Abstract
Purpose
Although a large number of anterior cruciate ligament (ACL) reconstructions are performed annually, there remains a considerable amount of controversy over whether an autograft or an allograft should be used. The aim of this meta-analysis was to compare the clinical outcomes of allograft and autograft in primary ACL reconstruction.
Methods
The authors systematically searched electronic databases to identify prospective studies which compared allografts with autografts for primary ACL reconstruction. The results of the eligible studies were analysed in terms of instrumented laxity measurements, Lachman test, Pivot Shift test, objective International Knee Documentation Committee (IKDC) Scores, Lysholm Scores, Tegner Scores, and clinical failures. Study quality was assessed and relevant data were extracted independently by two reviewers. A random effect model was used to pool the data. Statistical heterogeneity between trials was evaluated by the chi-square and I-square tests.
Results
Nine studies, with 410 patients in the autograft and 408 patients in the allograft group, met the inclusion criteria. Five studies compared bone-patellar tendon-bone (BPTB) grafts, and four compared soft-tissue grafts. Four studies were randomized controlled trials, and five were prospective cohort studies. The results of the meta-analysis showed that there were no significant differences between allograft and autograft on all the outcomes in terms of instrumented laxity measurements (P = 0.59), Lachman test (P = 0.41), Pivot Shift test (P = 0.88), objective IKDC Scores (P = 0.87), Lysholm Scores (P = 0.79), Tegner Scores (P = 0.06), and clinical failures (P = 0.68). These findings were still robust during the sensitivity analysis. However, a subgroup analysis of Tegner scores by involving only BPTB grafts showed a statistical difference in favour of autografts (P = 0.005).
Conclusions
There was insufficient evidence to identify which of the two types of grafts was significantly better for ACL reconstruction, though the subgroup analysis indicated that reconstruction with BPTB autograft might allow patients to return to higher levels of activity in comparison with BPTB allograft. More high-quality randomized controlled trials with specified age and activity level are highly required before drawing a reliable conclusion.
Introduction
Reconstruction of the anterior cruciate ligament (ACL) has become the gold-standard treatment for an ACL rupture to prevent knee instability, with an estimated 100,000 ACL reconstructions performed annually in the USA [1]. Despite the popularity of the procedure, there remains a considerable amount of controversy over whether an autograft or an allograft should be used for primary ACL reconstruction [2, 3]. Reconstruction with autografts has the major benefits of earlier incorporation and no rejection or disease transmission, but lead to potential donor-site morbidity. Allografts have the main advantages of eliminating donor-site morbidity, availability of multiple grafts, shorter operative times, less postoperative pain and faster rehabilitation [4, 5]. However, allografts have the major disadvantages of the risk for disease transmission, possible immunogenicity, and slower incorporation or ligamentization [6, 7]. To reduce the potential of disease transmission, gamma irradiation used to be a popular option for allografts secondary sterilization. However, many published studies have indicated that gamma irradiation significantly alters the initial biomechanical properties of allografts and may lead to poor clinical outcomes [8, 9]. Although the potential for disease transmission has been the main concern to patients and surgeons, the improved donor screening, modern procurement and sterilization techniques have significantly decreased the use of gamma irradiation to secondarily sterilize the allografts [10–12].
Over 50 published reports have evaluated the clinical results of allografts in comparison with autografts in the past 20 years. However, there is still considerable controversy regarding the use of allografts versus autografts in ACL reconstruction, because most of the publications are low-quality studies or different graft procurement and secondary sterilization techniques have been used in those studies. Until now, five previous studies systematically reviewed the clinical outcomes of allograft versus autograft for ACL reconstruction [13–17]. However, all the five systematic reviews were conducted over three years ago and the findings of those studies were compromised by the limited availability of high-quality trials. A number of new prospective comparative studies, especially randomized controlled trials, have been published since the latest systematic review. Therefore, we conducted an up-to-date meta-analysis of level I and II prospective studies that evaluated the clinical outcomes of allografts versus autografts for primary ACL reconstruction.
Methods
Search strategy
We searched the electronic databases Pubmed (1980 to October 31 2012), EMBASE (Ovid) (1988 to October 31 2012), Scopus (1980 to October 31 2012), Cochrane Central Register of Controlled Trials (until October 2012) and Cochrane Database of Systematic Reviews (2005 to October 2012). According to the search strategy of the Cochrane Collaboration, the search algorithm was “anterior cruciate ligament” or “ACL” in combination with “autograft” and “allograft”. Published studies in all languages were included for review. The full text was reviewed if the abstract indicated that the article might be a prospective comparative study with minimum two-year follow-up and non-irradiated allografts might have been used in the study. The references of these articles were also reviewed to identify potential additional publications.
Study selection
Eligibility Criteria: (1) A prospective comparative study (Level of Evidence I or II) [18]; (2) Patients with a unilateral ACL rupture requiring primary ACL reconstruction; (3) Bone-patellar tendon-bone (BPTB) autograft compared with BPTB allograft, or soft-tissue autograft compared with soft-tissue allograft; (4) Minimum two-year follow-up; (5) Including any clinically relevant subjective and objective outcomes, such as stability outcomes, functional outcomes, patient-oriented outcomes, or morbidity. Exclusion Criteria: (1) Case–control study, Retrospective cohort study or Case series; (2) Use of gamma irradiation in allografts; (3) BPTB grafts versus soft-tissue grafts.
Data extraction
Data were extracted independently from each eligible study by two reviewers using a pre-developed data extraction table. Any discrepancies between the extracted data were resolved by consensus. Where required, the corresponding authors were contacted for additional data. The following data were extracted from all eligible studies: the study design, population, when and where the trial was conducted, duration of follow-up, surgical techniques, properties of grafts, instrumented laxity measurements, Lachman test, Pivot Shift test, objective International Knee Documentation Committee (IKDC) scores, Lysholm scores, Tegner scores, and morbidity.
Assessment of study quality
Two investigators independently graded the methodological quality of each eligible study using the Detsky scale [19] for randomized controlled trials and the Newcastle-Ottawa Scale (NOS) [20] for prospective cohort studies. The quality scores obtained from the Detsky scale were converted into a percentage for ease of interpretation. Studies scoring >75 % on the Detsky scale were designated as high-quality randomized controlled trials [21]. The quality score of ≥7 on the 9-point NOS was chosen to represent high-quality prospective cohort studies [22].
Data analysis
Data analysis was performed with RevMan 5.1 (Cochrane Collaboration, Oxford, UK). A random-effect method was adopted for the pooling of results. Risk ratio (RR) was used as a summary statistic to perform statistical analysis of dichotomous variables, and the mean difference (MD) was used to analyse continuous variables. Both were reported with 95 % confidence intervals (CIs), and a P value of 0.05 was used as the level of statistical significance. Statistical heterogeneity between trials was evaluated by the chi-square and I-square tests, with significance set at P < 0.10. For data unable to be merged due to inconsistent data type, a descriptive analysis was performed. In situations where the standard deviations were not reported, the mean of the standard deviations from the other trials that reported this statistic was imputed [23]. To assess publication bias, a funnel plot was constructed for each outcome to examine the relationship between sample size and the magnitude of effect. A sensitivity analysis was conducted by excluding one study in each round and evaluating the influence of any single study on the primary meta-analysis estimate. In addition, a sensitivity analysis was also applied by only including those studies that did report the use of non-irradiated allografts, while excluding those studies underreporting the properties of allografts. We also performed a subgroup analysis to identify the potential differences in graft type (BPTB grafts or soft-tissue grafts).
Results
Literature search
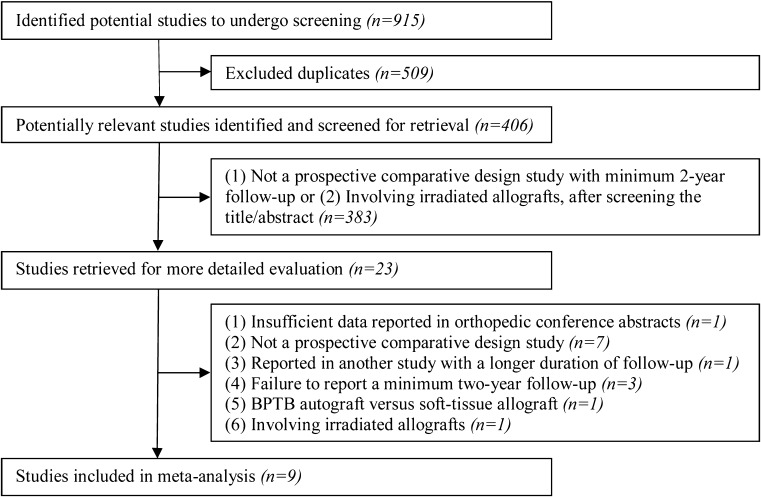
Our literature search generated 406 relevant citations after excluding the duplicates (n = 509) (Fig. 1). Subsequent review of the title/abstracts produced 23 articles that were retrieved for more detailed evaluation. One study was excluded because insufficient data reported in orthopaedic conference abstracts [24]. Seven studies were excluded because of not a prospective comparative study [25–31]. One study was excluded because data from the same patients were reported in another study with a longer duration of follow-up [32]. Three studies were excluded because of not a minimum two-year follow-up study [33–35]. One study was excluded because it compared BPTB autograft with soft-tissue allograft [36]. One study was excluded because of involving irradiated allografts [37]. Therefore, nine studies, with 410 patients in the autograft and 408 patients in the allograft group, were determined to be appropriate for this meta-analysis [38–46] (Table 1).
Fig. 1.
Selection process for meta-analysis of trials to compare allografts with autografts for anterior cruciate ligament reconstruction
Table 1.
Study descriptions
| First Author | Country | Date of publication | Study design | No. patient (auto/allo) | Mean age (yr) (auto/allo) | Mean follow-up (mo) (auto/allo) | Autograft | Allograft | Sterilization method | Detsky (%) or NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Edgar [41] | USA | 2008 | PCS | 37/46 | 27/31 | 52/48 | HT | HT | Non-irradiated | 9* |
| Kleipool [39] | Netherlands | 1998 | PCS | 26/36 | 28/28 | 52/46 | BPTB | BPTB | Non-irradiated | 8* |
| Leal-Blanquet [43] | Spain | 2011 | PCS | 15/16 | 29/25 | 36/34 | BPTB | BPTB | NA | 9* |
| Lawhorn [46] | USA | 2012 | RCT | 54/48 | 32/33 | 24/24 | HT | Anterior tibialis | Non-irradiated | 71 %# |
| Noh [44] | Korea | 2011 | RCT | 33/32 | 23/22 | 28/32 | HT | Free tendon Achilles | NA | 71 %# |
| Peterson [40] | USA | 2001 | PCS | 30/30 | 25/28 | 65/63 | BPTB | BPTB | Non-irradiated | 8* |
| Sun [42] | China | 2009 | RCT | 76/80 | 32/33 | 67/67 | BPTB | BPTB | Non-irradiated | 71 %# |
| Sun [45] | China | 2011 | RCT | 91/95 | 30/31 | 91/95 | HT | HT | Non-irradiated | 71 %# |
| Victor [38] | Belgium | 1997 | PCS | 48/25 | 28/28 | 24/24 | BPTB | BPTB | Non-irradiated | 7* |
PCS Prospective cohort studies; RCT Randomized controlled trials; auto Autograft; allo Allograft; yr Years; mo Months; BPTB Bone-patellar tendon-bone; HT Hamstring; NA Not available; NOS Newcastle-Ottawa Scale
*Points of 9; # Percentage of total 21 scores
Study characteristics and quality
Arthroscopic ACL reconstructions were conducted in all included studies. Within each study, the same surgical approaches and fixation methods were used for autograft and allograft, but one study did not report the specific fixation methods [38]. Similarly, within each study, postoperative rehabilitation was consistent for every patient. Five studies compared BPTB grafts [38–40, 42, 43], while two compared hamstring grafts [41, 45]; one compared hamstring autograft with anterior tibialis allograft [46] and one compared hamstring autograft with free tendon Achilles allograft [44]. Six of the nine eligible studies did report the use of non-irradiated allografts [39–42, 45, 46]. The corresponding authors of the other three studies were therefore contacted for the additional information of the allografts [38, 43, 44]. Only one author informed us that non-irradiated fresh-frozen allografts had been used in their study [38]. One study reported allografts (LifeNet Health Inc., Virginia Beach, VA, USA) had been used [44]. The detail information of allografts was not available in one study [43]. Seven studies used fresh-frozen allografts [38–40, 42, 44–46]; one study used both cryopreserved and fresh-frozen allografts [41]; and one study did not report the storage method [43]. All five prospective cohort studies were high-quality [38–41, 43], while none of the four randomized controlled trials was high-quality [42, 44–46] (Table 1).
Knee stability
KT-1000/2000 arthrometer
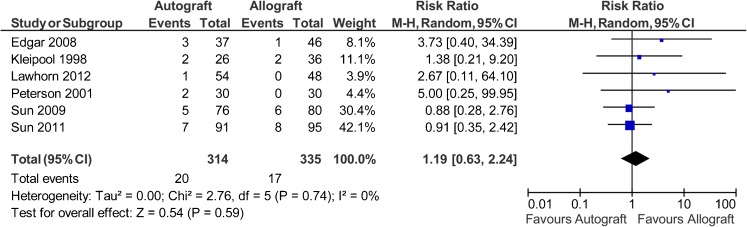
Eight studies used the instrumented laxity measurements as an outcome measure [38–43, 45, 46], but one study did not report the results [38] and one study only reported no significant difference on the mean side-to-side differences [43]. The authors of these studies were contacted for further information, but the data were unavailable. Therefore, only six studies reported data on 649 patients (314 autografts, 335 allografts) regarding the percentages of patients with a side-to-side difference >5 mm. The analysis showed the risk ratio for KT-1000/2000 side-to-side difference >5 mm was 1.19 in favour of allograft (95 % CI, 0.63 to 2.24; P = 0.59) (P = 0.74 for homogeneity) (Fig. 2).
Fig. 2.
Instrumented laxity measurement of >5 mm after anterior cruciate ligament reconstruction
Lachman test
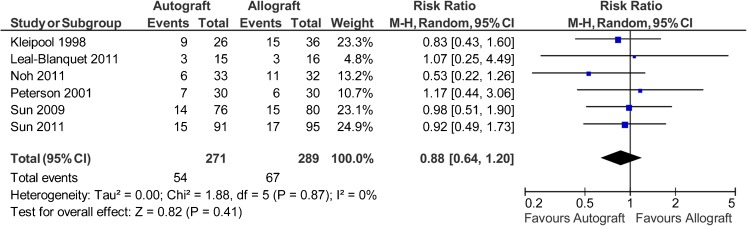
Six studies reported data on 560 patients (271 autografts, 289 allografts) regarding the manual Lachman test [39, 40, 42–45]. The analysis showed the risk ratio for abnormal Lachman test (grade >0) was 0.88 in favour of autograft (95 % CI, 0.64 to 1.2; P = 0.41) (P = 0.87 for homogeneity) (Fig. 3).
Fig. 3.
Abnormal Lachman test after anterior cruciate ligament reconstruction
Pivot shift test
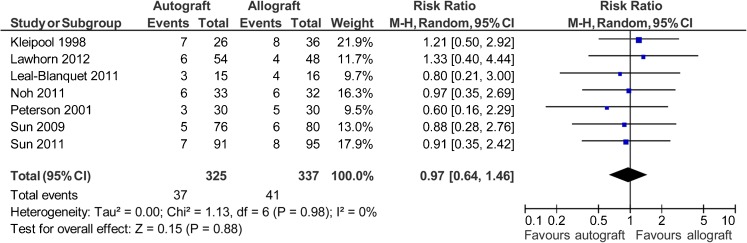
Seven studies reported data on 662 patients (325 autografts, 337 allografts) regarding the Pivot Shift test [39, 40, 42–46]. The analysis showed the risk ratio for abnormal Pivot Shift test (grade >0) was 0.97 in favour of autograft (95 % CI, 0.64 to 1.46; P = 0.88) (P = 0.98 for homogeneity) (Fig. 4).
Fig. 4.
Abnormal Pivot Shift test after anterior cruciate ligament reconstruction
Objective IKDC scores
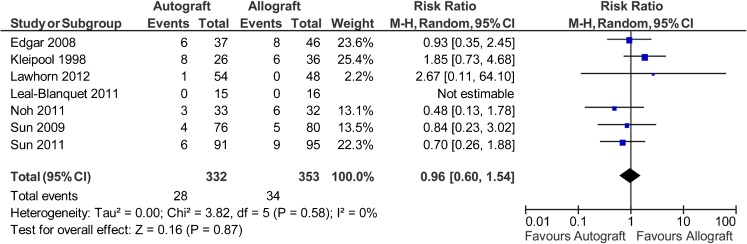
Seven studies reported data on 685 patients (332 autografts, 353 allografts) regarding the objective IKDC scores [39, 41–46]. For this analysis, the calculation of risk ratio was based on abnormal or severely abnormal versus normal or nearly normal (or IKDC C and D versus IKDC A and B). The analysis showed the risk ratio for abnormal or severely abnormal was 0.96 favouring autograft (95 % CI, 0.6 to 1.54; P = 0.87) (P = 0.58 for homogeneity) (Fig. 5).
Fig. 5.
Objective IKDC scores (abnormal or severely abnormal) after anterior cruciate ligament reconstruction
Lysholm and Tegner scores
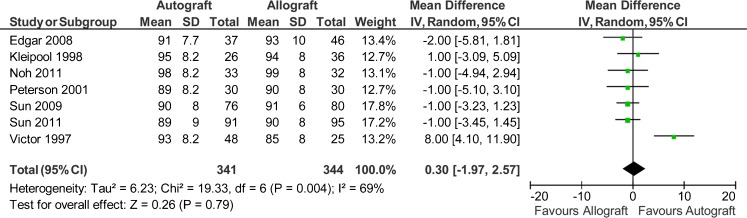
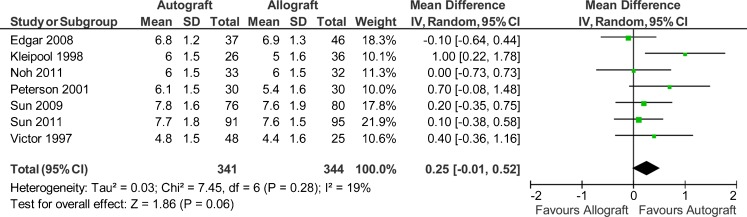
Seven studies reported data on 685 patients (341 autografts, 344 allografts) regarding the Lysholm scores and Tegner scores [38–42, 44, 45]. However, only three studies reported the standard deviations [41, 42, 45]. The authors of the other four studies were therefore contacted for further information, but the data were unavailable. So the imputed standard deviations were used for this meta-analysis. The analysis showed a mean difference of 0.3 on Lysholm scores in favour of autograft (95 % CI, −1.97 to 2.57; P = 0.79) (P = 0.004 for homogeneity) (Fig. 6). The analysis showed a mean difference of 0.25 on Tegner scores in favour of autograft (95 % CI, −0.01 to 0.52; P = 0.06) (P = 0.28 for homogeneity) (Fig. 7).
Fig. 6.
Lysholm scores after anterior cruciate ligament reconstruction
Fig. 7.
Tegner scores after anterior cruciate ligament reconstruction
Morbidity
Only five studies reported the donor-site symptoms [38–40, 43, 45]. Three studies reported data on anterior knee pain, and demonstrated no significant difference between autograft and allograft [38, 39, 43]. Peterson et al. reported that the rate of incisional site complaints was 53 % (16 of 30) in the autograft group and 7 % (2 of 30) in the allograft group [40]. Sun et al. reported that the rate of harvest site complaints was 2 % (2 of 91) in the autograft group and 0 % (0 of 95) in the allograft group [45].
There were seven studies reported the knee range of motion [39, 40, 42–46]. Only one study indicated that there was significant more extension loss in the autograft group in comparison with the allograft group [40]. The other six studies did not show a significant difference on the knee range of motion [39, 42–46]. Four studies evaluated the deep infection rate, but none of the patients was infected after autograft or allograft reconstruction [40–42, 45]. Similarly, within each individual study, there were no significant differences on arthrofibrosis [40, 41, 45, 46] and reoperation rates [39, 44–46] between autograft and allograft.
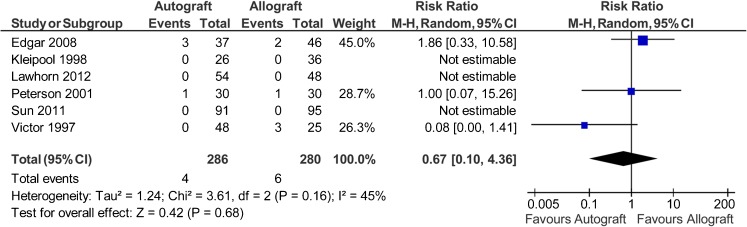
Data on graft clinical failures were available for 566 patients in six studies [38–41, 45, 46]. Clinical failures were reported in four of 286 patients in the autograft group (1.4 %) and six of 280 patients in the allograft group (2.1 %). The risk ratio of graft failure was 0.67 favouring autograft (95 % CI, 0.1 to 4.36; P = 0.68) (P = 0.16 for homogeneity) (Fig. 8).
Fig. 8.
Clinical failures after anterior cruciate ligament reconstruction
Publication bias
The funnel plots of each above outcome appeared mild asymmetrical about pooled estimates from the meta-analysis. Although the small number of studies available for comparison might have contributed to the asymmetry, it did suggest a possible publication bias.
Sensitivity analysis and subgroup analysis
A series of sensitivity analysis was conducted by omitting one of the eligible studies at a time. The results showed that there was not a particularly influential study among all selected studies, except the impact of the Victor’s trial on the Lysholm scores [38]. Exclusion of the Victor’s trial slightly altered the results on the Lysholm scores in the sensitivity analysis and estimated a mean difference of −0.92 favouring allograft (95 % CI, −2.19 to 0.35; P = 0.16) (P = 0.95 for homogeneity). However, there was still no statistic difference between autograft and allograft. Compared with the overall main analysis, pooled data only from those seven studies [38–42, 45, 46], which did report the use of non-irradiated allografts, gave consistent findings for stability outcomes, objective IKDC scores, Lysholm scores, Tegner scores and clinical failures (Table 2).
Table 2.
Sensitivity analysis was conducted by only pooling data from the seven studies that did report no use of gamma radiation in allografts
| Outcomes | Risk ratio or mean difference (95 % CI) | P value | Test for heterogeneity | No of patients | No of studies |
|---|---|---|---|---|---|
| Instrumented laxity | 1.19 (0.63, 2.24) | 0.59 | 0.74 | 649 | 6 |
| Lachman test | 0.94 (0.66, 1.33) | 0.73 | 0.95 | 464 | 4 |
| Pivot Shift test | 0.99 (0.61, 1.6) | 0.97 | 0.9 | 566 | 5 |
| IKDC scores | 1.07 (0.65, 1.76) | 0.8 | 0.63 | 589 | 5 |
| Lysholm scores | 0.52 (−2.08, 3.13) | 0.69 | 0.002 | 620 | 6 |
| Tegner scores | 0.29 (−0.01, 0.59) | 0.06 | 0.22 | 620 | 6 |
| Clinical failures | 0.67 (0.10, 4.36) | 0.68 | 0.16 | 566 | 6 |
IKDC International Knee Documentation Committee
Subgroup analysis was performed according to the graft types (BPTB graft or soft-tissue graft). However, this subgroup analysis did not change the findings in the majority of the outcomes, but the Tegner scores (Table 3). The analysis of Tegner scores by only pooling four studies involving BPTB grafts [38–40, 42] estimated a mean difference of 0.5 in favour of autograft (95 % CI, 0.15 to 0.85; P = 0.005) (P = 0.38 for homogeneity). The analysis of Tegner scores by only pooling three studies involving soft-tissue grafts [41, 45, 46] estimated a mean difference of 0.01 favouring autograft (95 % CI, −0.31 to 0.33; P = 0.95) (P = 0.86 for homogeneity).
Table 3.
Subgroup analysis was to identify the potential differences in graft sources
| Outcomes | BPTB grafts | Soft-tissue grafts | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk ratio or mean difference (95 % CI) | P value | Test for heterogeneity | No. of patients | No. of studies | Risk ratio or mean difference (95 % CI) | P value | Test for heterogeneity | No. of patients | No. of studies | |
| Instrumented laxity | 1.16 (0.46, 2.94) | 0.76 | 0.55 | 278 | 3 | 1.22 (0.52, 2.87) | 0.65 | 0.46 | 371 | 3 |
| Lachman test | 0.96 (0.64, 1.43) | 0.83 | 0.95 | 309 | 4 | 0.76 (0.45, 1.27) | 0.3 | 0.31 | 251 | 2 |
| Pivot Shift test | 0.92 (0.53, 1.61) | 0.77 | 0.84 | 309 | 4 | 1.03 (0.56, 1.89) | 0.93 | 0.88 | 353 | 3 |
| IKDC scores | 1.41 (0.66, 2.98) | 0.37 | 0.33 | 249 | 3 | 0.76 (0.42, 1.38) | 0.36 | 0.74 | 436 | 4 |
| Lysholm scores | 1.65 (–2.41, 5.71) | 0.43 | 0.001 | 351 | 4 | –1.23 (–3.06, 0.6) | 0.19 | 0.9 | 334 | 3 |
| Tegner scores | 0.5 (0.15, 0.85) | 0.005 | 0.38 | 351 | 4 | 0.01 (–0.31, 0.33) | 0.95 | 0.86 | 334 | 3 |
| Clinical failures | 0.29 (0.02, 3.77) | 0.35 | 0.2 | 195 | 3 | 1.86 (0.33, 10.58) | 0.48 | NA | 371 | 3 |
IKDC International Knee Documentation Committee; BPTB Bone-patellar tendon-bone; NA Not available
Discussion
Prospective comparative study has the main merits of the accuracy of data collection with regard to exposures, confounders and endpoints, and thus usually has fewer potential sources of bias and confounding than case–control study or retrospective study. Meta-analysis allows us to quantitatively analyse multiple similar prospective comparative studies to increase sample size and improve statistical power. Therefore, we performed this meta-analysis of prospective comparative studies to compare the curative effects of ACL reconstruction using either autografts or allografts, to provide a reference for the selection of graft sources. The results of the current meta-analysis showed that allografts ACL reconstruction could produce similar clinical outcomes in comparison with autografts.
The instrumented laxity measurements and Lachman test are typically used to examine the anterior-posterior stability of the knee, and the Pivot Shift test is commonly used to determine the rotational stability. Many factors during and after surgery can influence the knee stability: the surgical approaches, fixation methods, bone-to-bone versus tendon-to-bone healing, and postoperative rehabilitation. To reduce these confounding variables, this meta-analysis only pooled these studies that the surgical approaches, fixation methods and postoperative rehabilitation were consistent within each individual study, and excluded those studies comparing BPTB graft with soft-tissue graft [36]. In this meta-analysis, no significant difference could be found between autografts and allografts, regarding to the instrumented laxity measurements, Lachman test and Pivot Shift test. These findings were still robust during the sensitivity analysis and subgroup analysis, which varied the included studies on the basis of secondary sterilization technique and graft types.
The objective IKDC scores can provide an overall evaluation of postoperative ACL reconstruction outcomes. This meta-analysis demonstrated that there were no significant differences in objective IKDC scores between autografts and allografts, indicating that allografts could achieve similar outcomes in comparison with autografts. Similarly, the meta-analysis of Lysholm and Tegner scores indicated risk ratios were not significantly different. However, a subgroup analysis of Tegner scores by only involving BPTB grafts showed a statistical difference in favour of autografts. These findings suggested that although BPTB autograft and BPTB allograft ACL reconstructions exhibited similar knee stability and functional outcomes, BPTB autografts might be indicated to allow patients to return to higher levels of activity postoperatively without the sense of instability.
Until now, clinical failures of ACL reconstruction were not identically defined [47]. This meta-analysis only pooled the data from those six studies [38–41, 45, 46], in which the clinical failure was defined by the authors. There was no statistically significant difference on clinical failures between the allograft and autograft groups. The elimination of donor-site morbidity by using allografts is the main concern to patients and surgeons [2]. However, only five of the nine eligible studies reported the donor-site symptoms as an outcome measure. It was difficult to conduct a meta-analysis and make a conclusion, as there was no standardized method to report donor-site morbidity. Standardized outcomes for accessing the clinical failures and donor-site morbidity are highly needed for future studies.
With the advent of improved donor screening and modern procurement and sterilization techniques, the allografts can be processed aseptically without gamma irradiation [2, 10, 11]. Therefore, two prospective comparative studies with minimum two-year follow-up were excluded from this meta-analysis, due to involving the irradiated allografts [37, 48]. Unfortunately, although we tried our best to contact the corresponding authors of those three studies that did not report the detail information of allografts, we still did not know the secondary sterilization methods of allografts in two included studies [43, 44]. Therefore, we firstly included all nine studies that did not report use of gamma radiation in allografts. And then a sensitivity analysis was conducted by only including those seven studies that did report no use of gamma irradiation in allografts. However, this sensitivity analysis did not change all the findings in terms of stability outcomes, objective IKDC scores, Lysholm scores, Tegner scores and clinical failures.
A number of systematic reviews and meta-analysis have compared the clinical outcomes of allograft ACL reconstruction with those of autograft over the past few years. The first meta-analysis conducted by Prodromos et al. reported better stability with autograft reconstruction [13]. However, the findings of this study were compromised by the selection bias and questionable statistical methods. Another meta-analysis found that patients with allografts reconstruction might have increased joint laxity as measured by the KT-1000 arthrometer [16]. While no statistically significant differences were found on all the other outcomes between autograft and allograft ACL reconstruction. Although this meta-analysis included 56 studies, none of the eligible studies was a prospective comparative study (allograft versus autograft) [16]. As to the other three systematic reviews and meta-analyses, when those studies involving the irradiated allografts were excluded for analysis, their findings were consistent with the results of our study [14, 15, 17].
This study has several limitations. First, although five new prospective studies [42–46], including four randomized controlled trials, were added in this study since the latest systematic review, none of the nine eligible studies was high-quality randomized controlled trials. This might weaken the strength of the findings. Second, although only those studies with a minimum two-year follow-up were included for analysis, a previous study suggested that complete remodeling and cell replacement of the entire ACL grafts might take at least three years [6]. The findings of this study could only be generalized as short-term clinical outcomes of allograft ACL reconstruction. More high-quality randomized controlled trials with long-term follow-up are necessary to make a firm conclusion. Third, the impact of patients’ characteristics (such as age, sex, and activity level) on the outcomes could not be analysed in this meta-analysis due to the limited availability of data. Forth, the standard deviations were unavailable in some studies, so the imputed standard deviations were used for pooling data, which also compromised the findings of this meta-analysis.
In summary, the current evidence was insufficient to identify which of the two graft sources was significantly better for ACL reconstruction, though the subgroup analysis indicated that reconstruction with BPTB autograft might allow patients to return to higher levels of activity in comparison with BPTB allograft. More high-quality randomized controlled trials with specified age and activity level are highly required before drawing a firm conclusion.
Acknowledgments
Acknowledgments
This work was supported by The National Natural Science Foundation of China (No. 81171699).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Griffin LY, Agel J, Albohm MJ, Arendt EA, Dick RW, Garrett WE, Garrick JG, Hewett TE, Huston L, Ireland ML, Johnson RJ, Kibler WB, Lephart S, Lewis JL, Lindenfeld TN, Mandelbaum BR, Marchak P, Teitz CC, Wojtys EM. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Fu F, Christel P, Miller MD, Johnson DL. Graft selection for anterior cruciate ligament reconstruction. Instr Course Lect. 2009;58:337–354. [PubMed] [Google Scholar]
- 3.Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A (2012) An international survey on anterior cruciate ligament reconstruction practices. Int Orthop [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 4.Harner CD, Irrgang JJ, Paul J, Dearwater S, Fu FH. Loss of motion after anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20:499–506. doi: 10.1177/036354659202000503. [DOI] [PubMed] [Google Scholar]
- 5.Jackson DW, Grood ES, Goldstein JD, Rosen MA, Kurzweil PR, Cummings JF, Simon TM. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176–185. doi: 10.1177/036354659302100203. [DOI] [PubMed] [Google Scholar]
- 6.Malinin TI, Levitt RL, Bashore C, Temple HT, Mnaymneh W. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy. 2002;18:163–170. doi: 10.1053/jars.2002.30485. [DOI] [PubMed] [Google Scholar]
- 7.Mroz TE, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg. 2008;16:559–565. doi: 10.5435/00124635-200810000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Fideler BM, Vangsness CT, Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med. 1995;23:643–646. doi: 10.1177/036354659502300521. [DOI] [PubMed] [Google Scholar]
- 9.Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35:1653–1658. doi: 10.1177/0363546507302926. [DOI] [PubMed] [Google Scholar]
- 10.Vangsness CT, Jr, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31:474–481. doi: 10.1177/03635465030310032701. [DOI] [PubMed] [Google Scholar]
- 11.McAllister DR, Joyce MJ, Mann BJ, Vangsness CT., Jr Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35:2148–2158. doi: 10.1177/0363546507308936. [DOI] [PubMed] [Google Scholar]
- 12.Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26:1593–1601. doi: 10.1016/j.arthro.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15:851–856. doi: 10.1007/s00167-007-0328-6. [DOI] [PubMed] [Google Scholar]
- 14.Krych AJ, Jackson JD, Hoskin TL, Dahm DL. A meta-analysis of patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction. Arthroscopy. 2008;24:292–298. doi: 10.1016/j.arthro.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Carey JL, Dunn WR, Dahm DL, Zeger SL, Spindler KP. A systematic review of anterior cruciate ligament reconstruction with autograft compared with allograft. J Bone Joint Surg Am. 2009;91:2242–2250. doi: 10.2106/JBJS.I.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tibor LM, Long JL, Schilling PL, Lilly RJ, Carpenter JE, Miller BS. Clinical outcomes after anterior cruciate ligament reconstruction: a meta-analysis of autograft versus allograft tissue. Sports Health. 2010;2:56–72. doi: 10.1177/1941738109347984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster TE, Wolfe BL, Ryan S, Silvestri L, Kaye EK. Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? An evaluation of autograft versus allograft reconstruction results: a systematic review. Am J Sports Med. 2010;38:189–199. doi: 10.1177/0363546509356530. [DOI] [PubMed] [Google Scholar]
- 18.Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85-A:1–3. [PubMed] [Google Scholar]
- 19.Detsky AS, Naylor CD, O’Rourke K, McGeer AJ, L’Abbe KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992;45:255–265. doi: 10.1016/0895-4356(92)90085-2. [DOI] [PubMed] [Google Scholar]
- 20.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 10 Oct 2012
- 21.Sheth U, Simunovic N, Klein G, Fu F, Einhorn TA, Schemitsch E, Ayeni OR, Bhandari M. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94:298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 22.Simunovic N, Devereaux PJ, Sprague S, Guyatt GH, Schemitsch E, Debeer J, Bhandari M. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609–1616. doi: 10.1503/cmaj.092220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Alexander A, Garcia EA, Bynum EB, Sitler DF. Allograft versus autograft patellar tendon anterior cruciate ligament reconstruction: a prospective randomized study (early results) [Abstract] Ort Trans. 1996;20:912. [Google Scholar]
- 25.Harner CD, Olson E, Irrgang JJ, Silverstein S, Fu FH, Silbey M (1996) Allograft versus autograft anterior cruciate ligament reconstruction: 3- to 5-year outcome. Clin Orthop Relat Res:134–144 [DOI] [PubMed]
- 26.Stringham DR, Pelmas CJ, Burks RT, Newman AP, Marcus RL. Comparison of anterior cruciate ligament reconstructions using patellar tendon autograft or allograft. Arthroscopy. 1996;12:414–421. doi: 10.1016/S0749-8063(96)90034-1. [DOI] [PubMed] [Google Scholar]
- 27.Barrett G, Stokes D, White M. Anterior cruciate ligament reconstruction in patients older than 40 years: allograft versus autograft patellar tendon. Am J Sports Med. 2005;33:1505–1512. doi: 10.1177/0363546504274202. [DOI] [PubMed] [Google Scholar]
- 28.Wang K, Zhu L, Zeng C, Lu HD, Cai DZ. Comparative study on anterior cruciate ligament reconstruction with three different grafts in arthroscopy: a two-year follow-up. J Clin Rehabil Tissue Eng Res. 2007;11:5676–5679. [Google Scholar]
- 29.Landes S, Nyland J, Elmlinger B, Tillett E, Caborn D. Knee flexor strength after ACL reconstruction: comparison between hamstring autograft, tibialis anterior allograft, and non-injured controls. Knee Surg Sports Traumatol Arthrosc. 2010;18:317–324. doi: 10.1007/s00167-009-0931-9. [DOI] [PubMed] [Google Scholar]
- 30.Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26:S58–66. doi: 10.1016/j.arthro.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Tao H, Cho S, Chen S, Yao Z. Difference in graft maturity of the reconstructed anterior cruciate ligament 2 years postoperatively: a comparison between autografts and allografts in young men using clinical and 3.0-T magnetic resonance imaging evaluation. Am J Sports Med. 2012;40:1519–1526. doi: 10.1177/0363546512443050. [DOI] [PubMed] [Google Scholar]
- 32.Shelton WR, Papendick L, Dukes AD. Autograft versus allograft anterior cruciate ligament reconstruction. Arthroscopy. 1997;13:446–449. doi: 10.1016/S0749-8063(97)90122-5. [DOI] [PubMed] [Google Scholar]
- 33.Collette M, Dupont B, Peters M. Reconstruction of the anterior cruciate ligament with a free graft of the patellar tendon: allograft versus autograft. Acta Orthop Belg. 1991;57(Suppl 2):54–60. [PubMed] [Google Scholar]
- 34.Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17:464–474. doi: 10.1007/s00167-008-0714-8. [DOI] [PubMed] [Google Scholar]
- 35.Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States military academy. Am J Sports Med. 2012;40:1242–1246. doi: 10.1177/0363546512443945. [DOI] [PubMed] [Google Scholar]
- 36.Poehling GG, Curl WW, Lee CA, Ginn TA, Rushing JT, Naughton MJ, Holden MB, Martin DF, Smith BP. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21:774–785. doi: 10.1016/j.arthro.2005.04.112. [DOI] [PubMed] [Google Scholar]
- 37.Gorschewsky O, Klakow A, Riechert K, Pitzl M, Becker R. Clinical comparison of the Tutoplast allograft and autologous patellar tendon (bone-patellar tendon-bone) for the reconstruction of the anterior cruciate ligament: 2- and 6-year results. Am J Sports Med. 2005;33:1202–1209. doi: 10.1177/0363546504271510. [DOI] [PubMed] [Google Scholar]
- 38.Victor J, Bellemans J, Witvrouw E, Govaers K, Fabry G. Graft selection in anterior cruciate ligament reconstruction–prospective analysis of patellar tendon autografts compared with allografts. Int Orthop. 1997;21:93–97. doi: 10.1007/s002640050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleipool AE, Zijl JA, Willems WJ. Arthroscopic anterior cruciate ligament reconstruction with bone-patellar tendon-bone allograft or autograft. A prospective study with an average follow up of 4 years. Knee Surg Sports Traumatol Arthrosc. 1998;6:224–230. doi: 10.1007/s001670050104. [DOI] [PubMed] [Google Scholar]
- 40.Peterson RK, Shelton WR, Bomboy AL. Allograft versus autograft patellar tendon anterior cruciate ligament reconstruction: a 5-year follow-up. Arthroscopy. 2001;17:9–13. doi: 10.1053/jars.2001.19965. [DOI] [PubMed] [Google Scholar]
- 41.Edgar CM, Zimmer S, Kakar S, Jones H, Schepsis AA. Prospective comparison of auto and allograft hamstring tendon constructs for ACL reconstruction. Clin Orthop Relat Res. 2008;466:2238–2246. doi: 10.1007/s11999-008-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25:750–759. doi: 10.1016/j.arthro.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 43.Leal-Blanquet J, Alentorn-Geli E, Tuneu J, Valenti JR, Maestro A. Anterior cruciate ligament reconstruction: a multicenter prospective cohort study evaluating 3 different grafts using same bone drilling method. Clin J Sport Med. 2011;21:294–300. doi: 10.1097/JSM.0b013e31822153cb. [DOI] [PubMed] [Google Scholar]
- 44.Noh JH, Yi SR, Song SJ, Kim SW, Kim W. Comparison between hamstring autograft and free tendon Achilles allograft: minimum 2-year follow-up after anterior cruciate ligament reconstruction using EndoButton and Intrafix. Knee Surg Sports Traumatol Arthrosc. 2011;19:816–822. doi: 10.1007/s00167-010-1388-6. [DOI] [PubMed] [Google Scholar]
- 45.Sun K, Zhang J, Wang Y, Xia C, Zhang C, Yu T, Tian S. Arthroscopic reconstruction of the anterior cruciate ligament with hamstring tendon autograft and fresh-frozen allograft: a prospective, randomized controlled study. Am J Sports Med. 2011;39:1430–1438. doi: 10.1177/0363546511400384. [DOI] [PubMed] [Google Scholar]
- 46.Lawhorn KW, Howell SM, Traina SM, Gottlieb JE, Meade TD, Freedberg HI. The effect of graft tissue on anterior cruciate ligament outcomes: a multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28:1079–1086. doi: 10.1016/j.arthro.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Carey JL. Pediatric anterior cruciate ligament reconstruction with autograft or allograft. Clin Sports Med. 2011;30:759–766. doi: 10.1016/j.csm.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Sun K, Zhang J, Wang Y, Xia C, Zhang C, Yu T, Tian S. Arthroscopic anterior cruciate ligament reconstruction with at least 2.5 years’ follow-up comparing hamstring tendon autograft and irradiated allograft. Arthroscopy. 2011;27:1195–1202. doi: 10.1016/j.arthro.2011.03.083. [DOI] [PubMed] [Google Scholar]