Abstract
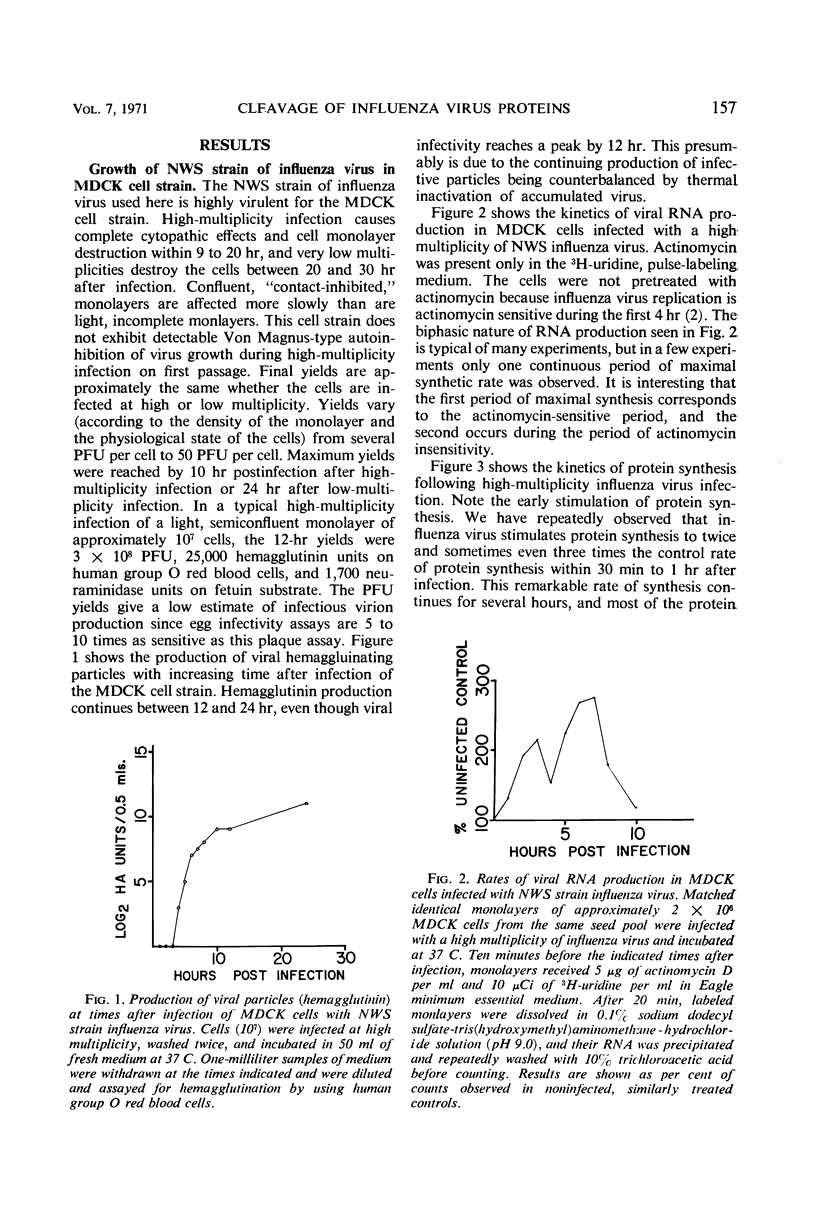
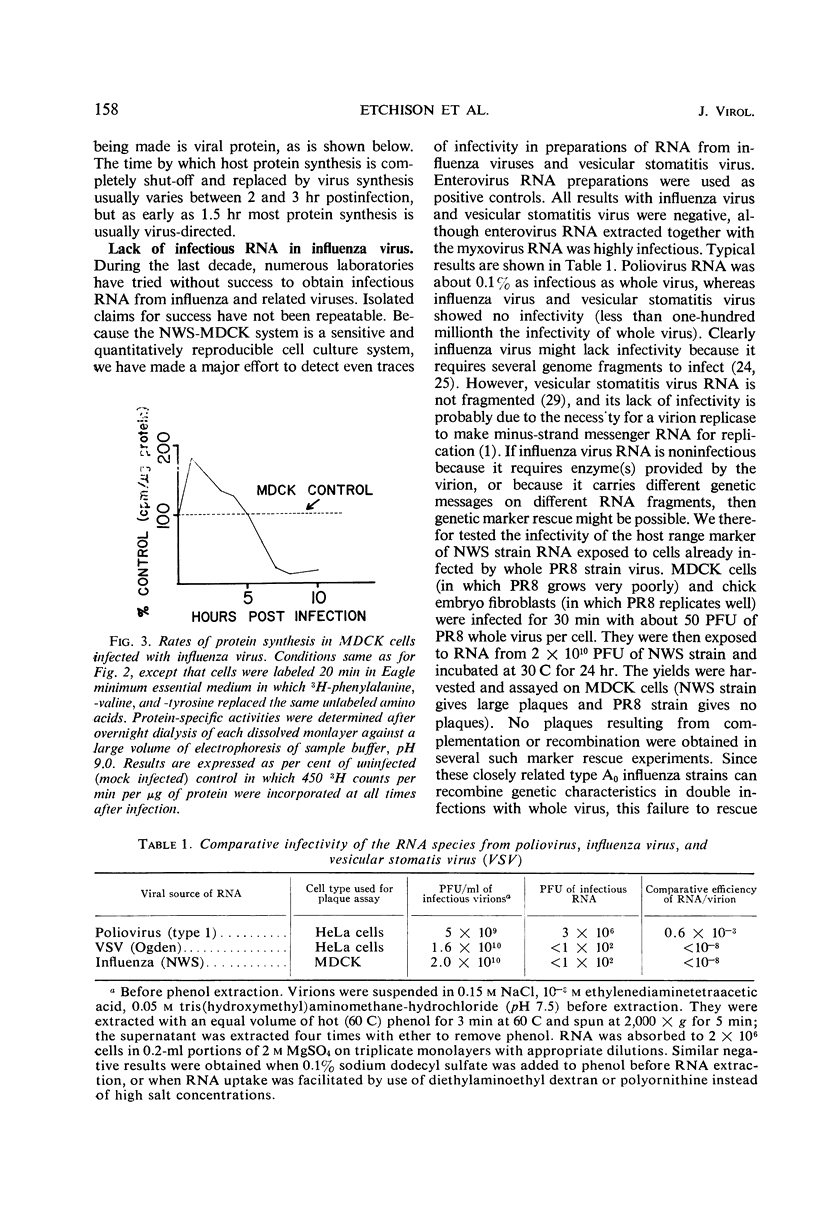
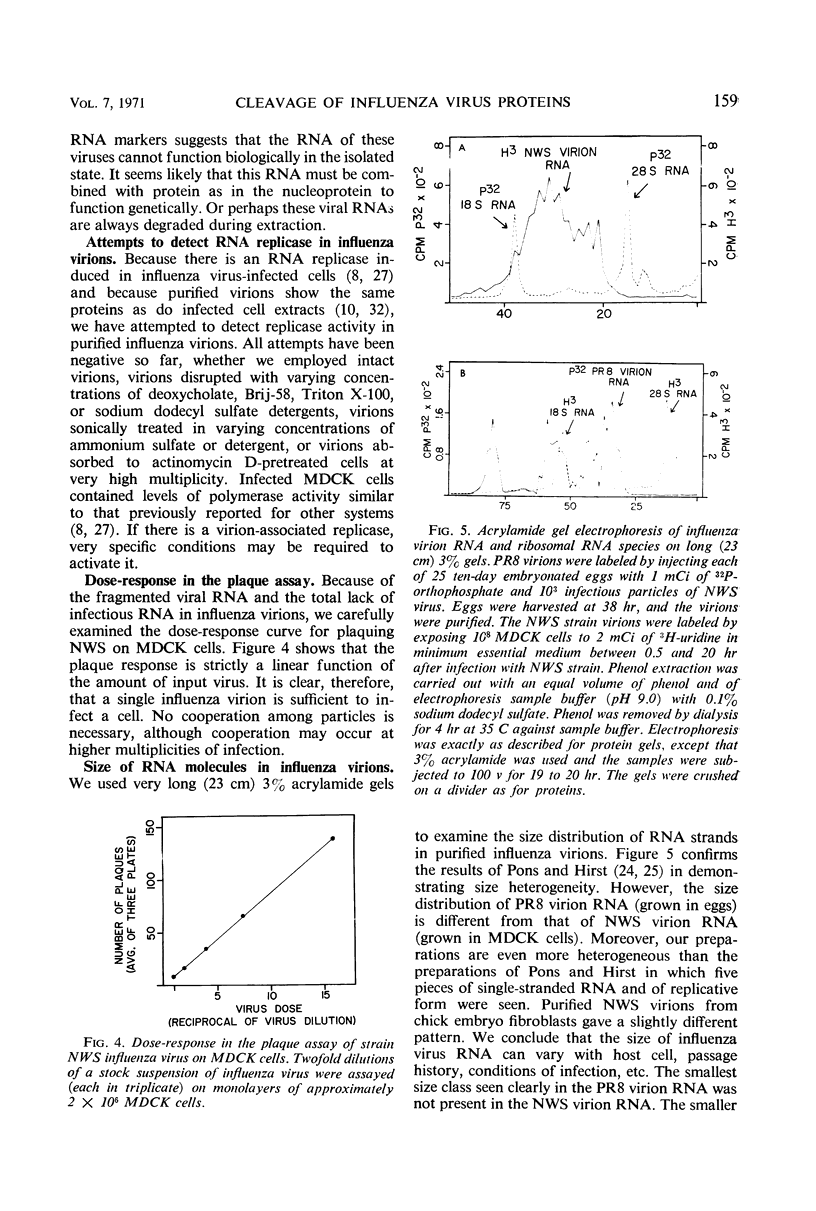
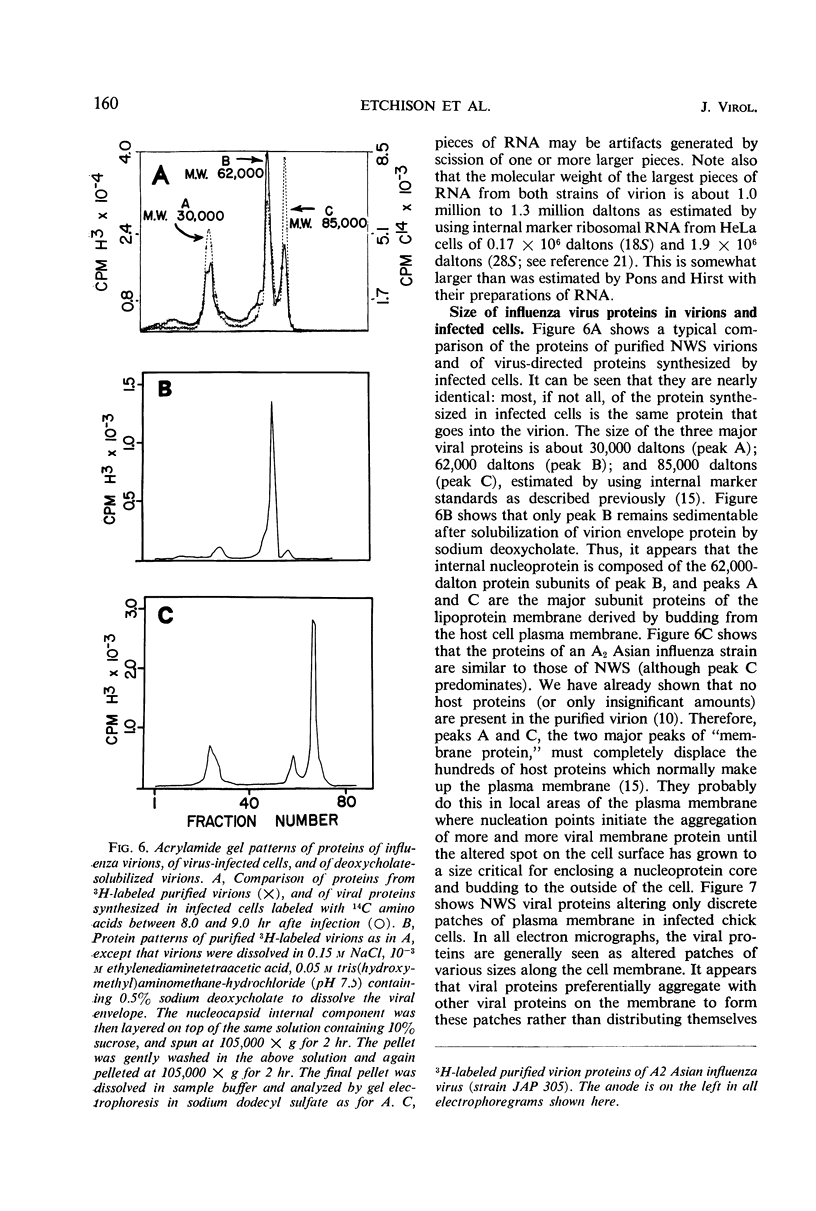
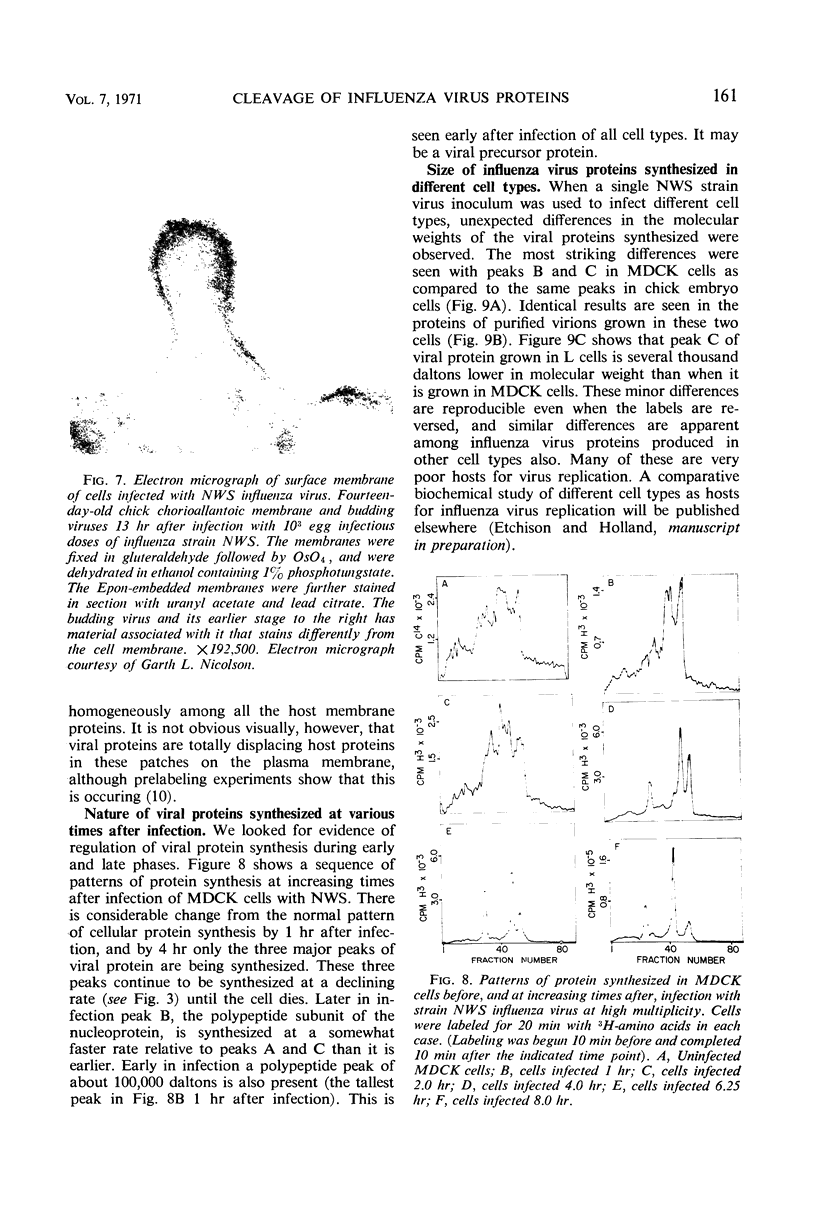
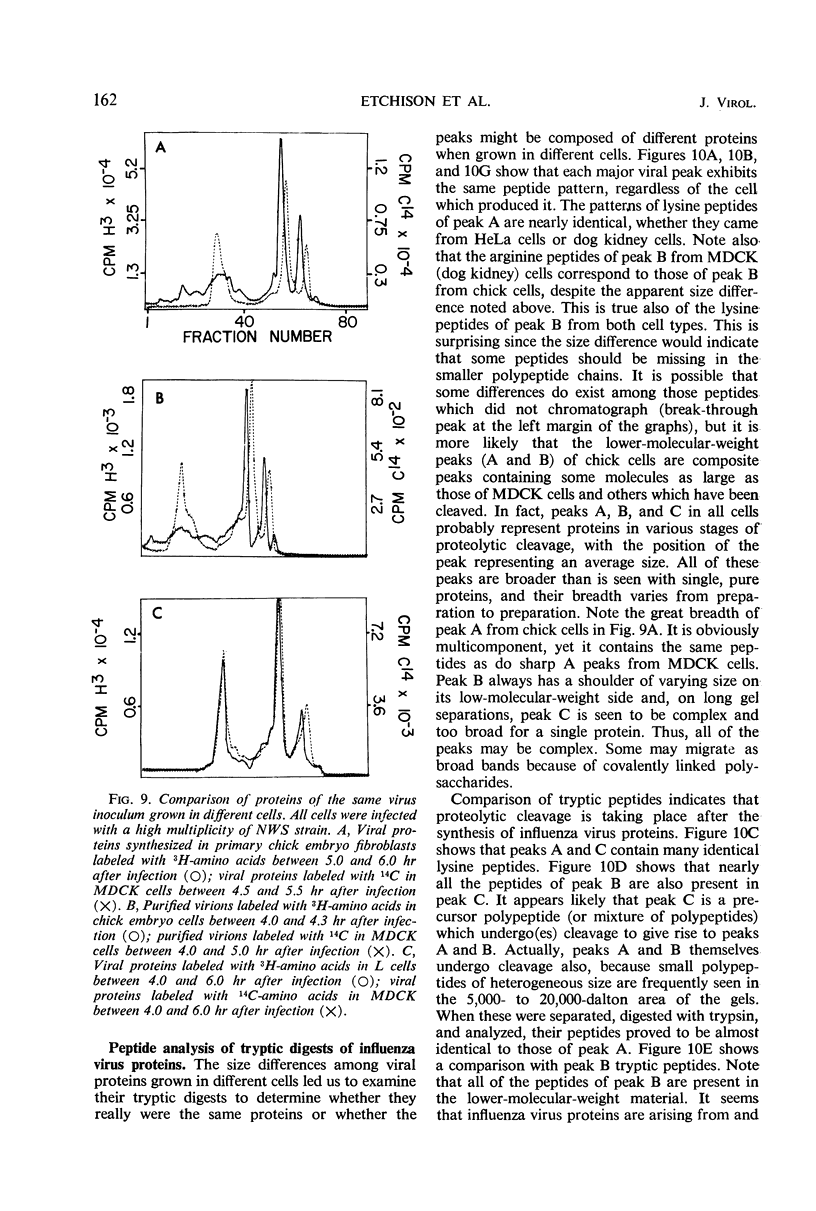
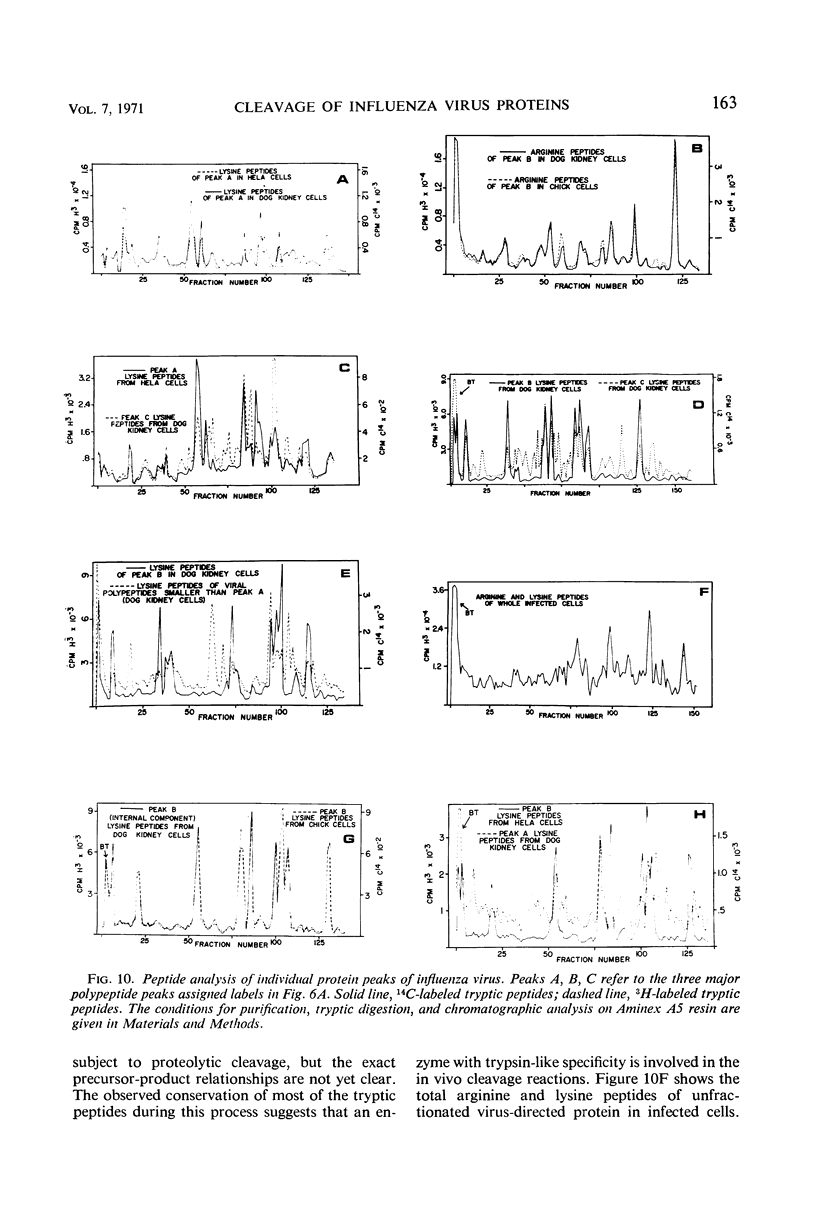
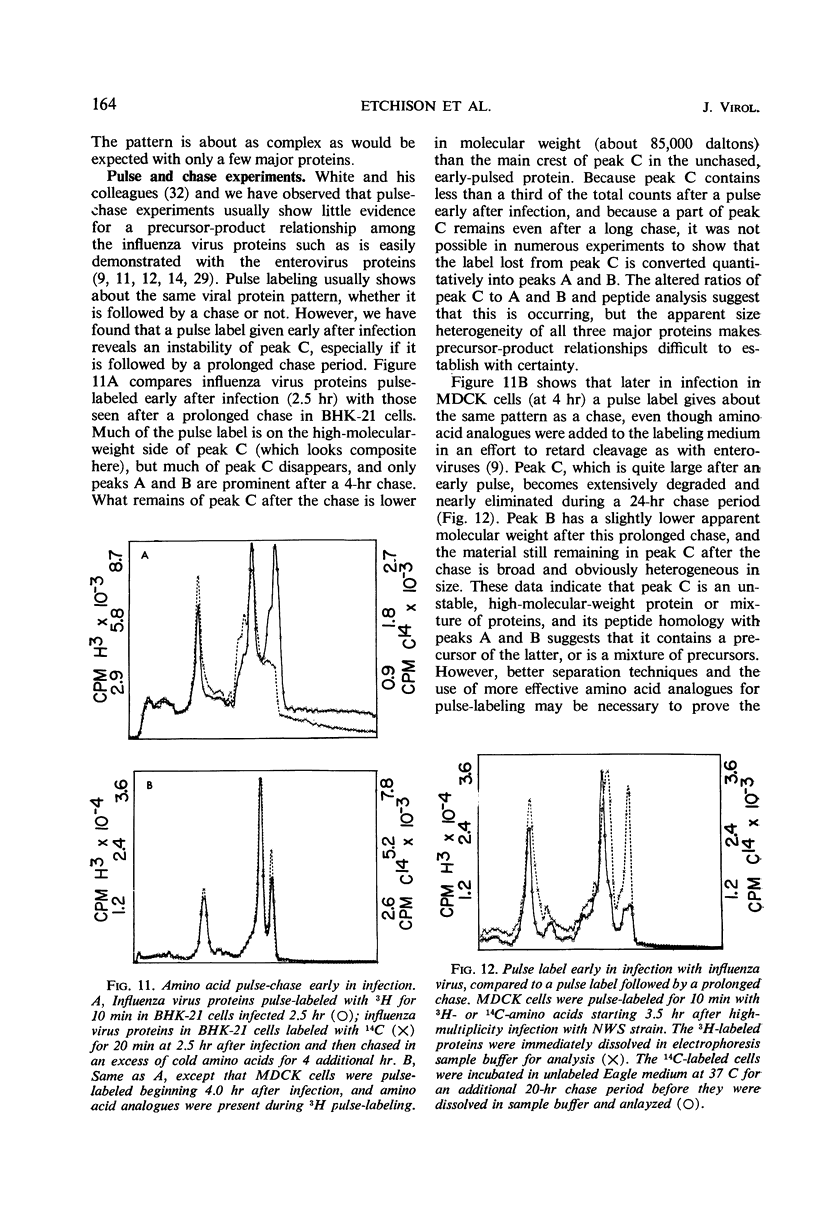
The NWS strain of influenza virus grows rapidly in and kills the MDCK dog kidney cell strain. Within 1 to 2 hr, the virus inhibits host cell protein synthesis and for 3 to 4 hr more it directs the synthesis of influenza virus proteins at a rate about twice that of uninfected cell synthesis. The rates of virus ribonucleic acid (RNA) and protein synthesis reach a maximum within the first few hours after infection and then drop. Plaque assays exhibit a linear dose-response, indicating that only one virion is necessary for productive infection. We have confirmed earlier reports regarding the fragmented nature of the RNA genome of purified influenza virions. However, high resolution gel electrophoresis indicated that each size class of viral RNA is heterogenous, so that there are at least 10 and probably more fragment sizes of RNA in these virions. Repeated attempts to detect infectivity in preparations of extracted viral RNA were completely negative (over a 108-fold loss of infectivity after extraction). Even infection of the “infectious” RNA-treated cells with intact, related, influenza viruses failed to support infectivity of the isolated RNA or to rescue a host range genetic marker of the RNA. Purified influenza virions exhibit only three major protein peaks based on separation according to molecular weights. These three major virion proteins are the only major virion proteins synthesized in infected cells. This is true throughout the infectious cycle from several hours after infection until the cells are dying. However, the molecular weight of these virion proteins differs slightly depending upon the cell type in which the virus is grown. No host membrane proteins are incorporated into the virions as they bud through the cell membrane. Pulse-chase labeling early after infection or prolonged chase experiments indicate that influenza virus proteins are cleaved from one or more precursor polypeptides. In fact, each of the three major peaks seems to be a heterogeneous mixture of polypeptides in various stages of cleavage. Peptide analysis confirms that the three major peaks share common peptides, but the exact precursor product relationships are not clear. There may be one or several precursor proteins. Also there could be overlapping messenger RNA molecules of varying length giving rise to polypeptides of various sizes and overlapping sequences. Late in infection, amino acid labeling shows a preponderance of internal nucleocapsid protein synthesis, indicating that either this protein is much more stable to cleavage in infection or it is made from a more stable messenger. There is no obvious relationship between virion RNA fragments and viral protein sizes, so these fragments may be artifacts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY R. D., IVES D. R., CRUICKSHANK J. G. Participation of deoxyribonucleic acid in the multiplication of influenza virus. Nature. 1962 Jun 23;194:1139–1140. doi: 10.1038/1941139a0. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough H. A., Merlie J. P. The lipids of incomplete influenza virus. Virology. 1970 Mar;40(3):685–692. doi: 10.1016/0042-6822(70)90213-8. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J. New virus-specific antigens in cells infected with influenza virus. Virology. 1969 Oct;39(2):224–234. doi: 10.1016/0042-6822(69)90042-7. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. The RNA of influenza virus. Proc Natl Acad Sci U S A. 1968 Mar;59(3):930–937. doi: 10.1073/pnas.59.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. P., Walters C. P. Influenza virus-induced ribonucleic acid nucleotidyltransferase and the effect of actinomycin D on its formation. Biochemistry. 1966 Jan;5(1):231–235. doi: 10.1021/bi00865a030. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Influenza virus effects on cell membrane proteins. Science. 1970 Jan 9;167(3915):202–205. doi: 10.1126/science.167.3915.202. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Kiehn E. D., Holland J. J. Membrane and nonmembrane proteins of mammalian cells. Synthesis, turnover, and size distribution. Biochemistry. 1970 Apr 14;9(8):1716–1728. doi: 10.1021/bi00810a010. [DOI] [PubMed] [Google Scholar]
- Kiehn E. D., Holland J. J. Synthesis and cleavage of enterovirus polypeptides in mammalian cells. J Virol. 1970 Mar;5(3):358–367. doi: 10.1128/jvi.5.3.358-367.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycosphingolipids of plasma membranes of cultured cells and an enveloped virus (SV5) grown in these cells. Proc Natl Acad Sci U S A. 1970 May;66(1):57–64. doi: 10.1073/pnas.66.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVER W. G. STRUCTURAL STUDIES ON THE PROTEIN SUBUNITS FROM THREE STRAINS OF INFLUENZA VIRUS. J Mol Biol. 1964 Jul;9:109–124. doi: 10.1016/s0022-2836(64)80094-2. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Kilbourne E. D. Identification in a recombinant influenza virus of structural proteins derived from both parents. Virology. 1966 Nov;30(3):493–501. doi: 10.1016/0042-6822(66)90125-5. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. The structure of influenza viruses. IV. Chemical studies of the host antigen. Virology. 1966 Sep;30(1):104–115. doi: 10.1016/s0042-6822(66)81014-0. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- McConkey E. H., Hopkins J. W. Molecular weights of some HeLa ribosomal RNA's. J Mol Biol. 1969 Feb 14;39(3):545–550. doi: 10.1016/0022-2836(69)90144-2. [DOI] [PubMed] [Google Scholar]
- Nayak D. P., Baluda M. A. Isolation and partial characterization of nucleic acid of influenza virus. J Virol. 1967 Dec;1(6):1217–1223. doi: 10.1128/jvi.1.6.1217-1223.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W., Hirst G. K. Polyacrylamide gel electrophoresis of influenza virus RNA. Virology. 1968 Feb;34(2):385–388. doi: 10.1016/0042-6822(68)90257-2. [DOI] [PubMed] [Google Scholar]
- Pons M. W., Hirst G. K. Polyacrylamide gel electrophoresis of the replicative form of influenza virus RNA. Virology. 1968 May;35(1):182–184. doi: 10.1016/0042-6822(68)90322-x. [DOI] [PubMed] [Google Scholar]
- Pons M. W., Schulze I. T., Hirst G. K., Hauser R. Isolation and characterization of the ribonucleoprotein of influenza virus. Virology. 1969 Oct;39(2):250–259. doi: 10.1016/0042-6822(69)90045-2. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Ribonucleic acid nucleotidyl transferase induced in chick fibroblasts after infection with an influenza virus. J Gen Virol. 1969 Jan;4(1):125–137. doi: 10.1099/0022-1317-4-1-125. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Hampson A. W., White D. O. The polypeptides of influenza virus. 1. Cytoplasmic synthesis and nuclear accumulation. Virology. 1969 Nov;39(3):419–425. doi: 10.1016/0042-6822(69)90090-7. [DOI] [PubMed] [Google Scholar]
- Webster R. G. Estimation of the molecular weights of the polypeptide chains from the isolated hemagglutinin and neuraminidase subunits of influenza viruses. Virology. 1970 Mar;40(3):643–654. doi: 10.1016/0042-6822(70)90209-6. [DOI] [PubMed] [Google Scholar]




