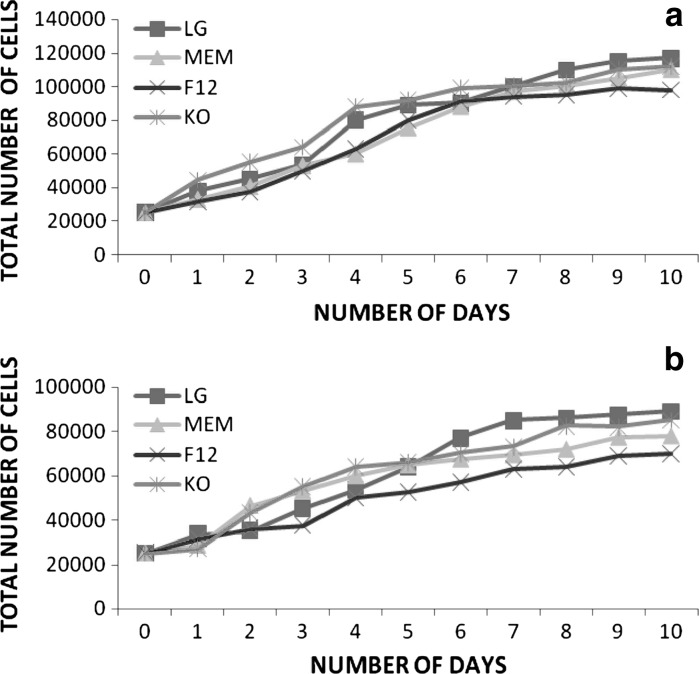
Evaluation of growth curve and PDT
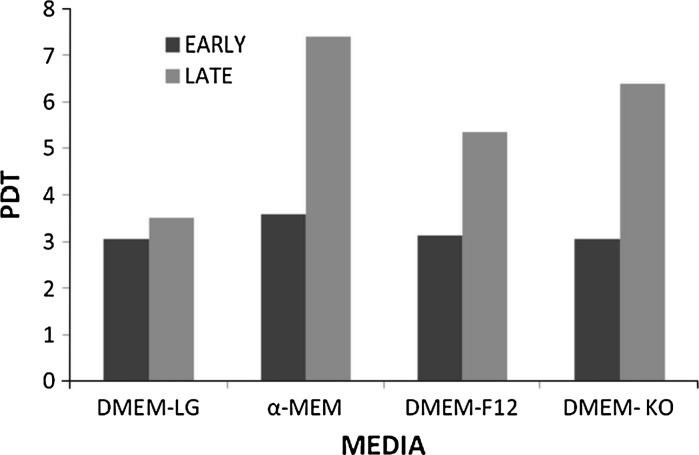
Growth characteristics of BMSCs (n = 3) were analyzed at early (P3) and late (P20) passages in DMEM-LG, α-MEM, DMEM-F12 and DMEM–KO media each supplemented with 10 % FBS and 1 % antibiotic. The growth of cells was calculated until day 10 on a daily basis (Fig. 6a, b). The growth curve symbolized exponential rise from day 2 till day 6 and existence of a lengthy stationary phase, with short lag phase. Population doubling time (PDT) was found to be lowest (3.04 days) in DMEM-LG and DMEM KO, intermediate (3.12 days) in DMEM-F12 and highest (3.57 days) in α-MEM for early passage. However, decrease in proliferation rate was seen at late passage with a PDT highest (7.4 days) in α-MEM, intermediate (6.38 and 5.34 days) in DMEM KO and DMEM F12, respectively, and lowest (3.51 days) in DMEM-LG (Fig. 7).
Fig. 6.
Growth curve of bone marrow derived MSC. Growth curve analysis of bone marrow derived MSC at early passage-P3 (a) and late passage-P20 (b) with respect to different media: LG (Low Glucose medium), MEM (Minimal Essential Medium), F12 (F12 medium), and KO (Knock Out medium)
Fig. 7.
Population doubling time analysis of Bone marrow derived MSC. Population doubling time analysis of bone marrow derived MSC at early-P3 and late -P20 passage with respect to different media: DMEM-LG, α-MEM, DMEM-F12 and DMEM–KO
Karyotyping
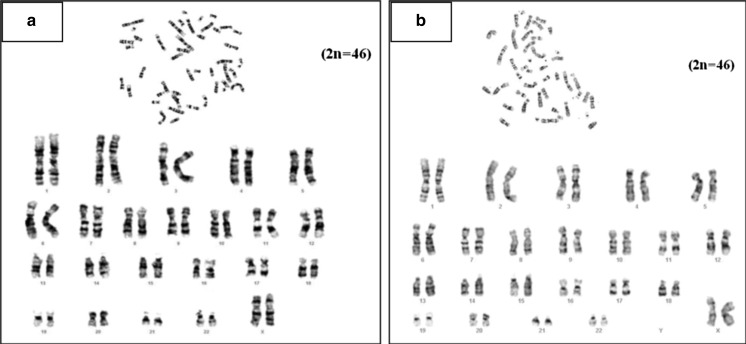
The structural and numerical normality of chromosomes in the cultured BMSCs was analysed by standard Giemsa staining method in DMEM LG and DMEM KO at late (P20) passage. GTG banding (Fig. 8a, b) confirmed the stability of chromosomes, as no anomaly was recorded in both allosomes and autosomes even at later passages.
Fig. 8.
Karyotyping of bone marrow derived MSC. Karyotype analysis of bone marrow derived MSC at late passage-P20 in DMEM-LG (a) and DMEM–KO (b)
Discussion
Being in the regenerative medicine epoch for treatment of degenerative diseases, it is important to address the inconclusive tribulations associated with the culture and proliferation of stem cells. Repair and regenerative potential together with tissue homing property of BMSC makes them prospective candidate for regenerative therapy (Gimble et al. 2003; Okura et al. 2009; Aggarwal and Pittenger 2005; Le Blanc et al. 2003; Majumdar et al. 2003). But, unclear experimental information on the grounds of upscaling and stemness maintenance along extensive culture conditions limits their clinical applicability. From our current study, it was found that bone marrow can be extensively cultured without losing its stem cell characteristics until P15. This was identified through its retention in morphology, expression profile, capacity to differentiate and normal karyotype analyzed at prolonged culturing. Our finding was in consistent with the work of Bernardo et al. (2007). On the other hand, our results were in contradictory to certain accumulated evidences which reported that BMSC could not withstand its characteristics for longer passages as they tend to increase its telomerase activity and undergo transformation (Pal et al. 2008; Miura et al. 2006).
However, we found that only one sample reached P20 and the remaining samples could not be passaged beyond P15, thereby providing evidence that bone marrow can be extensively cultured without losing its potency until P15. Evaluation of BMSC culture in different basal media led us to conclude DMEM LG and DMEM KO are suitable for the expansion of BMSC at early passages. However, unlike DMEM LG, DMEM KO produced a decrease in its proliferative potency at late passages, which was evident from the growth curve and population doubling time. Furthermore, from growth curve and PDT it was found that DMEM F12 and Alpha MEM are not suitable media for ex vivo expansion of BMSC.
In conclusion, from this study, we identified that sufficient number of bone marrow derived mesenchymal stem cells can be obtained through prolonged in vitro culture without losing their stemness and differentiation ability. Furthermore, the study revealed a normal karyotype of BMSC even at prolonged culturing; thereby elucidating the fact that BMSC had not undergone malignant transformation. Besides, from the results obtained, we found that DMEM LG served as an appropriate culture medium for prolonged culturing of BMSC. It can be identified thus, that lower glucose concentration might serve as most appropriate for extensive culturing of BMSC in vitro. However, although Alpha MEM, having the lower concentration of glucose, it does not serve as an appropriate medium in our study, when growth curve and PDT were considered. This can be a potential area of future research pursuit to exactly identify the difference in culture condition among these two media. Moreover, although all three samples could grow until P15, only one sample could reach P20. Hence, extensive work elucidating the stemness maintenance of BMSC cultures will provide incredible applications for cell based therapies in regenerative regimes.
Electronic supplementary material
Table S1 Comparison of surface antigenic expression of bone marrow derived MSC in different media. Statistical significance: * p<0.05, ** p<0.01 (DOC 1255 kb)
Acknowledgments
We thank Dr. Radhakrishnan for bone marrow sampling and the donors, who made this study possible.
References
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F. Human bone marrow-derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- Deda H, Inci M, Kurekci A, Kayihan K, Ozgun E, Ustunsoy G, Kocabay S. Treatment of chronic spinal cord injured patients with autologous bone marrow-derived hematopoietic stem cell transplantation: 1-year follow-up. Cytotherapy. 2008;10:565–574. doi: 10.1080/14653240802241797. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Schwab KE, Zillwood RM, Nguyen HPT, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM. Adipose tissue-derived therapeutics. Expert Opin Biol Ther. 2003;3:705–713. doi: 10.1517/14712598.3.5.705. [DOI] [PubMed] [Google Scholar]
- Hendrikx M, Hensen K, Clijsters C, Jongen H, Koninckx R, Bijnens E, Ingels M, Jacobs A, Geukens R, Dendale P, Vijgen J, Dilling D, Steels P, Mees U, Rummens JL. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation. Circulation. 2006;114:I-101–I-107. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- In't Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH (2003) Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102(4): 1548–1549 [DOI] [PubMed]
- Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, McGonagle D (2004) Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum 50(3):817–827 [DOI] [PubMed]
- Kuethe F, Richartz BM, Sayer HG, Kasper C, Werner GS, Hoffken K, Figulla HR. Lack of regeneration of myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans with large anterior myocardial infarctions. Int J Cardiol. 2004;97:123–127. doi: 10.1016/j.ijcard.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Kumar AA, Kumar SR, Narayanan R, Arul K, Baskaran M. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: a phase I/II clinical safety and primary efficacy data. Exp Clin Transplant. 2009;7:241–248. [PubMed] [Google Scholar]
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/S0301-472X(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo B-M, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- Okura H, Komoda H, Fumimoto Y, Lee C-M, Nishida T, Sawa Y, Matsuyama A. Transdifferentiation of human adipose tissue-derived stromal cells into insulin-producing clusters. J Artif Organs. 2009;12:123–130. doi: 10.1007/s10047-009-0455-6. [DOI] [PubMed] [Google Scholar]
- Pal R, Hanwate M, Totey SM. Effect of holding time, temperature and different parenteral solutions on viability and functionality of adult bone marrow-derived mesenchymal stem cells before transplantation. J Tissue Eng Regen Med. 2008;2:436–444. doi: 10.1002/term.109. [DOI] [PubMed] [Google Scholar]
- Rebelatto CK, Aguiar AM, Moreto MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med. 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155 [DOI] [PubMed]
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Comparison of surface antigenic expression of bone marrow derived MSC in different media. Statistical significance: * p<0.05, ** p<0.01 (DOC 1255 kb)