Abstract
Recent studies have shown that, in numerous species, systemically administered bone marrow-derived mesenchymal stem cells undergo site-specific differentiation. This suggests that osteoblasts, by means of cytokine secretion, may promote dental pulp stem cells (DPSCs) to undergo osteogenesis. The objective of this study was to assess the potential synergistic interaction effect of osteoblasts on DPSCs for promotion of osteogenesis. Stem cells, derived from dental pulp of healthy human donors, were co-cultured with calvaria osteoblasts using a culture insert system. The proliferation rate, calcium deposition, osteogenic-related gene expression of induced DPSCs, including Runx-2, bone sialoprotein, osteocalcin and collagen-1, were assayed using MTT, Alizarin Red S staining and reverse transcriptase polymerase chain reaction, respectively. Co-cultured DPSCs had the highest rate of proliferation compared with those cultured in absence of osteoblasts. The morphology and ultrastructure of DPSCs in the co-cultures showed improvement, with co-cultured DPSCs becoming more osteoblast-like as compared with DPSCs cultured alone, and the mineralization potential of co-cultured DPSCs was enhanced compared with DPSCs cultured alone. Furthermore, osteogenic-related genes were significantly over-expressed in co-cultured DPSCs after osteogenic induction. The results demonstrate that DPSCs successfully differentiate towards osteoblasts and that the paracrine interaction of osteoblasts is likely to contribute to DPSC differentiation. It is believed that this study demonstrates certain useful applications for DPSCs in bone tissue engineering.
Keywords: Dental pulp stem cells (DPSCs), Osteogenesis, Mineralized tissue, Co-culture
Introduction
There already exists an extensive body of research on the development of bone marrow-derived mesenchymal stem cell (BMSC) based therapies for the restoration of injured bone tissue. It has long been known that virtually all craniofacial structures are derivatives of mesenchymal cells originating from the neural crest (Mao et al. 2006). Because DPSCs originate from the neural crest, at present many researchers have the view that dental pulp stem cells (DPSCs) are a promising and versatile tool for bone tissue regeneration and have been used for therapeutic stem cell applications (Huang et al. 2009; Zheng et al. 2009; Gandia et al. 2008). DPSCs share similar characteristics with BMSCs. For example, DPSCs and BMSCs both have multiple differentiation and high proliferation capabilities (Gronthos et al. 2000). Furthermore, these cells can be transplanted across barriers with histo-compatibility complex, without the need for immune suppression. In fact, DPSCs and BMSCs have been found to actually induce immunosuppression (Pierdomenico et al. 2005; Jewett and Tseng 2011). It is also notable that DPSCs are superior to BMSCs in certain aspects, such as low morbidity at the anatomical site after pulp collection, as well as the high efficiency of the extraction procedure of stem cells from pulp tissue (Graziano et al. 2008). Previous studies have confirmed that DPSCs can differentiate into odontoblasts, osteoblasts, fat cells and nerve-like cells (Almushayt et al. 2006; Arthur et al. 2008). Above all, DPSCs can potentially be used for tissue restoration in clinical practice.
There exists good research coverage on stem-cell-mediated osteogenesis. Previous cell culture methods have successfully reported osteogenic differentiation of DPSCs. For example, Mori et al. successfully induced DPSCs into osteoblasts and detected DPSC osteoblast parameters using reverse transcriptase polymerase chain reaction (RT-PCR) and western blot analysis (Mori et al. 2010). When comparing the effects of various in vitro conditions on DPSC osteogenic differentiation, valproic acid and bFGF are known key factors for bone formation through osteogenesis (Karbanová et al. 2010). From these studies, it seems DPSCs cultured in different osteogenic media lead to different levels of osteogenesis. The contribution of these studies is to identify an optimal medium for bone tissue engineering and to confirm that the micro-environment plays an important role in the directional differentiation of DPSCs.
Previous studies suggested that stem cells interact in vivo with stromal cells or tissue cells. In 2003, Kim et al. succeeded in co-culturing osteoblasts with mesenchymal cells in direct cell-to-cell contact and confirmed that synergistic interactions exist between osteoblasts and MSCs (Kim et al. 2003). Co-culture of rabbit articular chondrocytes and rabbit BMSCs within a defined range of ratios promotes expression of a cartilaginous extra-cellular matrix. Here, the micro-environment formed by chondrocytes plays a crucial role in the chondrogenic differentiation of BMSCs (Qing et al. 2011). Zhou et al. utilized this co-culture method to observe osteoblasts controlling their mesenchymal precursors via the Wnt signaling pathway. The Wnt family consists of a large number of secreted glycoproteins. These signaling molecules are involved in regulating cell differentiation and osteoblast differentiation (Zhou et al. 2008). In the cell micro-environment, surface receptors of co-cultured cells come into direct physical contact with cytokines and the autocrine and paracrine factors secreted by one cell type readily interact with other cell types (Bigdeli et al. 2009). This theory clarifies the phenomenon that the interaction between stem cells and tissue cells exists. To date however, few studies have been performed to investigate the role of tissue cells on DPSC differentiation, based on the relationship between DPSCs and osteoblasts in vitro. This paper attempts to at least partially address this issue.
In this study, we co-cultured two cell types using a culture insert system to observe the effect of osteoblasts on DPSCs osteogenesis in vitro. Our aim was to explore a novel method to promote osteogenesis for bone engineering applications, which combines the technical requirements of both high efficiency and low consumption.
Materials and methods
DPSCs culture
Normal human permanent teeth were collected from patients 14–25 years of age at the dental clinic of the Second Affiliated Hospital of the Harbin Medical University. Each subject was assessed for systemic and oral infection or disease and only disease-free subjects were included in this study. All experiments on human subjects were carried out with the full understanding and written consent of the subjects and in the case of minor children, their parents or guardians. We also obtained approval from the Ethical Board of the Harbin Medical University before the extractions were performed. The DPSCs removed from human adult teeth were isolated enzymatically using the method described by Yuan et al. (2010). The dental pulp was immersed in the following digestive solution: 3 mg/mL type I collagenase, 4 mg/mL dispase in 4 mL 1x PBS, 100 U/mL penicillin, with 100 μg/mL streptomycin for 1 h at 37 °C. The solution was then filtered using a 70 μm strainer. Cells were collected by centrifugation and washed twice in Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12; Hyclone, Logan, UT, USA). The cells were seeded at 1 × 105 cells/cm2 in 25 cm2 culture flasks containing growth medium: DMEM/F12 supplemented with 20 % fetal bovine serum (Hyclone), 100 U/mL penicillin, 100 μg/mL streptomycin (Beyotime Institute of Biotechnology, Beijing, China), in a humidified atmosphere of 5 % CO2 and 95 % air at 37 °C. Medium was changed two to three times per week. DPSCs were cultured in this medium for at least one passage prior to differentiation and fibroblasts were removed from the DPSC population according to our previously described experimental method (Yuan et al. 2010). DPSCs at second passage (P2) were used for all experiments.
Osteoblast isolation and culture
Osteoblasts were obtained from the calvaria of 5 days old fetal Sprague–Dawley rats. The study was performed in accordance with a protocol approved by the Animal Care and Use Committee of the Harbin Medical University. After removal of the periosteum, several bone fragments were snipped off and cells were extracted from these by a 20 min digestion in 0.25 % trypsin and a 1 hour digestion in 1 mg/mL type II collagenase at 37 °C. Cell fractions were collected and re-suspended in growth medium and filtered through a 70 μm strainer. Cells were then seeded at 1 × 105 cells/cm2 in 75-mL flasks in growth medium as previously described and were incubated in a humidified atmosphere of 5 % CO2 and 95 % air at 37 °C. Culture medium was changed two to three times per week, and second passage (P2) osteoblasts were used for experiments.
Co-culture of DPSCs with osteoblasts
Both cell types were trypsinized and re-plated into the culture insert system (Greiner, Frickenhausen, Germany). DPSCs were plated at ~1–2 × 105 cells/well in the lower plate wells, and osteoblasts were plated at ~5–10 × 104 cells/well in the upper plate inserts (Mo et al. 2009). Cytokines and growth factors from the osteoblasts were able to disperse through the insert into the DPSC growth medium, which then became osteogenic medium. A control group comprising DPSCs cultured in growth medium in absence of osteoblasts was included.
Transmission electron microscopy (TEM)
DPSCs were collected and fixed in 2.5 % glutaraldehyde 0.1 M cacodylate buffer (pH = 7.4) for ~2–3 h at room temperature. Samples were post-fixed in 1 % osmium tetroxide and dissolved in a 0.1 M phosphate buffer (pH = 7.4) for 1 h at room temperature. The samples were dehydrated in ethanol and embedded in Epon 812. Ultra-thin sections were contrasted in aqueous uranyl acetate and lead-hydroxide, observed and photographed using a Hitachi 7000 TEM.
MTT assay
A methyl-tetra-zolium (MTT; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma, St. Louis, MO, USA) assay was used to evaluate the proliferation rate of osteogenic induced DPSCs. DPSCs and osteoblasts were seeded alone at 1.2 × 103 cells/well in 96-well plates, referred to as control groups. Co-cultured DPSCs and osteoblasts were each seeded at 0.6 × 103 cells/well within the same well and were referred to as the experimental group. They were analyzed on days 3, 6, 9, 12 and 15. Cells were incubated for 4 h with 500 μg/mL MTT reagent in the dark at 37 °C. After incubation, medium was removed and 100 μL dimethylsulfoxide (Kanto Chemical Co., Tokyo, Japan) was added. The plate was stirred using a microplate mixer (Amersham, Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK) and the absorbance was measured at a wavelength of 540 nm using a microplate reader Mycycler™ Thermal cycler (BIO-RAD, Hercules, CA, USA).
Alizarin red S staining
The capability of induced DPSCs to form mineralized nodules in vitro was assessed by Alizarin red S staining. Both DPSC control and experimental groups were cultured for 28 days in growth medium. As mentioned previously, this growth medium became osteogenic medium if DPSCs were co-cultured in the presence of osteoblasts. Media in all groups were changed twice weekly with fresh growth medium. After 28 days, cells were rinsed three times using PBS and fixed with 95 % ethanol for 10 min. Cells were then stained with 1 % Alizarin red S (Sigma) for 5 min. Distilled water was used to rinse the stain prior to observation.
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis
Performance analysis was carried out on samples from two DPSCs groups at days 5 and 15 and repeated three times. Osteogenic-related genes in the stem cells were detected by RT-PCR. Total RNA was extracted from 10 × 105 induced cells at two time points using Trizol (Sigma) according to the manufacturer’s instructions and was stored at −20 °C for 40 min before performance analysis. cDNA synthesis was carried out from total RNA by reverse transcriptase (Gene Copoeia, Germantown, PA, USA). Polymerase chain reaction (PCR) amplification of target messenger RNA was performed using the ComWin PCR kit (ComWin Biotech Co., Beijing, China). The PCR oligonucleotide primers and annealing temperatures are listed in Table 1. The products were electrophoresed on 2 % agarose gels.
Table 1.
Primers of BSP, Runx-2, OCN, Col-1 for RT-PCR
| Gene | Primer sequences | Annealing temperature (Tm) |
|---|---|---|
| BSP (60 bp) | Forward: TGCCTTGAG CCTGCTTCCT | 48.2 |
| Reverse: CTGAGCAAAATTAAAGCAGTCTTCA | ||
| Runx-2 (289 bp) | Forward: TCACCTCAGGCATGTCCCTCGGTAT | 60.1 |
| Reverse: TGGCTTCCATCAGCGTCAACACC | ||
| OCN (119 bp) | Forward: ACCCAGGCGCTACCTGTATCAATG | 53.9 |
| Reverse: CGATGTGGTCAGCCAACTCGTCA | ||
| Col-1 (314 bp) | Forward: TCTACTGGCGAAACCTGTATCCG | 51.8 |
| Reverse: GCAACAAGTTCAACATCATTAGAGCC |
Statistical analysis
MTT analysis and expression of osteogenic-related genes were reported as mean ± standard deviation. One-way ANOVA was used to assess differences between treatment groups. When a difference between groups was identified by ANOVA, we further compared the indices in the co-cultured (experimental) group and the control groups. All statistical analyses were performed using SPSS (SPSS Inc, Chicago, IL, USA).
Results
Isolation of DPSCs and osteoblast populations
DPSCs and osteoblasts were isolated from humans and rats, repsectlively. Dental pulp stem cells proliferated rapidly, appearing spindle shaped; osteoblasts proliferated rapidly with tapered profile.
Morphology and ultrastructure analysis of DPSCs
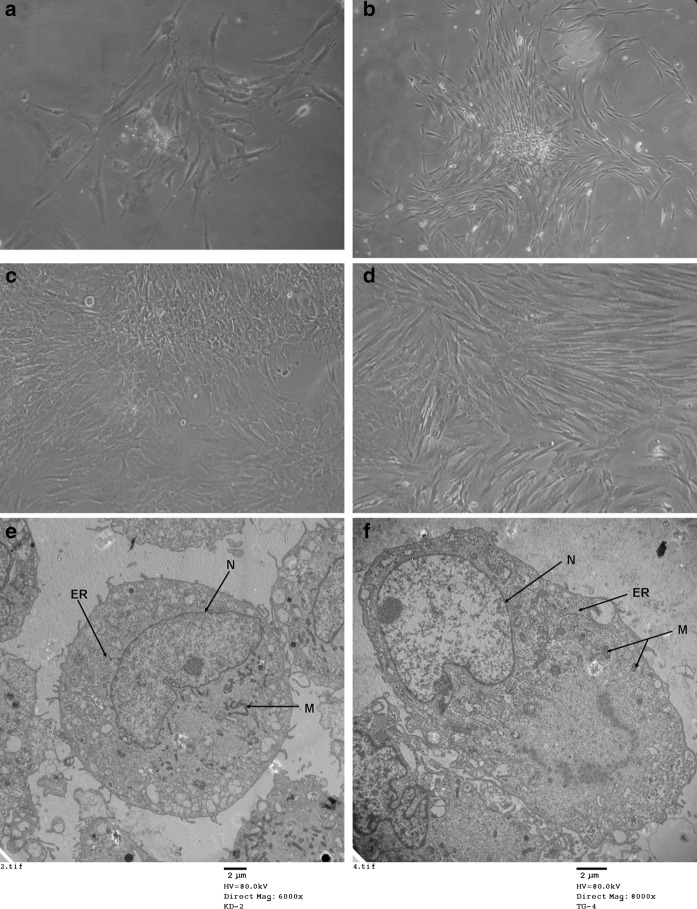
Primary DPSC colony cultured for 48 h displayed no obvious morphological changes. At day 7, short spindle-shaped primary cells became more elongated (Fig. 1a–b). The second passage DPSCs were cultured under two different conditions: (1) in the presence of osteoblasts, which produced an osteogenic medium; (2) in standard growth medium. No differences in size or shape between cells cultured in the two conditions were observed until the 28th day. Some co-cultured DPSCs were observed displaying an osteoblast-like morphology: their fusiform morphology had changed partly into a polygonal shape, with many bumps and an opaque body; however, DPSCs cultured alone showed almost no change. (Fig. 1b–e).
Fig. 1.
Phase contrast microscopy showing the morphology of DPSCs (a–d ×100). a Primary cultured DPSC were cultured for 48 h. b Primary cultured DPSC cultured until day 7. c The morphology of DPSCs in the presence of osteoblast co-cultures at day 28. d The morphology of DPSCs cultured alone at day 28. TEM analysis was used to assess the ultrastructural appearance of DPSCs in the presence and absence of osteoblast co-cultures at day 15, N for nucleus, ER for endoplasmatic reticulum, M for mitochondria. (Fig. 1e–f). e DPSCs cultured alone were round in shape, with a basal nucleus and thin nuclear elongations; the cytoplasm contained scattered mitochondria, lysosome-like organelles and a rudimental reticulum (original magnification ×6,000). f Ultrastructure of induced DPSCs in the co-culture groups displayed nuclear polarity, with a round shape and basal nucleus. The nucleolus became bigger and an extremely extended rough endoplasmic was observed. A large Golgi apparatus and multiple coated matrix vesicles were also observed (original magnification ×8,000)
The influence of osteogenic medium on DPSC morphology was assessed using TEM analysis. At day 15, DPSCs in all conditions were mainly spherical or anomalous in shape, with small cell volumes, containing one or two nucleoli in the center, amounting to between 70 and 80 % of the total cytoplasm. Other morphological features did not change significantly. However, the DPSCs cell volumes in the co-cultured groups were greater than in the cells cultured alone groups. Induced DPSCs in the experimental groups had a basal nucleus, an evident nucleolus and an extremely extended rough endoplasmic reticulum, polarity of cytoplasmic organelles, numerous coated vesicles and a large Golgi apparatus (Fig. 1e, f).
Proliferation assay
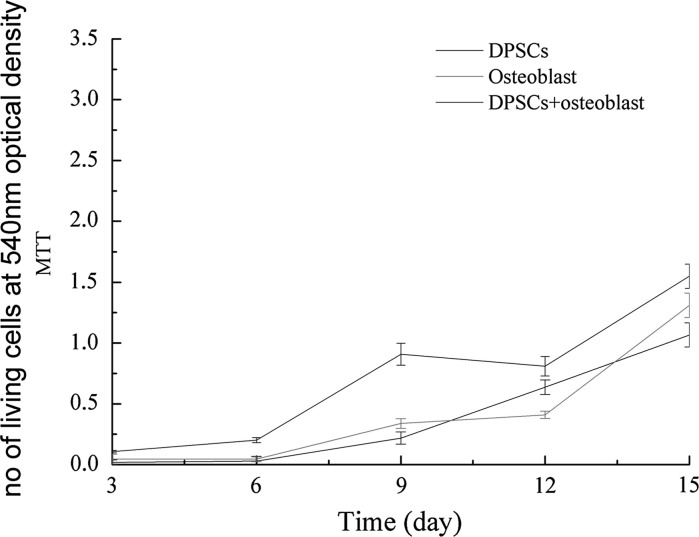
An MTT proliferation assay was performed to determine whether osteogenic factors secreted by osteoblasts were biologically active. Co-cultured DPSCs with osteoblasts (experimental groups) exhibited a higher rate of proliferation compared with DPSCs and osteoblasts cultured alone (control groups). Cell growth of the co-cultured groups was greater in comparison to those cultured alone (Fig. 2).
Fig. 2.
The cell groups exhibited different growth rates. Cell proliferation in all groups increased from day 3–15. Proliferation of the osteoblasts and DPSCs cultured alone (control groups) were comparable; however, DPSCs in the co-cultured group proliferated at the fastest rate throughout culture
Calcium assay
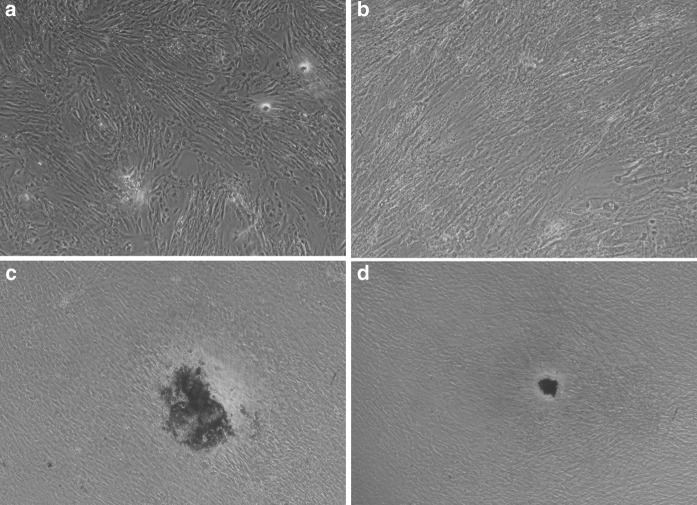
Mineralization occurs in the final stages of osteogenesis. DPSCs were stained with Alizarin red S at days 10 and 28. In all groups calcium deposition was absent at day 10 (Fig. 3a, b). At day 28, significant calcium depositions were identified at large, round, stained nodules in the co-cultured DPSC and osteoblast group. There were fewer and smaller mineralized nodules in the DPSC group cultured alone at day 28 (Fig. 3c, d). The amount of calcium deposition greatly increased after the day 28 in the DPSC co-cultured groups.
Fig. 3.
Phase contrast microscopy (×100) showing Alizarin red stained DPSCs in experimental and control groups at days 10 and 28 (a–d). At day 10, few mineralized nodules are present in both the co-cultured DPSC experimental group (a) and DPSCs cultured alone group (b). After induction for 28 days, Alizarin red S staining demonstrated that the DPSC co-cultured group generated more mineralize nodules (c) than the DPSCs cultured alone group (d)
RT-PCR
To evaluate the influence of osteoblasts on the expression level of osteogenic-related genes in DPSCs, the most specific and common gene markers of the osteogenic lineage were investigated using RT-PCR at days 5 and 15. Osteogenic-related genes, including Runt-related transcription factor (Runx-2, also known as core binding factor), bone sialoprotein (BSP), collagen type I (COL-1) and osteocalcin (OCN), were expressed during DPSC osteogenic differentiation, and levels were more significant in co-cultured DPSCs compared with DPSCs cultured alone from day 5 to 15. Co-cultured DPSCs expressed normal levels of osteogenic-related genes when differentiating into osteoblasts at days 5–15. As the data illustrate, levels of Runx-2 and BSP expression were higher at day 5 compared with day 15, while levels of OCN and COL-1 expression were up-regulated at day 15 compared with day 5. This experiment indicates that induced DPSCs express many of the genes and transcription factors necessary for osteogenesis (Table 2).
Table 2.
The levels of osteogenic related genes were expressed in the co-culturing and DPSCs conditions at the 5th and 15th days using RT-PCR (3/group)
| Gene | Culture method | Time point | |
|---|---|---|---|
| 5 days | 15 days | ||
| Runx-2 | Co-culture | 0.432 ± 0.079 | 0.060 ± 0.008a |
| DPSCs culture | 0.132 ± 0.013 | 0.049 ± 0.009a | |
| BSP | Co-culture | 30.421 ± 3.214 | 9.807 ± 1.135a |
| DPSCs culture | 11.401 ± 0.246 | 9.795 ± 1.014 | |
| Col-1 | Co-culture | 1.579 ± 0.198 | 2.913 ± 0.310a |
| DPSCs culture | 0.491 ± 0.612 | 2.754 ± 0.351a | |
| OCN | Co-culture | 0.251 ± 0.311 | 0.382 ± 0.421a |
| DPSCs culture | 0.148 ± 0.225 | 0.334 ± 0.562 | |
aThe the level of osteogenic related genes was statistically different from that at the 5th days
Discussion
In this study, we could confirm that osteoblasts can promote the osteogenic differentiation ability and proliferation capacity of DPSCs. Combining cellular and biochemical parameters, we simulated an in vitro model of the osteoblast niche that provides a physiological quiescent micro-environment. DPSCs were induced into osteogenic differentiation by the secretions from osteoblasts, suggesting that DPSCs could potentially be differentiated along other specific lineages if co-cultured with the appropriate primary cell type. DPSCs co-cultured with osteoblasts exhibited enhanced cell proliferation compared with DPSCs cultured alone. At day 28, the fusiform body morphology of induced DPSCs showed a partly polygonal and opaque body which resembled osteoblasts. DPSCs have previously been shown to consist of several sub-populations which are able to differentiate into odontoblasts and form mineralized nodules during cells passaging (Yu et al. 2010). Mineralized nodules were detected in both groups, but to a greater extent in the co-cultured DPSC group. It is likely that the osteoblasts performed a crucial role in this process. Extracellular matrix mineralization is a physiological process required for the formation of bones and teeth. The appearance of a mineralized matrix in the co-cultured DPSC group offers confirmation of the effect of osteoblast.
After 5 and 10 days co-culture of DPSCs with osteoblasts, DPSCs expressed vast amounts of mRNA of several osteogenic markers, including Runx-2, BSP, OCN and COL-1. Runx-2 is a member of the runt homology domain family of transcription factors and is expressed during the early differentiation phase (Seong et al. 2010). Previous studies have confirmed that Runx-2 knocked out leads to the formation of defects in fibrous cells (Yu et al. 2010). Thus, Runx-2 is clearly a crucial gene in the process of osteoblastic differentiation. In fact, high expression of Runx-2 at day 5 is closely associated with the process of ossification and is regarded as a useful factor during early differentiation of DPSCs. Furthermore, BSP, an osteogenic-specific gene, is an acidic, noncollagenous glycoprotein abundantly expressed in mineralized tissues and was significantly up-regulated in DPSC co-cultures.
In the later phase of differentiation, bone markers COL-1 and OCN were also highly expressed. Collagen I and hydroxyapatite crystals occupy more than 90 % of the bone extracellular matrix. Taken together, the results from the RT-PCR analysis show that osteogenic-related genes were identified at higher levels in DPSCs co-cultures, all of which promote the processes of bone development.
The use of osteoblastic cytokines in the culture of DPSCs can induce DPSCs to address bone tissue repair problems. As we know, bone morphogenetic proteins (BMPs) are members of the transforming growth factor-β superfamily (Sowa et al. 2004). Fetal bovine bone cells are reported to transcribe TGF-β mRNA, and synthesize and secrete this peptide. As early as 1987, researchers confirmed that bone cells can be stimulated by TGF-β (Robey et al. 1987). BMPs promote DPSCs osteogenesis by playing a pivotal role in endochondral bone formation (Yang et al. 2009; Lavery et al. 2008). Osteopontin (OPN) is a key sialoprotein, which together with BSP is essential for the process of mineralization (Sodek et al. 2000). MSCs are able to migrate and home into sites of injury, a process closely related to OPN (Zou et al. 2011). Furthermore, BSP has a positive function in Saos-2 cell bone induction and bone morphogenesis (Anderson et al. 2002) and subsequently regulates the formation of hydroxyapatite (Gordon et al. 2007). BMPs, OPN and BSP are all derived from osteoblasts.
We therefore conclude that osteogenic proteins secreted by osteoblasts contribute to osteogenesis. We believe the synergistic effect of the secreted proteins could be superior to any single protein. Distinct differences exist between our study and previous studies. First, Ueno et al. co-cultured dental pulp cells with osteoblasts and found pulp cells were able to mineralize (Ueno et al. 2006). In our study we extracted DPSCs from the pulp cells and co-cultured them with osteoblasts in the same medium. This limits direct cell–cell contact between the two cell types. Osteoblasts in the upper insert secrete proteins which diffuse through the pores into the lower well containing DPSCs. By simulating the in vivo micro-environment, we found that DPSCs play a major role in mineralization and explored the role these secreted proteins have on DPSCs osteogenic differentiation. Furthermore, it is clear that the ratio of cell types within the co-cultures is important. In one study, an osseous phenotype was histologically detected only in 2:1 ratio of co-cultured MSCs and chondrocytes (Mo et al. 2009; Takagi et al. 2007). The optimal ratio of co-cultured DPSCs and osteoblasts was carried out in our experiment so as to achieve best results.
However, some limitations exist in our study. First, rat osteoblasts were included in the co-cultures because they are reported to have the highest homology with their human counterparts. However, it is not clear whether the same results would be obtained if human osteoblasts were used. Second, whether this novel culture method can be utilized in an in vitro three-dimensional frame is unclear for the moment.
Optimal in vitro culture conditions are critical for DPSCs induction. Tissue engineering and therapies arising from DPSC research are broadening the application for cell culture technology. Using a novel co-culture system, DPSCs can be directly modulated by paracrine signals from osteoblasts; this is a valid way for bone formation and tissue function repair applications. These results might be beneficial to future maxillofacial regeneration therapies. Ongoing work in our laboratory aims to compare this co-culture method with other osteogenic induction methods.
Footnotes
Jie Yao and Mengtong Yuan have equally contributed to this work.
References
- Almushayt A, Narayanan K, Zaki AE, George A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 2006;13:611–620. doi: 10.1038/sj.gt.3302687. [DOI] [PubMed] [Google Scholar]
- Anderson HC, Reynolds PR, Hsu HH, Missana L, Masuhara K, Moylan PE, Roach HI (2002) Selective synthesis of bone morphogenetic proteins-1, -3, -4 and bone sialoprotein may be important for osteoinduction by Saos-2 cells. J Bone Miner Metab 20:73–82 [DOI] [PubMed]
- Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- Bigdeli N, Karlsson C, Strehl R, Concaro S, Hyllner J, Lindahl A (2009) Coculture of human embryonic stem cells and human articular chondrocytes results in significantly altered phenotype and improved chondrogenic differentiation. Stem Cells 27:1812–1821 [DOI] [PubMed]
- Graziano A, d’Aquino R, Laino G, Papaccio G (2008) Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev 4:21–26 [DOI] [PubMed]
- Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, Sanchez-Torrijos J, Payá R, Mirabet V, Carbonell-Uberos F, Llop M, Montero JA, Sepúlveda P (2008) Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 26:638–645 [DOI] [PubMed]
- Gordon JA, Tye CE, Sampaio AV, Underhill TM, Hunter GK, Goldberg HA (2007) Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone 41:462–473 [DOI] [PubMed]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630 [DOI] [PMC free article] [PubMed]
- Huang GT. Pulp and dentin tissue engineering and regeneration: current progress. Regen Med. 2009;4:697–707. doi: 10.2217/rme.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett A, Tseng HC. Tumor induced inactivation of natural killer cell cytotoxic function; implication in growth, expansion and differentiation of cancer stem cells. J Cancer. 2011;2:443–457. doi: 10.7150/jca.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbanová J, Soukup T, Suchánek J, Mokrý J (2010) Osteogenic differentiation of human dental pulp-derived stem cells under various ex vivo culture conditions. Acta Medica (Hradec Králové) 53:79–84 [DOI] [PubMed]
- Kim H, Lee JH, Suh H. Interaction of mesenchymal stem cells and osteoblasts for in vitro osteogenesis. Yonsei Med J. 2003;44:187–197. doi: 10.3349/ymj.2003.44.2.187. [DOI] [PubMed] [Google Scholar]
- Lavery K, Swain P, Falb D, Alaoui-Ismaili MH. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem. 2008;283:20948–20958. doi: 10.1074/jbc.M800850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, Shi S (2006) Craniofacial tissue engineering by stem cells. J Dent Res 85:966–979 [DOI] [PMC free article] [PubMed]
- Mo XT, Guo SC, Xie HQ, Deng L, Zhi W, Xiang Z, Li XQ, Yang ZM (2009) Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone 45:42–51 [DOI] [PubMed]
- Mori G, Centonze M, Brunetti G, Ballini A, Oranger A, Mori C, Lo Muzio L, Tetè S, Ciccolella F, Colucci S, Grano M, Grassi FR (2010) Osteogenic properties of human dental pulp stem cells. J Biol Regul Homeost Agents 24:167–175 [PubMed]
- Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C, Alviano F, Fossati V, Staffolani N, Franchina M, Grossi A, Bagnara GP (2005) Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 80:836–842 [DOI] [PubMed]
- Qing C, Wei-ding C, Wei-min F. Co-culture of chondrocytes and bone marrow mesenchymal stem cells in vitro enhances the expression of cartilaginous extracellular matrix components. Braz J Med Biol Res. 2011;44:303–310. doi: 10.1590/s0100-879x2011007500026. [DOI] [PubMed] [Google Scholar]
- Robey PG, Young MF, Flanders KC, Roche NS, Kondaiah P, Reddi AH, Termine JD, Sporn MB, Roberts AB (1987) Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol 105:457–463 [DOI] [PMC free article] [PubMed]
- Seong JM, Kim BC, Park JH, Kwon IK, Mantalaris A, Hwang YS (2010) Stem cells in bone tissue engineering. Biomed Mater 5:062001 [DOI] [PubMed]
- Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Hendy GN, Canaff L, Komori T, Sugimoto T, Chihara K (2004) Menin is required for bone morphogenetic protein 2- and transforming growth factor beta-regulated osteoblastic differentiation through interaction with smads and runx2. J Biol Chem 24:40267–40275 [DOI] [PubMed]
- Takagi M, Umetsu Y, Fujiwara M, Wakitani S. High inoculation cell density could accelerate the differentiation of human bone marrow mesenchymal stem cells to chondrocyte cells. J Biosci Bioeng. 2007;103:98–100. doi: 10.1263/jbb.103.98. [DOI] [PubMed] [Google Scholar]
- Ueno A, Yamashita K, Miyoshi K, Horiguchi T, Ruspita I, Abe K, Noma T. Soluble matrix from osteoblastic cells induce mineralization by dental pulp cells. J Med Invest. 2006;53:297–302. doi: 10.2152/jmi.53.297. [DOI] [PubMed] [Google Scholar]
- Yang X, van der Kraan PM, Bian Z, Fan M, Walboomers XF, Jansen JA. Mineralized tissue formation by BMP2-transfected pulp stem cells. J Dent Res. 2009;88:1020–1025. doi: 10.1177/0022034509346258. [DOI] [PubMed] [Google Scholar]
- Yu J, He H, Tang C, Zhang G, Li Y, Wang R, Shi J, Jin Y. Differentiation potential of STRO-1 + dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010;11:32. doi: 10.1186/1471-2121-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan MT, Hu WP, Zhou HY (2010) Observation of dental pulp stem cells from human young permanent teeth of cloning subculture in vitro. J Oral Sci Res 26:624–627
- Zheng Y, Liu Y, Zhang CM, Zhang HY, Li WH, Shi S, Le AD, Wang SL. Stem cells from deciduous tooth repair mandibular defect in swine. J Dent Res. 2009;88:249–254. doi: 10.1177/0022034509333804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Mak W, Zheng Y, Dunstan CR, Seibel MJ (2008) Osteoblasts directly control lineage commitment of mesenchymal progenitor cells through Wnt signaling. J Biol Chem 283:1936–1945 [DOI] [PubMed]
- Zou C, Song G, Luo Q, Yuan L, Yang L (2011) Mesenchymal stem cells require integrin β1 for directed migration induced by osteopontin in vitro. In Vitro Cell Dev Biol Anim 47:241–250 [DOI] [PubMed]