Abstract
Vibrio sp. V26 isolated from mangrove sediment showed 98 % similarity to 16S rRNA gene of Vibrio cholerae, V. mimicus, V. albensis and uncultured clones of Vibrio. Phenotypically also it resembled both V. cholerae and V. mimicus. Serogrouping, virulence associated gene profiling, hydrophobicity, and adherence pattern clearly pointed towards the non—toxigenic nature of Vibrio sp. V26. Purification and characterization of the enzyme revealed that it was moderately thermoactive, nonhemagglutinating alkaline metalloprotease with a molecular mass of 32 kDa. The application of alkaline protease from Vibrio sp. V26 (APV26) in sub culturing cell lines (HEp-2, HeLa and RTG-2) and dissociation of animal tissue (chick embryo) for primary cell culture were investigated. The time required for dissociation of cells as well as the viable cell yield obtained by while administering APV26 and trypsin were compared. Investigations revealed that the alkaline protease of Vibrio sp. V26 has the potential to be used in animal cell culture for subculturing cell lines and dissociation of animal tissue for the development of primary cell cultures, which has not been reported earlier among metalloproteases of Vibrios.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-012-9472-z) contains supplementary material, which is available to authorized users.
Keywords: Alkaline protease, Vibrio sp., Animal cell culture, Primary cell culture, Virulence genes, Trypsin
Introduction
Protease constitutes one of the most important groups of industrial enzymes, accounting for more than 65 % of the total (Johnvesly and Naik 2001). One of the fields, where proteases find application is in animal cell culture. The use of trypsin to detach growing cells from plasma clots was first introduced by Rous and Jones 1916, a method that preceded the use of proteolytic enzymes for preparing separated cells from tissue fragments for primary cell culture. Trypsin has since remained a popular agent for primary dissociation of tissues for detaching cells from monolayers for subsequent passaging. Lee et al. (1986) described the application of fig tree extracts in the subculture of monolayers of fish cell lines. The use of enzymes such as pancreatin, elastase (Rinaldini 1958) and accutase (Bajpai et al. 2008) in tissue culture has also been investigated. As the above are of animal origin, their sources are restricted and they turn out to be expensive as well. Trypsin’s use is often limited by its narrow range of pH for action. Meanwhile collagenase (Hilfer 1973), pronase (Foley and Aftonomos 1970), dispase (Kitano and Okada 1983) and TrypLE™ Express a recombinant fungal trypsin-like protease (Nestler et al. 2004) are the microbial enzymes that have application in cell culture. Each of these enzymes has its own limitations, as collagenase acts only on tissues containing collagen, while Pronase, with regard to its completeness of dispersion of certain continuous epithelial cell lines is inferior to trypsin (Foley and Aftonomos 1970). In this context we report, a novel alkaline metallo-protease from a non-pathogenic mangrove isolate of Vibrio sp. V26 possessing dissociating properties on animal cell culture monolayers and on animal tissues with low toxicity. An added advantage of the enzyme is that it is devoid of the limitations of the other enzymes meant for the purpose. This is the first report of the application of a metalloprotease from Vibrio for animal cell culture.
Materials and methods
Microorganism
The organism used in this study was Vibrio sp. V26 isolated from mangrove sediments of Puduvyppu, Cochin, Kerala, India and maintained in the Microbiology Laboratory of the Department of Marine Biology, Microbiology and Biochemistry, Cochin University of Science and Technology (CUSAT) (Venugopal 2004).
Identification
Phenotypic characterization of the isolate was done as per the standard keys (Alsina and Blanch 1994; Farmer and Janda 2005).
Molecular identification
Total genomic DNA extraction was carried out following the method of Lee et al. (2003a) with slight modification. Identity of the above isolate was ascertained by sequencing a 1500 bp fragment of the 16S rRNA gene using the primers NP1F 5′-GAGTTTGATCCTGGCTCA-3′ and NP1R 5′-ACGGCTACCTTGTTACGACTT-3′ (Reddy et al. 2000). Bacterial DNA (50 ng) amplification was carried out in a thermal cycler (Master Cycler, Eppendorf, Hamburg/D) which involved 1 × 95 °C for 5 min followed by 35 × (94 °C for 20 s, 58 °C for 20 s, 72 °C for 90 s) and finally 1 × 72 °C for 10 min. The amplified product was separated on 1 % agarose gel, purified using QIAEX II gel purification kit (Qiagen, New Delhi, India) and sequenced using the primer walking service of Microsynth AG, Balgach, Switzerland. All sequences obtained were matched with the database in Genbank using the BLAST algorithm (Altschul et al. 1990). Nucleotide sequence has been submitted to the Genbank data base and assigned the accession no: FJ665509.
Serogrouping
Serogrouping was done using Vibrio cholerae O1 polyvalent antisera as per manufacturer’s protocol (Murex Diagnostics Limited, Darford, UK).
Putative virulence traits
Virulence genes
Virulence-associated factors such as cholera toxin (ctxA), outer membrane protein (ompU), zonula occludens toxin (zot), toxin-coregulated pilus (tcpA), ToxR regulatory protein (toxR) and hemolysin (hlyA) were investigated in the isolate Vibrio sp. V26. The primers (Table 1) synthesized by Bioserve Biotechnologies, Hyderabad, India were employed for both multiplex PCR for hlyA and tcpA (classical and E1Tor) and simple PCR for ctx A, ompU, zot and toxR based on the works of Fields et al. (1992) and Rivera et al. (2001).
Table 1.
Primers used in amplifying toxin genes in Vibrio sp. V26
| Gene(s), primers, and sequences (5′–3′) | Amplicon size (bp) | Reference |
|---|---|---|
| ctxA and ompU (CT subunit A and outer membrane protein) | 564 (ctxA) 869 (ompU) |
Fields et al. (1992) Rivera et al. (2001) |
| 94F, CGG GCA GAT TCT AGA CCT CCT G 614R, CGA TGA TCT TGG AGC ATT CCC AC 80F, ACG CTG ACG GAA TCA ACC AAA G 906R, GCG GAA GTT TGG CTT GAA GTA G | ||
| zot (zonula occludens toxin) | 947 | Rivera et al. (2001) |
| 225F, TCG CTT AAC GAT GGC GCG TTT T 1129R, AAC CCC GTT TCA CTT CTA CCC A | ||
| toxR (operon ToxR) | 779 | Rivera et al. (2001) |
| 101F, CCT TCG ATC CCC TAA GCA ATA C 837R, AGG GTT AGC AAC GAT GCG TAA G | ||
| tcpA (TCP A [Classical and El Tor]) | 451 (El Tor) 620 (Classical) |
Rivera et al. (2001) |
| 72F, CAC GAT AAG AAA ACC GGT CAA GAG 477R, CGA AAG CAC CTT CTT TCA CGT TG 647R, TTA CCA AAT GCA ACG CCG AAT G | ||
| hlyA (hemolysin [Classical and El Tor]) | 481 (El Tor) 738/727 (ET/Clas) |
Rivera et al. (2001) |
| 489F, GGC AAA CAG CGA AAC AAA TAC C 744F, GAG CCG GCA TTC ATC TGA AT 1184R, CTC AGC GGG CTA ATA CGG TTT A |
The PCR was carried out in 0.2 ml PCR tubes, using 25 μl reaction mixture consisting of 2.5 μl 10× Thermopol buffer (New England Biolabs, Ipswich, MA, USA—Standard buffer), 2 μl (250 μM) each of dATP, dCTP, dGTP and dTTP), 2 μl (10 μM) primer, 1.5 μl template (50 ng/μl) and 1 μl Taq DNA Polymerase (0.5 U, New England Biolabs) and Milli Q (to a final volume of 25 μl). The amplification was carried out in a thermal cycler (Master Cycler, Eppendorf) which involved 1 × 95 °C for 5 min followed by 30 × (95 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min) and final incubation at 72 °C for 10 min. The amplified products were separated on 1 % agarose gel and stained with ethidium bromide. The DNA from V. cholerae MTCC 3906 was used as the positive control, and the reaction mixture containing Milli Q was used as negative control.
Hydrophobicity
Cell surface hydrophobicity of the organism was evaluated using the bacterial adhesion to hydrocarbons test (BATH) (Rosenberg et al. 1980) and salt aggregation test (SAT) (Lindahl et al. 1981).
Adherence assay
The isolate was examined for their adherence to HEp-2 cells following the method of Snoussi et al. (2008) with slight modifications. The adherence patterns were examined using an inverted phase contrast microscope (Leica DMIL, Wetzlar, Germany). The adhesion index was recorded as: NA = non adhesion (0–10 bacterial cells/HEp 2 cells); W = weak adhesion (10–20 bacterial cells/HEp 2 cells); M = medium adhesion (20–50 bacterial cells/HEp 2 cells); S = strong adhesion (50–100 bacterial/HEp 2 cells).
Purification of the enzyme
Protease production was carried out in nutrient broth supplemented with gelatin. The cell free supernatant was recovered by centrifugation (8,000g, 4 °C, 15 min). The partial purification of the enzyme was carried out by precipitation of the crude enzyme with ammonium sulphate between 40 and 80 % saturation. The precipitate obtained was collected by centrifugation (8,000g, 4 °C, 15 min) and dissolved in minimum quantity of Tris–Cl buffer (pH 8.5). This preparation was treated as partially purified enzyme which was diafiltered using an Amicon UF Stirred Cell (Model 8010) with 10 kDa cut-off membrane against Tris–Cl buffer (pH 8.5). The enzyme was then loaded onto a DEAE cellulose column (C 10/20 column, AKTA prime, Amersham, Chennai, India) pre-equilibrated with the 20 mM Tris–Cl buffer pH 8.5. The column was washed with the same buffer to remove the unbound proteins. The bound protein was then eluted by applying a linear gradient of 0–0.8 M NaCl in the same buffer at flow rate of 0.5 ml/min and monitored at 280 nm. The peak protein fractions were analyzed for protease activity. The active fractions were pooled and used for further studies.
Assay of protease activity and protein determination
Protease activity was measured by the modified method of Kembhavi et al. (1993) using casein as substrate. An aliquot of 500 μl of suitably diluted enzyme was added to 500 μl of casein (1 %) prepared in 100 mM Tris–Cl buffer (pH 9) and incubated at 60 °C for 30 min. The reaction was stopped by the addition of 500 μl of 20 % tricholoroacetic acid (TCA). The mixture was allowed to stand for 15 min at room temperature and then centrifuged at 8,000g for 15 min. Suitable controls were placed. The absorbance of the supernatant was measured at 280 nm spectrophotometrically. One unit of protease activity is defined as the amount of enzyme required to liberate 1 μg tyrosine per millilitre per minute under the standard assay conditions. Protein content was measured by the method of Hartree-Lowry (1972) with bovine serum albumin (BSA) as the standard. Meanwhile, for cell culture application the assays were done at pH 7 and temperature at 37 °C.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS PAGE)
SDS-PAGE was carried out for determination of molecular mass of the protease in a 10 % resolving gel and a 4 % stacking gel according to the method of Laemmli (1970). Electrophoresis was carried out at a constant current of 12 mA. Broad range molecular marker (Bangalore Genei, Bangalore, India) was used as standard. After electrophoresis, gels were stained with 0.025 % Coomassie Brilliant Blue R-250 and then destained with a solution containing 5 % methanol and 7 % acetic acid.
Effect of pH on enzyme activity and stability
The effect of pH on protease activity was evaluated over a pH range 7-12, using different buffers such as Sodium phosphate 0.1 M (pH 7), 0.1 M Tris–Cl (pH 8–9) and 0.1 M Glycine NaOH (pH 11–12) in the reaction mixture. The activity of the sample was expressed in terms of relative activity calculated. Stability of the enzyme at various pH was studied by pre-incubating the enzyme in buffers of different pH (7–12) for 1 h and the residual enzyme activity (%) was measured. The percentage residual activity was calculated by comparing the activity of treated enzyme with that of the untreated enzyme (control), taken as 100 %.
Effect of temperature on the enzyme activity and stability
The effect of temperature on the enzyme activity was assessed by carrying out the assay at different temperatures (30–80 °C). The temperature stability of the enzyme was determined by pre-incubating the enzyme at temperatures (30–80 °C) for an hour and then assaying the residual activity (%) under standard assay conditions. The activity of untreated enzyme (control) was taken as 100 %.
Effect of metal ions and inhibitors on enzyme activity
The influence of various metal ions on the purified enzyme was investigated by incubating the enzyme in their presence (ZnCl2, CaCl2, MgCl2, MnCl2, PbCl2, CoCl2, HgCl2, BaCl2, and CuSO4) at final concentration of 1 mM and 5 mM at 60 °C for 30 min. The percentage relative activity was calculated by considering the activity of the enzyme in the absence of metal ions as 100 %.
To study the effect of different protease inhibitors on the purified enzyme, aliquots of enzymes were pre-incubated with the different enzyme inhibitors such as phenylmethylsulphonyl fluoride (PMSF) (5 mM), iodo acetic acid (IAA) (1 mM), ethylene-diamine tetraacetic acid (EDTA) (5 mM) and 1, 10 phenanthroline (5 mM) for 30 min at room temperature. Residual activities (%) were measured. Suitable controls were placed (without inhibitors).
Hemagglutination assay
The hemagglutinating activity was assayed using human (O type) and chick erythrocytes. The cells were washed twice in Alsever’s solution (dextrose 2.05 g, sodium citrate 0.8 g, sodium chloride 0.42 g, citric acid 0.05 g in 100 ml distilled water) and resuspended in fresh 5 % (v/v) solution. Two fold serial dilutions of the purified alkaline protease in Alsever’s solution were made in round-bottomed microtitre plates, and aliquots of equal volume (25 μl) of 5 % (v/v) suspension of erythrocytes were added and mixed. After 45 min of incubation at room temperature (28 ± 2 °C) the extent of agglutination was examined and reported in terms of hemagglutination titre, the highest dilution at which agglutination was visible.
Application in animal cell culture
Cell lines and tissues used
HEp-2 (Human larynx epithelial cells), HeLa (Human cervical carcinoma) and RTG-2 (gonadal cell line derived from Rainbow trout (Oncorhynchus mykiss)) cell lines were used in the investigation. Cell lines were maintained in Eagles MEM along with 2 mM glutamine, 1.5 g/l sodium bicarbonate and 10 % FBS. HEp-2 and HeLa cells were maintained at 37 °C while RTG-2 cells were grown at 25 °C. Nine day old chick embryos were used for the development of chick embryo fibroblastic primary cell culture. The methods followed for the application of the enzyme in cell cultures and their maintenance was as per the standard protocols described by Freshney (2000).
Enzymes for cell culture
Alkaline protease of Vibrio sp. V26 (APV26): The cell free supernatant was precipitated with ammonium sulphate (40–80 %) and dissolved in minimum amounts of Tris–Cl buffer (pH 8.5). This was then diafiltered and concentrated using Amicon UF stirred cell (Model 8010) with 10 kDa cut off membrane. The concentrated enzyme was lyophilized and dissolved in phosphate buffered saline (pH 7.2). This was treated as alkaline protease stock. The stock was suitably diluted with PBS to get the desired enzyme concentrations (400, 300, 200, 100 and 50 U at 37 °C).
Trypsin from Sigma Aldritch Inc., was used as the control for comparison. Aliquot of 0.025 % trypsin was found to have an activity of ~400 U. From this stock further dilution were made to obtain 300, 200, 100 and 50 U working trypsin concentrations.
Dissociation of monolayer
The cells were seeded into 24 well plates at a density of 1 × 105 cells ml−1. The plates were incubated till confluent monolayers were established. Different concentrations of APV26 were tested on the cell lines in-order to determine an ideal concentration of the enzyme for cell dissociation. Cells were treated with each of these concentrations (400, 300, 200, 100 and 50 U) until they dissociated/detached from the wells. Time required for detachment was noted at each concentration. For a comparative study the same was done using trypsin as well. Cells were then washed to stop the action of the enzyme and the viable count noted at each concentration of APV26 and trypsin.
Primary cell culture
A 9 days old chick embryo was carefully dissected to remove head, appendages and viscera retaining the body alone. The body was cut into two parts using a sterile scalpel. Each half was further minced into smaller pieces. One half (~100 mg) of the cut pieces was transferred into a tube containing 1 ml (200 U) of APV26 and the other half to the same quantity of trypsin (0.025 %). Tubes were incubated at 37 °C until the tissue pieces were fully dissociated. The suspension was allowed to stand for 1 min at 4 °C for the sedimentation of un-dissociated smaller tissue pieces. The supernatant of cell suspension was carefully transferred to another test tube and centrifuged for 10 min at 200 g at 4 °C. The supernatant was discarded and deposited cells resuspended in fresh medium. Viable counts were made by trypan blue exclusion method and the number adjusted and seeded into cell culture bottles, incubated at 37 °C in 5 % CO2 atmosphere.
Viable count-trypan blue dye exclusion method
Cell suspensions obtained after enzyme treatment (APV26 and trypsin) were centrifuged and re-suspended in fresh medium. This was then mixed with an equal volume of trypan blue (0.4 %) prepared in PBS having the same osmolarity of the medium. The sample was loaded into a counting chamber (Improved Neubauer) and viable and dead cells were counted, as the dead cells absorbed the stain and the live ones remained unstained.
Results
Identification of the isolate
The isolate V26 was identified as Vibrio based on the phenotypic characters. To confirm the identity at species level, the 1,500 bp fragment of 16S rRNA gene was amplified and partially sequenced. This nucleotide sequence has been submitted to the GenBank database and assigned the Accession no: FJ665509. When the sequence of this strain was compared with the GenBank database using the BLAST algorithm, 98 % similarity (98 % query coverage) was obtained to 16S rRNA gene of Vibrio cholerae, V. mimicus, V. albensis and certain uncultured Vibrio clones.
Serogrouping
No agglutination was observed with Vibrio cholerae O1 polyvalent antisera. This revealed that the isolate Vibrio sp. V26 did not belong to the O1 serogroup.
Putative virulence traits
Pathogenicity is contributed by a combination of virulence associated factors. Therefore determination of the virulence associated gene profile, hydrophobicity and adherence pattern of Vibrio sp. V26 were undertaken.
Virulence genes
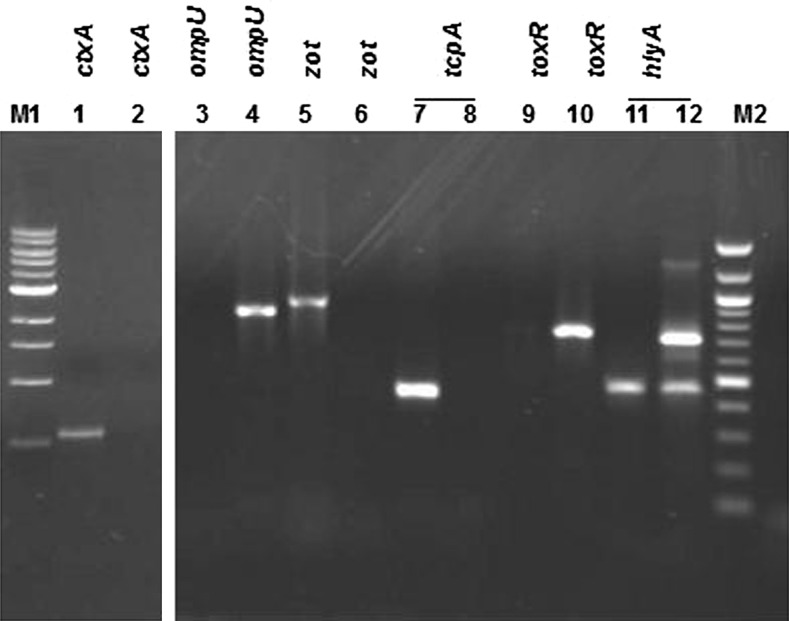
The virulence profiles (Fig. 1) of the environmental isolate Vibrio sp. V26 and the type strain MTCC 3906 are as follows
Vibrio sp. (V26) ctxA−zot−tcp A–hly AET+ompU+tox R+
MTCC 3906 ctx A +zot+tcp A+hly AET+ompU −tox R+
Fig. 1.
Analysis of PCR products of virulence genes. Lane M1 10 kb DNA ladder; lane M2 100 bp DNA ladder; lanes: 1, 3, 5, 7 and 11V. cholerae MTCC 3906; lanes 2, 4, 6, 8, 10 and 12Vibrio V26; lanes: 1–6, 9–10 simple PCR for ctxA, ompU, zot and toxR; lane 9toxR negative control; lanes: 7, 8, 11 and 12 amplicons obtained using multiplex PCR for tcpAhlyA
Adherence and Hydrophobicity
Vibrio sp. V26 exhibited weak adherence to the HEp-2 cell lines as the number of adhering bacteria were only 10–20 per cell. Moreover, the pattern of adherence was of the diffuse type. On assessing hydrophobicity by SAT assay no bacterial aggregation could be observed in the range of 0.05–4.0 mol l−1 ammonium sulphate, and by BATH assay the adherence value to xylene was 14.04 %, pointing to the lack of surface hydrophobicity.
Purification and characterization
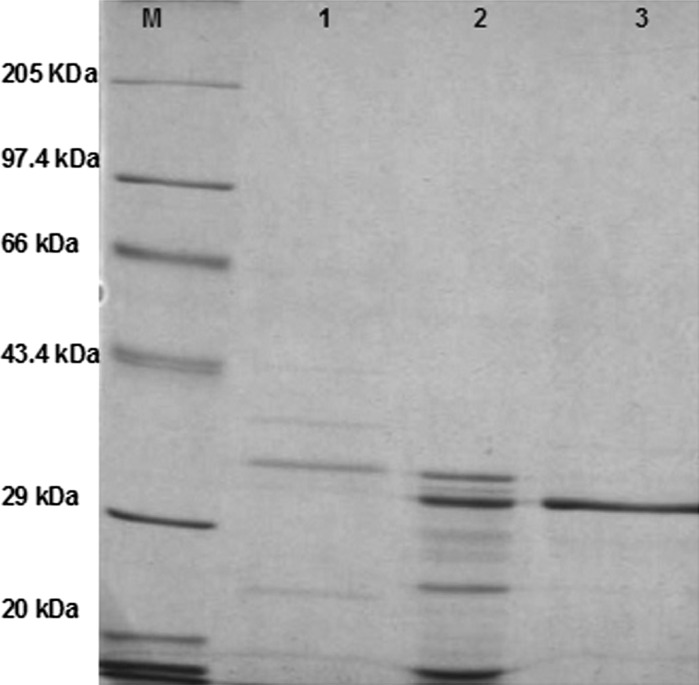
Protease purification was successfully achieved to homogeneity, as evidenced by a single band corresponding to 32 kDa on SDS-PAGE (Fig. 2).
Fig. 2.
SDS-PAGE of the Vibrio sp. V26 protease M-molecular mass markers; lane 1 crude enzyme; lane 2 40–80 % ammonium sulphate saturation fraction; lane 3 purified protease
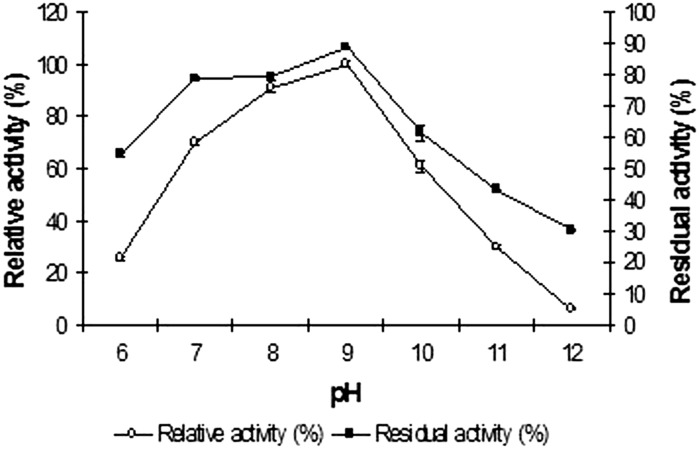
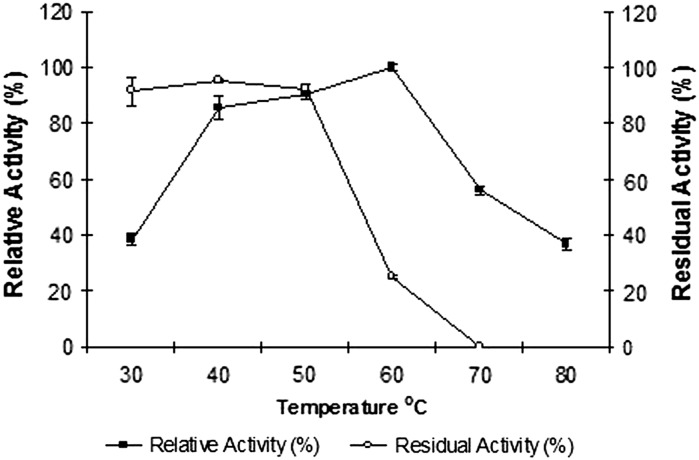
The purified enzyme was active in the pH range of 6.0–11.0, with an optimum at pH 9 Fig. 3. The highest residual activity was also found at the same pH (Fig. 3) indicating the enzyme is an alkaline protease. The alkaline protease of Vibrio sp. V26 was active at a range of temperatures (30–80 °C) tested, with maximum recorded at 60 °C, qualifying it to be designated as a moderately thermo-active protease. A sharp decline in activity at temperatures above 60 °C was noted. The enzyme’s temperature stability profile (Fig. 4) revealed a great deal of stability in the temperature range 30–50 °C. However the protease was found to be unstable at its optimal temperature on prolonged exposure.
Fig. 3.
Effect of pH on the activity and stability of the protease from Vibrio sp. V26
Fig. 4.
Effect of temperature on the activity and stability of the protease form Vibrio sp. V26
Results of the effects of metal ions on the activity of the protease are presented in Table 2. In the presence of Ca2+ (1 mM) and Ba2+ (1 mM) the activity of the protease was not significantly different from the untreated control (p > 0.05). Hg2+ and Cu2+ were found to be inhibitory at both concentrations, while Zn2+ had a negative effect at 5 mM concentration. All metal ions at 5 mM concentration were found to have a negative influence on the activity. The inhibitory studies conducted with EDTA (5 mM), 1, 10 phenanthroline (5 mM), PMSF (5 mM) and IAA (1 mM) indicated the enzyme as zinc-metallo protease (Table 2).
Table 2.
Effect of inhibitors and metal ions on the enzyme
| Ingredient | Concentration (mM) | Residual activity (%) |
|---|---|---|
| Inhibitors | ||
| None | 100 | |
| IAA | 1 mM | 87.86 ± 2.09 |
| 1,10 Phenanthroline | 5 mM | 0 |
| EDTA | 5 mM | 47.55 ± 3.65 |
| PMSF | 5 mM | 94.48 ± 0.54 |
| Metal ions | ||
| ZnCl2 | 1 mM | 66.04 ± 3.25 |
| 5 mM | 7.94 ± 0.24 | |
| MnCl2 | 1 mM | 86.23 ± 2.24 |
| 5 mM | 30.35 ± 0.34 | |
| CaCl2 | 1 mM | 93.51 ± 2.47 |
| 5 mM | 70.85 ± 1.54 | |
| Pb(NO3)2 | 1 mM | 71.50 ± 3.62 |
| 5 mM | 11.89 ± 0.58 | |
| MgCl2 | 1 mM | 88.92 ± 1.80 |
| 5 mM | 72.88 ± 1.05 | |
| HgCl2 | 1 mM | 12.85 ± 0.92 |
| 5 mM | 1.43 ± 0.39 | |
| BaCl2 | 1 mM | 98.09 ± 11.80 |
| 5 mM | 65.38 ± 1.15 | |
| CuSO4 | 1 mM | 12.24 ± 0.41 |
| 5 mM | 3.03 ± 0.63 | |
| CoCl2 | 1 mM | 86.51 ± 1.41 |
| 5 mM | 34.40 ± 1.85 | |
Neither the ammonium sulphate fraction nor the purified enzyme was able to agglutinate human (O blood group) and chick RBCs.
Application in animal cell culture
Cell dissociation and primary cell culture development
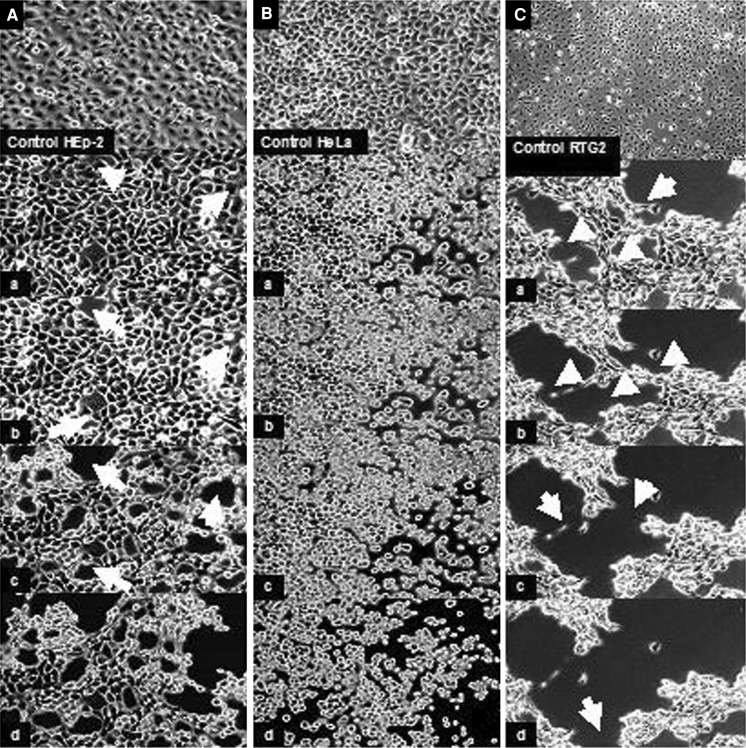
APV26 was effective in dissociating the monolayers of the three cell lines tested (HeLa, HEp-2 and RTG2) as presented in Fig. 5. It was also found to disperse cells from chick embryonic tissue effectively at 37 °C. The dispersed cells when seeded into tissue culture bottles were seen to attach with in about 2 h and grow as confluent mono layers (Fig. 6).
Fig. 5.
Time lapse image showing the cell dissociating and detaching property of APV26 (200U): a HEp-2 cell lines, b HeLa cell lines, c RTG-2 cell lines (images a–d taken after every 10 s of exposure)
Fig. 6.
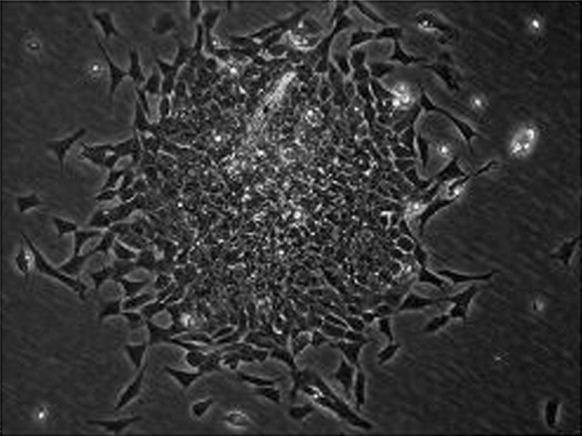
Primary culture of chick embryo fibroblast after 24 h
With the increase in the concentration of APV26 the time required for cell dissociation was found to have decreased (Table 3). HeLa required the longest enzyme exposure to get dissociated by both APV26 and Trypsin. Two-way ANOVA indicated that the time required by APV26 for the detachment of the cell lines except RTG-2 was significantly (p < 0.01) lower than that of trypsin, but independent of the concentration.
Table 3.
Comparison of time required by APV26 and trypsin for monolayer dissociation
| Cell lines | Enzyme concentration (U) | Time required for monolayer dissociation (min) | |
|---|---|---|---|
| APV26 | Trypsin | ||
| HeLa | 50 | 7 | 12 |
| 100 | 4 | 12 | |
| 200 | 1 | 10.4 | |
| 300 | 0.5 | 10.4 | |
| 400 | 0.4 | 10.4 | |
| HEp-2 | 50 | 1.3 | 7.4 |
| 100 | 1.3 | 6 | |
| 200 | 1.3 | 6 | |
| 300 | 1 | 6 | |
| 400 | 0.56 | 6 | |
| RTG-2 | 50 | 4.3 | 1.22 |
| 100 | 1.3 | 1.22 | |
| 200 | 0.56 | 0.51 | |
| 300 | 0.36 | 0.35 | |
| 400 | 0.31 | 0.26 | |
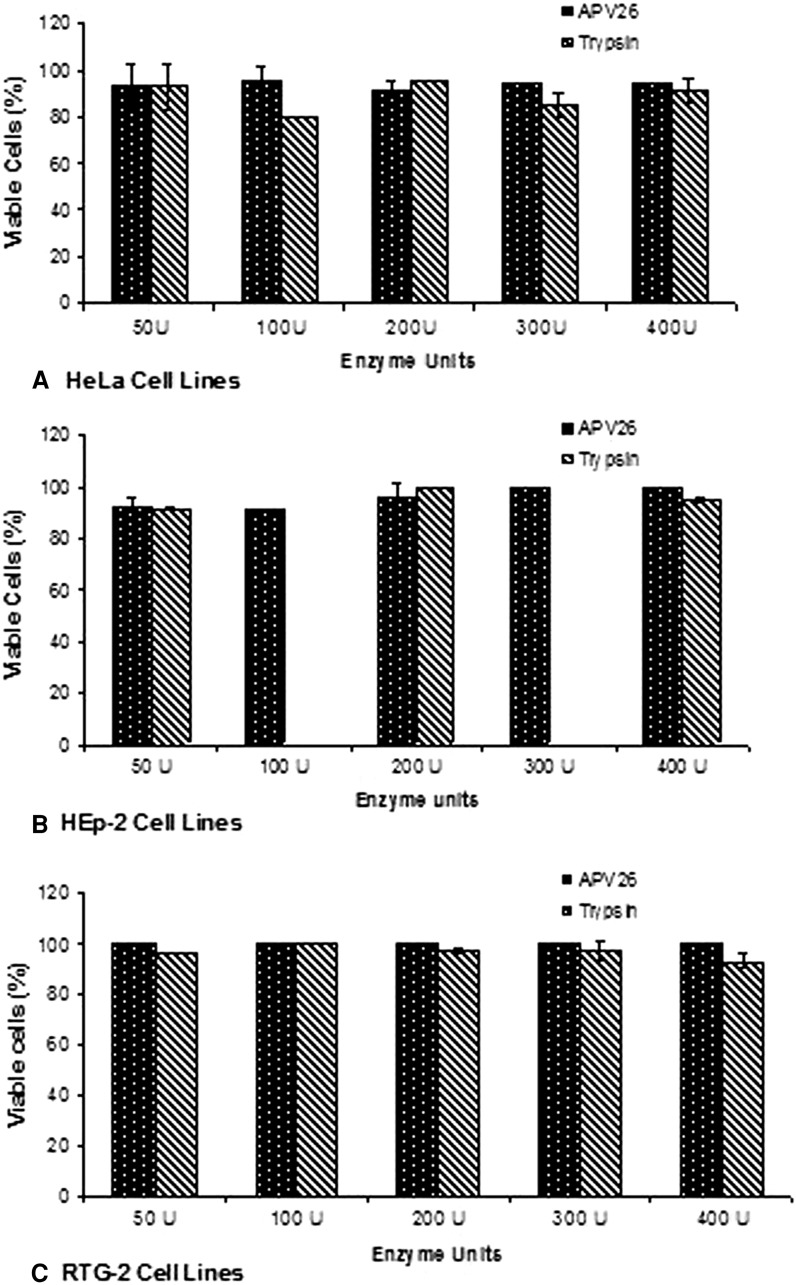
The viable cell yield obtained by administering APV26 and trypsin was compared (Fig. 7). With APV26, it was 94 % for HeLa, 96 for HEp-2 and 100 for RTG-2. Meanwhile, with trypsin it was 89, 95 and 97 %. The overall average yield of cells by administering APV26 was 96.7 % and with that of trypsin 93.8 %. Two-way ANOVA indicated that there was no significant (p > 0.05) effect of concentration with in the range 50–400 units of both enzymes on the viable cell yield. However, a better viable cell yield was obtained when APV26 was applied on RTG-2. Accordingly, it could be concluded that viable cell yield obtained using APV26 was comparable to that of trypsin.
Fig. 7.
Comparison of viable cell yield obtained by applying APV26 and Trypsin
Discussion
Supplementing the phenotypic characteristics of the isolate, 16S rRNA gene sequencing was employed to identify it to species (Thompson et al. 2004). Results of the 16S rRNA gene partial sequence analysis confirmed that the isolate belonged to the Genus Vibrio exhibiting 98 % similarity to V. cholerae, V. mimicus, V. albensis and uncultured Vibrio clones. Kita-Tsukamoto et al. (1993) pointed out based on their study on 16S rRNA gene sequences of Vibrionaceae that at least 99.3 % 16S rRNA gene similarity must be obtained for the organism to be designated to a particular species. Accordingly, in this study, only 98 % similarity could be obtained, and that too with more than one species and therefore confirmation of the precise identity of the isolate to species could not be accomplished. V. cholerae, V. albensis and V. mimicus share a great degree of similarity with regard to their nucleotide sequences and putative virulence traits (Davis et al. 1981; Ruimy et al. 1994). Moreover, distribution of V. cholerae virulence genes among other species of vibrios has also been reported (Sechi et al. 2000). Therefore a protocol for analysis of putative virulence traits was designed and executed based on those of V. cholerae. However, prior to examining the virulent traits serological grouping of the isolate was carried out.
Serogrouping revealed that the isolate did not belong to O1 serogroup suggesting its environmental origin. Saravanan et al. (2007) observed that majority of V. cholerae present in sea food and its environment in Mangalore, India are of non-O1 serogroup, which is an autochthonous microflora of aquatic environment and their presence is not related to fecal contamination.
The isolate Vibrio sp. V26 was found to lack the genes ctxA and zot involved in toxin production as observed among several environmental Vibrio isolates (Iyer et al. 2000; Sechi et al. 2000; Karunasagar et al. 2003; Bag et al. 2008). The absence of toxin genes (Fig. 1) in Vibrio sp. V26 indicated its non-pathogenic nature (Levine et al. 1982). It also lacked the tcpA gene, the absence of which gained significance in the light that TCP was the only colonizing factor of V. cholerae whose importance in human disease had been proven (Kaper et al. 1995) and that it also acted as receptor for the phage CTXΦ (Levin and Tauxe 1996). Karunasagar et al. (2003) in their study too have stated that any strain of V. cholerae lacking ctxA, zot and tcpA is less likely to be toxigenic.
However, Vibrio sp. V26 was positive for hlyA (both 481 bp and 738 bp amplified fragments), ompU and toxR. Such a dual amplification fragment pattern in the case of hly A has been reported among E1 Tor biotypes and even among non-toxigenic V. cholerae O1 and V. cholerae O139 strains (Rivera et al. 2001). Hemolysin genes are found in both pathogenic and non pathogenic (O1, O139 and non O1/non O139) isolates of V. cholerae. Levine et al. (1988) in their study showed that cytolysin/hemolysin was not the probable cause of diarrhea and the isolate Vibrio sp. V26 by being positive for hlyA gene could not be considered pathogenic. Investigations made by Nakasone and Iwanaga (1998) on the outer membrane protein OmpU suggested that it was not involved in adhesion of V. cholerae to the intestinal epithelium and that the ompU gene product had more of a physiological role (Provenzano et al. 2001; Rivera et al. 2001; Mathur and Waldor 2004). Vibrio sp. V26 was positive for toxR, consistent with the previous studies (Ghosh et al. 1997; Rivera et al. 2001) where toxR was detected in all isolates of V. cholerae. In nonpathogenic strains, the ToxR protein (“master switch”) controls only the biosynthesis of the outer membrane proteins OmpU and OmpT unlike pathogenic strains where it is involved in the regulation of ctxA, TCP colonizing factor, ompU and at least 17 other distinct genes (Miller et al. 1987; Peterson and Mekalanos 1988; Drita 1992; Smirnova et al. 2007). As Vibrio sp. V26 is devoid of ctxA and tcpA the presence of toxR alone is unlikely to contribute to pathogenicity. The enteropathogenicity of non-O1, non-O139 Vibrio cholerae as well as V. mimicus is multifactorial, and the presence of a single factor should not be considered as the cause of pathogenicity (Ramamurthy et al. 1993).
Hydrophobicity plays an important role in the adherence of bacteria to various surfaces (Smyth et al. 1978; Magnusson et al. 1980) and this adhesive property of Vibrio spp. in turn is a key factor of their pathogenicity (Olafsen 2001). The isolate Vibrio sp. V26 exhibited only weak adherence to HEp-2 cell lines and the pattern was diffuse. The weak adherence could be correlated with its non-hyrophobic nature (as determined by BATH and SAT assay) and also to the absence of tcpA gene. Therefore, taking into account of all the properties of the isolate Vibrio sp. V26, we concluded that the isolate was non-toxigenic with non-pathogenic features.
The molecular mass of the protease was found to be 32 kDa in close agreement with the observation of previous workers on V. cholerae (Finkelstein and Hanne, 1982; Ichinose et al., 1992, Vaikkevicius, 2007), V. mimicus (Chowdhury et al., 1990) as well as other Vibrios (Lee et al. 1997; Venugopal and Saramma 2006; Jellouli et al. 2009).
The protease from Vibrio sp. V26 recorded maximum activity as well as maximum stability at pH 9, which entailed it to be an alkaline protease. The enzyme was active over a wide range of temperature with optimal activity at 60 °C unlike that of most other vibrios (Ishihara et al. 2002; Lee et al. 2002, 2003b; Venugopal and Saramma 2006). Meanwhile it has high degree of stability in the range 30–50 °C which qualifies it to be used under such temperature conditions for long durations. However, it was found that the alkaline protease from Vibrio sp. V26 was quite unstable when pre-incubated at its optimum temperature (60 °C) for an hour. The proteases from Salinivibrio sp., V. fluvialis, and Bacillus strain SAL1 too have been found to be unstable at their optimum temperatures for action (Karbalaei-Heidari et al. 2007; Wang et al. 2007; Almas et al. 2009). A drop in activity of the proteases on prolonged exposure to temperatures above 50 °C has been reported among vibrios (Lee et al. 2002, 2003b). Even alkaline protease used in commercial detergents tends to get inactivated on extended exposures to temperature of 60 °C or more.
At 1 mM concentration, the effect of ions such as Ba2+ and Ca2+ were not significantly different from the control indicating practically no effect of these ions on the protease. The inhibitory potential of Zn2+ was more prominent at higher concentration which indicated that it was most likely a zinc metallo protease (Larsen and Auld 1991). Enzyme inhibition studies primarily give an insight into the nature of the enzyme, its cofactor requirements and the nature of the active centre (Sigma and Moser 1975). In the present study, the protease was completely inhibited by 1, 10 phenanthroline (5 mM), the zinc specific chelator, and up to 53 % by EDTA (5 mM) by which the enzyme was classified as alkaline metalloprotease. Neither the ammonium sulphate fraction nor the purified alkaline protease from Vibrio sp. V26 displayed hemagglutination property in contrary to the observations made by Benitez et al. (2001) in V. cholerae and V. mimicus.
The alkaline protease (APV26) produced by Vibrio sp. V26 has been found useful in animal cell culture especially for subculturing (HEp-2, HeLa and RTG-2) and dissociation of cells from tissues (chick embryo). The time required for the action of APV26 was found to be concentration dependent. The mechanism by which APV26, a metalloprotease, brings about detachment is likely to be different from that of trypsin which is a serine protease.
On considering the duration of exposure of cell lines to proteolytic enzymes, APV26 required less time (2.6 min for HeLa and 1.09 min for HEp-2) to dislodge cells compared to trypsin (11.0 min and 6.28 for HeLa and HEp-2, respectively) except in the case of RTG-2. In the case of RTG-2, though APV26 took a slightly longer time (1.36 min) than trypsin (0.71) it was less toxic in terms of cell viability. The average viable cell yield (%) of APV26 was 94, 96 and 100 for HeLa, HEp-2 and RTG-2 cell lines, respectively, with an overall average viable yield of 96.7 %, slightly higher than that of trypsin (93.7 %). Whereas the TrypLE™ Express, a high purity recombinant fungal enzyme with cell dissociating property was found to have cell viability of 95 % (Nestler et al. 2004). The time taken by APV26 for the dissociation of chick embryo for developing primary cell culture was the same as by trypsin. However, the yield of viable cells was 25 % greater than that of the standard trypsin treatment. The dispersed chick fibroblast cells when seeded into fresh culture bottles, were found to be capable of attaching and multiplying.
Reports on similar grounds are available. The ability of alkaline protease from Conidiobolus (Chiplonkar et al. 1985) and Pronase (mixture of proteinases) from Streptomyces griseus (Foley and Aftonomos 1970) to dislodge cell lines have been compared to trypsin and found as possible substitutes for trypsin in animal cell culture. Dispase from Bacillus polymyxa (Kitano and Okada 1983) and Collagenase from Clostridium histolyticum are some of the other microbial proteases that have been put to various cell culture applications. APV26 is the first reported alkaline metalloprotease from Vibrio sp. useful in animal cell culture.
To arrest the activity of trypsin, media containing metal ions such as calcium and magnesium and serum are used. As APV26 was not inhibited by the above, simple washing with the growth medium would be sufficient to arrest its activity. Moreover trypsin acts only over a narrow pH range (7.2–7.4) while APV26 has broader pH range (6–9) facilitating it to act in cell culture media with pH, ranging from 6 to 7.5. The alkaline protease used in this study retains activity and stability over wide range of temperatures; even in this regard too it has an advantage over trypsin, which is highly temperature sensitive. Having all these facts in the background, we conclude that the alkaline metalloprotease from Vibrio sp. V26 has great potential in animal cell culture as a tissue dissociating and cell dislodging agent.
Electronic supplementary material
Acknowledgments
This work was carried out with the financial assistance from the Department of Biotechnology, Government of India, under Programme Support in Marine Biotechnology (BT/PR4012/AAQ/03/204/2003).The first author thanks the University Grants Commission for Fellowship.
References
- Almas S, Hameed A, Shelly D, Mohan P. Purification and characterization of a novel protease from Bacillus strain SAL1. Afr J Biotechnol. 2009;8:3603–3609. [Google Scholar]
- Alsina M, Blanch AR. Improvement and update of a set of keys for biochemical identification of Vibrio species. J Appl Bacteriol. 1994;77:719–721. doi: 10.1111/j.1365-2672.1994.tb02824.x. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bag PK, Bhowmik P, Hajra TK, Ramamurthy T, Sarkar P, Majumder M, Chowdhury G, Das SC. Putative virulence traits and pathogenicity of Vibrio cholerae non-O1, non-O139 isolates from surface waters in Kolkata, India. Appl Environ Microbiol. 2008;74:5635–5644. doi: 10.1128/AEM.00029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai R, Lesperance J, Kim M, Terskikh AV. Efficient propagation of single cells accutase-dissociated human embryonic stem cells. Mol Reprod Dev. 2008;75:818–827. doi: 10.1002/mrd.20809. [DOI] [PubMed] [Google Scholar]
- Benitez J, Silva A, Finkelstein R. Environmental signals controlling production of hemagglutinin/protease in Vibrio cholera. Infect Immun. 2001;69:6549–6553. doi: 10.1128/IAI.69.10.6549-6553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiplonkar JM, Gangodkar SV, Wagh UV, Ghadge GD, Rele MV, Srinivasan MC. Applications of alkaline protease from Conidiobolus in animal cell culture. Biotechnol Lett. 1985;7:665–668. doi: 10.1007/BF01040206. [DOI] [Google Scholar]
- Chowdhury MAR, Miyoshi S-I, Shinoda S. Purification and characterization of a protease produced by Vibrio mimicus. Infect Immun. 1990;58:4159–4162. doi: 10.1128/iai.58.12.4159-4162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BR, Fanning GR, Madden JM, Steigerwalt AG, Bradford HB, Jr, Smith HL, Jr, Brenner DJ. Characterization of biochemically atypical Vibrio cholerae strains and designation of a new pathogenic species, Vibrio mimicus. J Clin Microbiol. 1981;14:631–639. doi: 10.1128/jcm.14.6.631-639.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drita VJ. Coordinate control of virulence gene expression by Tox R in Vibrio cholerae. Mol Microbiol. 1992;6:451–458. doi: 10.1111/j.1365-2958.1992.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Farmer JJ, III, Janda JM. Family 1 Vibrionaceae. In: Brenner DJ, Kreig NR, Stanley JT, editors. Bergey’s manual of systematic bacteriology, vol 2. 2. NY: Springer Science + Bussiness Media Inc.; 2005. [Google Scholar]
- Fields PI, Popovic T, Wachsmuth K, Olsvik O. Use of polymerase chain reaction for detection of toxigenic Vibrio cholerae O1 strains from the Latin American cholera epidemic. J Clin Microbiol. 1992;30:2118–2121. doi: 10.1128/jcm.30.8.2118-2121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R, Hanne L. Purification and characterization of the soluble hemagglutinin (cholera lectin) produced by Vibrio cholerae. Infect Immun. 1982;36:1199–1208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JF, Aftonomos B. The use of pronase in tissue culture: a comparison with trypsin. J Cell Physiol. 1970;75:159–161. doi: 10.1002/jcp.1040750204. [DOI] [PubMed] [Google Scholar]
- Freshney RI. Culture of animal cells: a manual of basic techniques. NY: Wiley-Liss; 2000. [Google Scholar]
- Ghosh C, Nandy RK, Dasgupta SK, Nair GB, Hall RH, Ghose AC. A search for cholera toxin (CT), toxin coregulated pilus (TCP), the regulatory element ToxR, and other virulence factors in non-O1/non-O139 Vibrio cholerae. Microb Pathog. 1997;22:199–208. doi: 10.1006/mpat.1996.0105. [DOI] [PubMed] [Google Scholar]
- Hartree EE. Determination of protein; a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972;48:422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hilfer SR. Tissue culture: methods and applications. New York: Academic Press; 1973. [Google Scholar]
- Ichinose Y, Ehara M, Utsunomiya A. Purification of protease from Vibrio cholerae O1 and its partial characterization. Trop Med. 1992;34:121–125. [Google Scholar]
- Ishihara M, Kawanishi A, Watanabe H, Tomochika KI, Miyoshi SI, Shinoda S. Purification of a serine protease of Vibrio parahaemolyticus and its characterization. Microbiol Immunol. 2002;46:299–303. doi: 10.1111/j.1348-0421.2002.tb02699.x. [DOI] [PubMed] [Google Scholar]
- Iyer L, Vadivelu J, Puthucheary SD. Detection of virulence associated genes, haemolysin and protease amongst Vibrio cholerae isolated in Malaysia. Epidemiol Infect. 2000;125:27–34. doi: 10.1017/S0950268899004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellouli K, Bougatef A, Manni L, Agrebi R, Siala R, Younes I, Nasri M. Molecular and biochemical characterization of an extracellular serine-protease from Vibrio metschnikovii J1. J Ind Microbiol Biotechnol. 2009;36:939–948. doi: 10.1007/s10295-009-0572-5. [DOI] [PubMed] [Google Scholar]
- Johnvesly B, Naik GR (2001) Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Process Biochem 37:139–144
- Kaper JB, Morris JG, Jr, Levine MM. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbalaei-Heidari HR, Ziaee A–A, Schaller J, Amoozegar MA. Purification and characterization of an extracellular haloalkaline protease produced by the moderately halophilic bacterium, Salinivibrio sp. strain AF-2004. Enzyme Microb Technol. 2007;40:266–272. doi: 10.1016/j.enzmictec.2006.04.006. [DOI] [Google Scholar]
- Karunasagar I, Rivera I, Joseph B, Kennedy B, Shetty VR, Huq A, Karunasagar I, Colwel RR. ompU genes in non-toxigenic Vibrio cholerae associated with aquaculture. J Appl Microbiol. 2003;95:338–343. doi: 10.1046/j.1365-2672.2003.01984.x. [DOI] [PubMed] [Google Scholar]
- Kembhavi AA, Kulharni A, Pant AA. Salt-tolerant and thermostable alkaline protease from Bacillus subtilis NCIM No 64. Appl Biochem Biotechnol. 1993;38:83–92. doi: 10.1007/BF02916414. [DOI] [PubMed] [Google Scholar]
- Kitano Y, Okada N. Separation of the epidermal sheet by dispase. Br J Dermatol. 1983;108:555–600. doi: 10.1111/j.1365-2133.1983.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Kita-Tsukamoto K, Oyaizu H, Nanba K, Simidu U. Phylogenetic relationships of marine bacteria, mainly members of the family Vibrionaceae, determined on the basis of 16S rRNA sequences. Int J Syst Bacteriol. 1993;43:8–19. doi: 10.1099/00207713-43-1-8. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen KS, Auld DS. Characterization of an inhibitory metal binding site in carboxypeptidase A. Biochem. 1991;30:2610–2613. doi: 10.1021/bi00224a007. [DOI] [PubMed] [Google Scholar]
- Lee LEJ, Pochmursky V, Bols NC. Effect of corticosteroids on the morphology and proliferation of two Salmonid cell lines. Gen Comp Endocrin. 1986;64:373–380. doi: 10.1016/0016-6480(86)90071-7. [DOI] [PubMed] [Google Scholar]
- Lee K, Yu S, Liu P. Alkaline serine protease is an exotoxin of Vibrio alginolyticus in kuruma prawn, Penaeus japonicus. Curr Microbiol. 1997;34:110–117. doi: 10.1007/s002849900153. [DOI] [PubMed] [Google Scholar]
- Lee C-Y, Cheng M-F, Yu M-S, Pan M-J. Purification and characterization of a putative virulence factor, serine protease, from Vibrio parahaemolyticus. FEMS Microbiol Letts. 2002;209:31–37. doi: 10.1111/j.1574-6968.2002.tb11105.x. [DOI] [PubMed] [Google Scholar]
- Lee YK, Kim HW, Liu CL, Lee HK. A simple method for DNA extraction from marine bacteria that produce extracellular materials. J Microbiol Meth. 2003;52:245–250. doi: 10.1016/S0167-7012(02)00180-X. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Ahn SH, Lee E-M, Kim Y-O, Lee S-J, Kong I-S. Characterization of the enzyme acitivity of an extracellular metalloprotease (VMC) from Vibrio mimicus and its C-terminal deletions. FEMS Microbiol Lett. 2003;223:293–300. doi: 10.1016/S0378-1097(03)00401-4. [DOI] [PubMed] [Google Scholar]
- Levin BR, Tauxe RV. Cholera: nice bacteria and bad viruses. Curr Biol. 1996;6:1389–1391. doi: 10.1016/S0960-9822(96)00738-5. [DOI] [PubMed] [Google Scholar]
- Levine MM, Black RE, Clements ML, Cisneros L, Saah A, Nalin DR, Gill DM, Craig JP, Young CR, Ristaino P. The pathogenicity of nonenterotoxigenic Vibrio cholerae serogroup O1 biotype El Tor isolated from sewage water in Brazil. J Infect Dis. 1982;145:296–299. doi: 10.1093/infdis/145.3.296. [DOI] [PubMed] [Google Scholar]
- Levine MM, Kaper JB, Herrington D, Losonsky G, Morris JG, Clements ML, Black RE, Tall B, Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988;56:161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M, Faris A, Wastrom T, Hjerten S. A new test based on ‘salting out’ to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981;677:471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Magnusson KE, Davies J, Grundstrom T, Kihlstrom E, Normark S. Surface charge and hydrophobicity of Salmonellae, E. coli and Gonococci in relation to their tendency to associate with animal cells. Scand J Infect Dis. 1980;24:135–140. [PubMed] [Google Scholar]
- Mathur J, Waldor MK. The Vibrio cholerae ToxR-regulated porin OmpU confers resistance to antimicrobial peptides. Infect Immun. 2004;72:3577–3583. doi: 10.1128/IAI.72.6.3577-3583.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VL, Taylor RK, Mekalanos JJ. Cholera toxin transcriptional activator ToxR is a trans membrane DNA binding protein. Cell. 1987;48:271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- Nakasone N, Iwanaga M. Characterization of outer membrane protein OmpU of Vibrio cholerae O1. Infect Immun. 1998;66:4726–4728. doi: 10.1128/iai.66.10.4726-4728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler L, Evege E, McLaughlin J, Munroe D, Tan T, Wagner K, Stiles B. TrypLE™ express: a temperature stable replacement for animal trypsin in cell dissociation applications. Quest. 2004;1:42–47. [Google Scholar]
- Olafsen JA. Interaction between fish larvae and bacteria in marine aquaculture. Aquaculture. 2001;200:223–247. doi: 10.1016/S0044-8486(01)00702-5. [DOI] [Google Scholar]
- Peterson KM, Mekalanos JJ. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect Immun. 1988;56:2822–2829. doi: 10.1128/iai.56.11.2822-2829.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano D, Lauriano CM, Klose KE. Characterization of the role of the ToxR-modulated outer membrane porins OmpU and OmpT in Vibrio cholerae virulence. J Bacteriol. 2001;183:3652–3662. doi: 10.1128/JB.183.12.3652-3662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy T, Bag PK, Pal A, Bhattacharya SK, Bhattacharya MK, Sen D, Shimada T, Takeda T, Nair GB. Virulence patterns of V. cholerae non-O1 isolated from hospitalized patients with acute diarrhea in Calcutta, India. J Med Microbiol. 1993;39:310–317. doi: 10.1099/00222615-39-4-310. [DOI] [PubMed] [Google Scholar]
- Reddy GSN, Aggarwal RK, Matsumoto GI, Shivaji S. Arthrobacter flavus sp. nov., a psychrophilic bacterium isolated from a pond in McMurdo Dry Valley, Antarctica. Int J Syst Evol Microbiol. 2000;50:1553–1561. doi: 10.1099/00207713-50-4-1553. [DOI] [PubMed] [Google Scholar]
- Rinaldini LMJ. The isolation of living cells from animal tissues. Int Rev Cytol. 1958;7:587–647. doi: 10.1016/S0074-7696(08)62696-0. [DOI] [Google Scholar]
- Rivera ING, Chun J, Huq A, Sack RB, Colwell RR. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. Appl Environ Microb. 2001;67:2421–2429. doi: 10.1128/AEM.67.6.2421-2429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M, Gutnick D, Rossenberg E. Adherence of bacteria to hydrocarbons a simple method for measuring cell surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. doi: 10.1111/j.1574-6968.1980.tb05599.x. [DOI] [Google Scholar]
- Rous P, Jones FS. A method for obtaining suspensions of living cells from the fixed tissues, and for the plating out of individual cells. J Exp Med. 1916;23:549–555. doi: 10.1084/jem.23.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruimy R, Breittmayer V, Elbaze P, Lafay B, Boussemart O, Gauthier M, Christen R. Phylogenetic analysis and assessment of the genera Vibrio, Photobacterium, Aeromonas, and Plesiomonas deduced from small subunit rRNA sequences. Int J Syst Bacteriol. 1994;44:416–426. doi: 10.1099/00207713-44-3-416. [DOI] [PubMed] [Google Scholar]
- Saravanan V, Kumar SH, Karunasagar I, Karunasagar I. Putative virulence genes of Vibrio cholerae from seafoods and the coastal environment of Southwest India. Int J Food Microbiol. 2007;119:329–333. doi: 10.1016/j.ijfoodmicro.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Sechi LA, Dupre` I, Deriu A, Fadda G, Zanetti S. Distribution of Vibrio cholerae virulence genes among different Vibrio species isolated in Sardinia, Italy. J Appl Microbiol. 2000;88:475–481. doi: 10.1046/j.1365-2672.2000.00982.x. [DOI] [PubMed] [Google Scholar]
- Sigma DS, Moser G. Chemical studies of enzyme active sites. Ann Rev Biochem. 1975;44:889–931. doi: 10.1146/annurev.bi.44.070175.004325. [DOI] [PubMed] [Google Scholar]
- Smirnova NI, Nefedov KS, Osin AV, Livanova LF, Ya Krasnov M. A study of the distribution of regulatory genes controlling an expression of virulence genes among strains of Vibrio Cholerae biovar Eltor differing in their pandemic potential. Mol Gen Microbiol Virol. 2007;22:16–23. doi: 10.3103/S089141680701003X. [DOI] [PubMed] [Google Scholar]
- Smyth CJ, Jonsson P, Olsson E, Soderlind O, Rosengren J, Hjerton S, Wadstrom T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978;22:462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoussi M, Noumi E, Cheriaa J, Usai D, Sechi LA, Zanetti S, Bakhrouf A. Adhesive properties of environmental Vibrio alginolyticus strains to biotic and abiotic surfaces. New Microbiol. 2008;31:489–500. [PubMed] [Google Scholar]
- Thompson FL, Iida T, Swings J. Biodiversity of vibrios. Microbiol Mol Biol Res. 2004;68:403–431. doi: 10.1128/MMBR.68.3.403-431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaikkevicius K. Effects of Vibrio cholerae protease and pigment production on environmental survival and host interaction. Sweden: Umea University; 2007. [Google Scholar]
- Venugopal M (2004) Alkaline proteases from bacteria isolated from Cochin estuary India. Cochin University of Science and Technology, Ph D thesis, p 196
- Venugopal M, Saramma AV. Characterization of alkaline protease from Vibrio fluvialis strain VM 10 isolated from mangrove sediment sample and its application as a laundry detergent additive. Process Biochem. 2006;41:1239–1243. doi: 10.1016/j.procbio.2005.12.025. [DOI] [Google Scholar]
- Wang S-L, Chio Y-H, Yen Y-H, Wang C-L. Two novel surfactant-stable alkaline protease from Vibrio fluvialis TKU005 and their applications. Enzyme Microb Technol. 2007;40:1213–1230. doi: 10.1016/j.enzmictec.2006.09.012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.